Abstract
OBJECTIVE
To assess whether red blood cell (RBC) docosahexaenoic acid and eicosapentaenoic acid (DHA+EPA) levels have a protective association with the risk of dementia in older women.
METHODS
RBC DHA+EPA levels were assessed at baseline, and cognitive status was evaluated annually in a cohort of 6706 women aged ≥65 years who participated in the Women’s Health Initiative Memory Study (WHIMS). Cox regression was used to quantify the association between RBC DHA+EPA and the risk of probable dementia, independent of major dementia risk factors.
RESULTS
During a median follow-up period of 9.8 years, 587 incident cases of probable dementia were identified. After adjusting for demographic, clinical, and behavioral risk factors, a one standard deviation increase in DHA+EPA levels was associated with a significantly lower risk of dementia (HR = 0.92, 95% CI: 0.84, 1.00; p < 0.05). This effect estimate did not meaningfully change after further adjustment for baseline cognitive function and APOE genotype. For women with high DHA+EPA exposure (1 SD above mean) compared to low exposure (1 SD below mean), the adjusted 15-year absolute risk difference for dementia was 2.1% (95% CI: 0.2%, 4.0%). In secondary analyses, we also observed a protective association with longitudinal change in Modified Mini-Mental State (3MS) Exam scores, but no significant association with incident MCI, PD/MCI, or baseline 3MS scores.
DISCUSSION
Higher levels of DHA+EPA may help protect against the development of dementia. Results from prospective randomized controlled trials of DHA+EPA supplementation are needed to help clarify whether this association is causal.
Keywords: All cognitive disorders/dementia, Alzheimer’s Disease, Cohort studies, Omega-3 fatty acids, Women, biomarkers
INTRODUCTION
With the aging of the U.S. population, developing interventions to prevent and treat AD/D has become an increasingly important public health priority. Due to their longer life expectancy, the disease burden of AD/D is especially high for women. For a woman aged 65 years, the subsequent lifetime risk of dementia is 20%.[1]
No truly effective pharmacological or non-pharmacological strategy exists for the prevention and/or treatment of Alzheimer’s disease and related dementias.[2, 3] Interest in a potential role of long-chain, marine omega-3 fatty acids (FA) including eicosapentaenoic and docosahexaenoic acids (EPA and DHA, respectively) has grown based on a variety of observations. These include the structural presence of DHA in neural tissues,[4] the anti-inflammatory properties of these FAs,[5] and the ability of the DHA metabolite resolvin-D1 to increase phagocytosis of amyloid-β by monocytes.[6] In addition, epidemiologic studies have shown that diets richer in fish are associated with reduced risk for dementia[7, 8] and less neuropathology.[9]
In the present study, we evaluated the association between DHA+EPA exposure and incident dementia in the Women’s Health Initiative (WHI) Memory Study (WHIMS), a large and well-characterized cohort of older U.S. women. Secondary analyses explored relationships with other cognitive outcomes, including incident mild cognitive impairment (MCI) and longitudinal changes in Modified Mini-Mental State (3MS) scores. We hypothesized that higher levels of RBC DHA+EPA would have protective associations with these outcomes.
METHODS
Study Population
This study was a secondary analysis of longitudinal data collected for the WHIMS study cohort. WHIMS was an ancillary study of cognitive outcomes in 7479 older women who participated in the WHI randomized trials of hormone therapy (HT). In the trials, women with a prior hysterectomy were randomized to receive 0.625 mg conjugated equine estrogens (CEE) or placebo daily, and women with an intact uterus received a combination of 0.625 mg CEE and 2.5 mg progestin (CEE+P) or placebo daily.[10, 11] All participants gave informed consent, and institutional review boards approved the study protocols. At the time of enrollment in WHIMS, participants were 65–80 years of age and free of dementia. The CEE+P and CEE-only trials were terminated in 2002 and 2004, respectively, when it was determined that the risk-benefit ratio was unfavorable for participants assigned to active treatment.[12, 13] Observational follow-up continued after the end of the trial interventions.
For the present study, we excluded women who were missing biomarker data on omega-3 FA exposure, had no longitudinal data available on cognitive status following enrollment, or who had missing covariate values (Figure 1). Median length of follow-up was approximately 10 years (maximum =20.8) in WHIMS and its subsequent extension study (WHIMS-ECHO).
Figure 1.
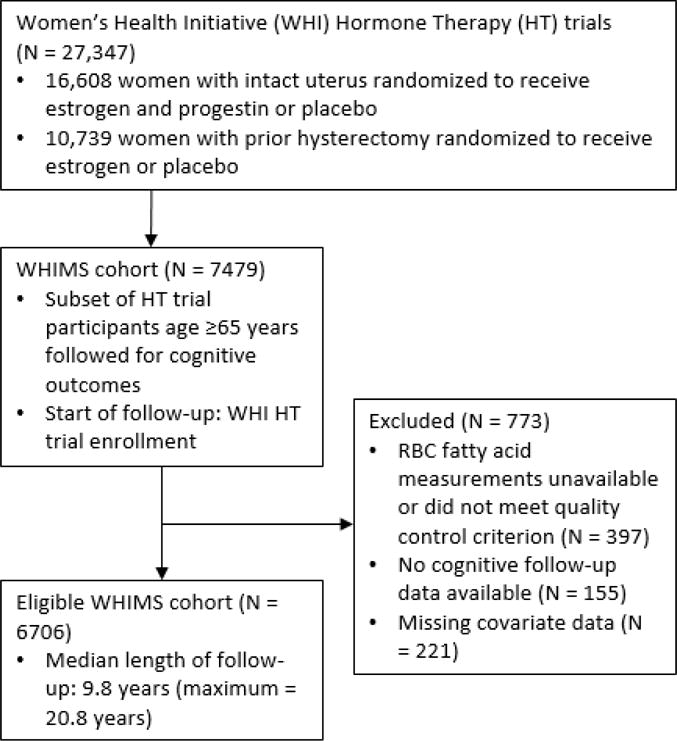
Flow diagram showing identification of eligible Women’s Health Initiative Memory Study (WHIMS) participants.
Outcomes
WHIMS participants’ cognitive status was evaluated at annual follow-up assessments. Participants were classified as having probable dementia (PD), mild cognitive impairment (MCI), or no cognitive impairment based on criteria from the DSM-IV.[14] During the WHIMS study period (1995–2007), participants were screened in person for dementia with the 100-point Modified Mini-Mental State (3MS) exam on an annual basis. Those who screened positive underwent additional cognitive testing and clinical evaluation.[15] All potential cases were centrally adjudicated based on neuropsychiatric test results, questionnaire data, and relevant contextual information on recent health events (e.g., stroke). Participants were classified as having probable dementia (PD), mild cognitive impairment (MCI), or no cognitive impairment based on criteria from the DSM-IV.[14] Details on outcome ascertainment and case adjudication have been described previously.[10, 11, 16] After the original in-person follow-up phase of WHIMS finished, extended follow-up (2008–2016) was conducted under the WHIMS-ECHO (Epidemiology of Cognitive Health Outcomes) protocol where cognitive evaluation was conducted by telephone interview. Participants were screened for dementia with the Modified Telephone Interview for Cognitive Status (TICS-M).[17] The Dementia Questionnaire,[18] a validated instrument that assesses cognitive, behavioral, and functional status, was administered to participants who screened positive. In addition, Dementia Questionnaire proxy interviews were used if a woman’s cognitive status could not be assessed directly. As occurred during WHIMS, potential cases were centrally adjudicated based on DSM-IV criteria using on all available information, which included scores from the TICS-M and Dementia Questionnaire, and information on recent health events.
In the present study, the primary endpoint was incident PD ascertained over the WHIMS and WHIMS-ECHO study periods. Secondary endpoints included a composite endpoint of PD or MCI, MCI alone, longitudinal changes in 3MS scores, and 3MS scores at baseline. Participants were censored at their last cognitive assessment.
Exposure Assessment
Participants’ baseline omega-3 FA status was assessed using a validated RBC biomarker.[19, 20] RBC FA composition was analyzed using gas chromatography with flame ionization detection. The individual RBC FAs were quantified and expressed as a percent of total identified FAs. The primary exposure of interest was the combination of RBC DHA and EPA content. RBC DHA+EPA, referred to as “the omega-3 index” in prior literature, has been shown to be associated with reduced risks of dementia and cardiovascular disease, and with larger cerebral and hippocampal tissue volumes in prior research.[19, 21–23] The intra-assay coefficient of variation for RBC DHA+EPA was <5.0%. There were 7299 participants with analyzed RBC samples. During aliquoting, the RBC samples were stored improperly at −20 C for a period of approximately two weeks, causing oxidative degradation of the polyunsaturated FAs before measurement. The original FA levels were estimated with multiple (i.e., 10) imputations using independent data on FA degradation rates and the length of time the samples were exposed to −20 C, as described previously by Pottala et al.[24] After correction and the exclusion of samples not meeting quality-control criteria described by Pottala et al., 7082 participants were eligible for analysis. Among these women, correlations between the corrected RBC biomarkers and participants’ intakes of DHA and EPA, as estimated from food frequency questionnaires, were similar to those reported in a sample of participants in the Nurses’ Health Study.[25, 26] Descriptive statistics on the RBC FA profiles for the study cohort are provided in Appendix Table A1. Parameter estimates were then pooled using Rubin’s technique to account for the uncertainty in the corrected DHA+EPA measurements.[27]
Covariates
Covariates reflecting participant HT trial assignment, demographics, health conditions, and health behaviors were selected a priori based on their importance as predictors of cognitive and cardiovascular health. (See Tables 1 and 2.) As with DHA+EPA status, all covariates were assessed at study entry.
Table 1.
Baseline characteristics of study participants stratified by red blood cell docosahexaenoic acid and eicosapentaenoic acid (RBC DHA+EPA) content (N = 6706).
| Characteristic | RBC DHA+EPA Tertile
|
P value | |||
|---|---|---|---|---|---|
| Total (N=6706) | Lowest (RBC content: 2.0–4.5%; N=2235) | Middle (RBC content: 4.5–5.6%; N=2236) | Highest (RBC content: 5.6–15.6%; N=2235) | ||
| Hormone therapy (HT) trial | <.001 | ||||
| Estrogen alone | 2630 (39.2) | 919 (41.1) | 907 (40.6) | 804 (36.0) | |
| Estrogen + progestin | 4076 (60.8) | 1316 (58.9) | 1329 (59.4) | 1431 (64.0) | |
| HT trial treatment assignment | 0.151 | ||||
| Active treatment | 3315 (49.4) | 1100 (49.2) | 1140 (51.0) | 1075 (48.1) | |
| Placebo | 3391 (50.6) | 1135 (50.8) | 1096 (49.0) | 1160 (51.9) | |
| Age in years at enrollment | <.001 | ||||
| Median (min–max) | 70 (63–81) | 69 (63–81) | 70 (64–79) | 70 (63–79) | |
| Baseline 3MS score | 0.575 | ||||
| Median (min–max) | 96 (66–100) | 96 (68–100) | 96 (68–100) | 96 (66–100) | |
| APOE genotype | 0.160 | ||||
| No ε4 alleles | 3984 (59.4) | 1353 (60.5) | 1384 (61.9) | 1247 (55.8) | |
| 1+ ε4 alleles | 1349 (20.1) | 496 (22.2) | 442 (19.8) | 411 (18.4) | |
| Unavailable | 1373 (20.5) | 386 (17.3) | 410 (18.3) | 577 (25.8) | |
| Education | <.001 | ||||
| College or postgraduate degree | 2014 (30.0) | 495 (22.1) | 632 (28.3) | 887 (39.7) | |
| Some post-secondary education | 2715 (40.5) | 948 (42.4) | 926 (41.4) | 841 (37.6) | |
| High school or less | 1977 (29.5) | 792 (35.4) | 678 (30.3) | 507 (22.7) | |
| Race/ethnicity | <.001 | ||||
| Black | 456 (6.8) | 61 (2.7) | 145 (6.5) | 250 (11.2) | |
| White | 5867 (87.5) | 2058 (92.1) | 1998 (89.4) | 1811 (81.0) | |
| Other | 383 (5.7) | 116 (5.2) | 93 (4.2) | 174 (7.8) | |
| Health conditions and behaviors | |||||
| Cardiovascular disease | 1344 (20.0) | 443 (19.8) | 452 (20.2) | 449 (20.1) | 0.945 |
| Hypertension | 3581 (53.4) | 1208 (54.0) | 1195 (53.4) | 1178 (52.7) | 0.666 |
| Diabetes | 744 (11.1) | 255 (11.4) | 267 (11.9) | 222 (9.9) | 0.086 |
| Current smoker | 459 (6.8) | 214 (9.6) | 127 (5.7) | 118 (5.3) | <.001 |
| Past alcohol user | 1314 (19.6) | 499 (22.3) | 444 (19.9) | 371 (16.6) | <.001 |
| Body-mass Index (BMI), kg/m2 | <.001 | ||||
| Median (min–max) | 27.6 (13.8–66.7) | 28.0 (13.8–66.7) | 27.9 (17.0–64.9) | 26.9 (15.0–66.1) | |
| Alcohol servings per week | <.001 | ||||
| Median (min–max) | 0.2 (0.0–64.7) | 0.0 (0.0–49.2) | 0.4 (0.0–64.2) | 0.4 (0.0–64.7) | |
Participants were ranked and divided into DHA+EPA tertiles based on the mean of the 10 imputed RBC DHA+EPA values. Summary statistics are the group median (range) for continuous variables or category count (%) for categorical variables. P values for between-group differences across tertiles are based on Kruskal-Wallis and chi-square test results for continuous and categorical variables, respectively. Participants with missing APOE genotype data were excluded from chi-square test for differences in APOE genotype across tertiles. For simplicity, the 11 educational attainment levels self-reported by WHIMS participants were categorized as follows: a doctoral degree, master’s degree, some post-graduate or professional education, and college graduate as “college or postgraduate degree”; some college or associate’s degree, and vocational or training school as “some post-secondary education”; and high school diploma or GED, some high school, and grade school as “high school or less.”
Table 2.
Associations between red blood cell docosahexaenoic acid and eicosapentaenoic acid (RBC DHA+EPA) content at baseline and the relative hazards (HRs) of incident probable dementia (PD), mild cognitive impairment (MCI), or the composite outcome (PD or MCI) during WHIMS and WHIMS-ECHO.
| Time period | Exposure | Model | PD* (587 cases total; 213 in WHIMS; 374 in WHIMS-ECHO) | MCI (671 cases total; 325 in WHIMS; 346 in WHIMS-ECHO) | PD or MCI (1047 cases total; 455 in WHIMS; 592 in WHIMS-ECHO) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| HR | 95°% CI | P value | HR | 95°% CI | P value | HR | 95°% CI | P value | ||||||
| WHIMS (1995–2007) and WHIMS-ECHO (2008–2016) | DHA+EPA† (per 1 SD) | 1 | 0.92 | 0.84 | 1.00 | 0.0434 | 0.96 | 0.89 | 1.05 | 0.3751 | 0.95 | 0.89 | 1.02 | 0.1399 |
| 2 | 0.92 | 0.84 | 1.00 | 0.0454 | 0.97 | 0.89 | 1.05 | 0.3880 | 0.95 | 0.90 | 1.02 | 0.1463 | ||
| 3 | 0.92 | 0.84 | 1.00 | 0.0498 | 0.97 | 0.89 | 1.05 | 0.4466 | 0.96 | 0.90 | 1.02 | 0.1741 | ||
| S1 | 0.91 | 0.83 | 0.99 | 0.0293 | 0.95 | 0.88 | 1.03 | 0.2284 | 0.94 | 0.88 | 1.00 | 0.0647 | ||
| S2 | 0.92 | 0.83 | 1.01 | 0.0929 | 1.00 | 0.90 | 1.10 | 0.9501 | 0.96 | 0.89 | 1.04 | 0.3430 | ||
| DHA (per 1 SD) | 3 | 0.92 | 0.85 | 1.01 | 0.0799 | 0.97 | 0.90 | 1.06 | 0.5226 | 0.96 | 0.90 | 1.02 | 0.2032 | |
| EPA (per 1 SD) | 3 | 0.93 | 0.85 | 1.02 | 0.1244 | 0.97 | 0.89 | 1.05 | 0.4376 | 0.97 | 0.91 | 1.03 | 0.3357 | |
| WHIMS | DHA+EPA (per 1 SD) | 3 | 0.85 | 0.73 | 0.99 | 0.0429 | 0.95 | 0.86 | 1.07 | 0.4098 | 0.96 | 0.87 | 1.05 | 0.3575 |
| WHIMS-ECHO | DHA+EPA (per 1 SD) | 3 | 0.95 | 0.86 | 1.05 | 0.3162 | 0.98 | 0.87 | 1.09 | 0.6704 | 0.95 | 0.88 | 1.03 | 0.2344 |
Model 1 = RBC omega-3 FA content, HT trial arm (4 levels: estrogen-only trial: active treatment or placebo; estrogen and progestin trial: active treatment or placebo), age at baseline, education, race/ethnicity (black, white or other). Educational attainment was analyzed as an 11-level ordinal variable, ranging from 1 (no formal education) to 11 (doctoral degree). For simplicity, these 11 levels were collapsed to three broader categories in Table 1. Cubic spline transformations of age and education with knots at the 10th, 50th and 90th percentiles were used to allow for non-linear relationships with the study outcomes.
Model 2 = Model 1 + cardiovascular disease, hypertension, diabetes.
Model 3 = Model 2 + BMI (cubic spline transformation), current smoking status, status as prior alcohol user, current average servings of alcohol per week (cubic spline transformation).
Model S1 (sensitivity analysis 1) = Model 3 + baseline 3MS score (cubic spline transformation).
Model S2 (sensitivity analysis 2) = Model 3 + APOE genotype (none vs. 1+ ε4 alleles). Note: the S2 sensitivity analysis included only participants for whom data on APOE genotype were available.
Probable dementia was the primary endpoint.
RBC DHA+EPA was the primary exposure.
In sensitivity analyses, baseline 3MS score and APOE status were added as potential covariates. Because 3MS score at baseline may reflect the accrued effects of higher DHA+EPA levels over one’s lifetime, we did not adjust for 3MS in our main analyses. APOE genotype could be imputed only for white participants based on genome-wide association study (GWAS) results. APOE status was not included as a covariate in our main analyses because it was available only for a subset (80%) of otherwise eligible participants.
Several tests for effect modification were performed in pre-specified secondary analyses. In these analyses, we evaluated whether the relationship between DHA+EPA and cognitive status was modified by APOE genotype, baseline cognitive function (as measured by the 3MS), HT trial assignment, and time since trial enrollment.
Statistical Methods
Kaplan-Meier survival analysis and Cox proportional hazards regression were used to evaluate the relationship between RBC DHA+EPA levels at baseline and time-to-incident-PD. DHA+EPA was analyzed as a continuous variable in our regression models. Three Cox regression models with increasing degrees of covariate adjustment were used to estimate the effect of DHA+EPA on the relative hazard of PD. In model 1, we adjusted for trial assignment and patient demographics; in model 2 we additionally adjusted for cardiometabolic comorbidities; in model 3 we additionally adjusted for health behaviors (Tables 1–2). This approach was repeated for the survival analyses of the MCI and composite PD/MCI endpoints. Univariate survival plots were inspected to check for meaningful violations of the proportional hazards assumption. Changes in risk estimates and in cohort composition over the course of the study period were evaluated to assess the possible impact of loss to follow-up on our results.
To describe differences in absolute risk of PD across levels of RBC DHA+EPA, we used model 3 to estimate the predicted probability of PD for all cohort members at year 15 of follow-up under two DHA+EPA exposure scenarios: (a) one standard deviation (SD) above the mean (6.79% RBC content) and (b) one SD below the mean (3.75% RBC content). This contrast was intended to represent, at the population level, the estimated impact of DHA+EPA biostatus on the cumulative incidence of PD after adjustment for baseline covariates. Bootstrapping with 1000 replicate samples was used to estimate 95% confidence intervals for the risk difference.
Linear mixed models were used to estimate the relationships between DHA+EPA and baseline 3MS scores and the change in 3MS scores over the follow-up period. All covariates in the Cox survival analyses were included as fixed effects and as interactions with time in the 3MS models; in addition, random intercept and time effects were included to account for within-person correlations in test scores.
A two-tailed p value <0.05 was considered statistically significant. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
After restricting to WHIMS participants with information on baseline RBC DHA+EPA levels and longitudinal data on cognitive status, 6706 women were eligible for analysis (Figure 1). At enrollment, the median participant age was 70 years (range: 63, 81) and median 3MS score was 96 (range: 66, 100). Mean RBC DHA+EPA content was 5.27% (SD = 1.52%). Compared to women in the lowest DHA+EPA tertile, women in the highest tertile were slightly older, twice as likely to have graduated from college (40% vs. 22%), and more likely to be non-white (19% vs. 8%). They were also slightly healthier in terms of diabetes prevalence, average body weight, and current smoking status. There was no significant univariate association between DHA+EPA tertile and global cognitive function, as measured by the 3MS, at baseline (Table 1).
Primary Endpoint: Incident Probable Dementia
Over a median of 9.8 years of follow-up (maximum = 20.8), 587 incident PD cases were identified. The Kaplan-Meier cumulative incidence of PD in the WHIMS cohort was 1.5% at year 5, 4.0% at year 10, and 11.7% at year 15. In unadjusted analyses, higher DHA+EPA levels were associated with a lower risk of incident PD, but this association was not significant (log-rank test p value: 0.15; see Figure 2). In Cox proportional hazard models, after adjustment for trial assignment and demographic, health status, and health behavior variables (model 3), a 1-SD increase in DHA+EPA was associated with a reduced risk of incident dementia (HR = 0.92, 95% CI: 0.84, 1.00; p < 0.05). This effect estimate did not change meaningfully after further adjustment for baseline 3MS score or APOE genotype. Full results are provided in Table 2.
Figure 2. Kaplan-Meier plots of dementia-free survival stratified by red blood cell docosahexaenoic acid and eicosapentaenoic acid (RBC DHA+EPA) tertile.
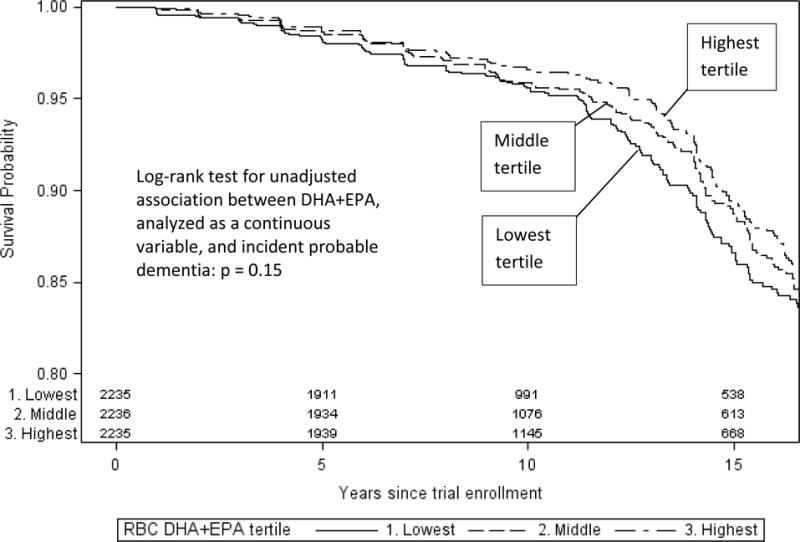
Kaplan-Meier plots of dementia-free survival by RBC DHA+EPA levels at baseline. Incident cases of probable dementia were counted as events. Participants were censored at loss to follow-up, the end of the study period or death. Numbers at the bottom of the graph reflect the number of participants remaining in the risk set at a given follow-up interval.
To quantify the absolute risk reduction associated with higher levels of DHA+EPA, we contrasted the projected cumulative incidence of PD if all participants had a DHA+EPA level 1-SD above the sample mean (“high exposure”), or 1-SD below the sample mean (“low exposure”). The HR for this 2-SD contrast, based on regression model 3, was 0.84 (95% CI: 0.71, 1.00; p < 0.05). The 15-year cumulative incidence of PD was estimated to be 12.1% with high DHA+EPA exposure compared to 14.2% with low DHA+EPA exposure (absolute risk difference = 2.05%, 95% CI: 0.24%, 3.97%).
Several possible sources of effect modification of the relationship between RBC DHA+EPA and the relative hazard of PD were explored. We found no evidence for effect modification (on the log HR scale) by HT trial assignment (CEE-only trial: p = 0.98; CEE+P trial: p = 0.57, baseline 3MS score (p = 0.40), or APOE genotype (p = 0.32). A marginally significant interaction between DHA+EPA and years since trial enrollment was observed (p = 0.11): the inverse association between a 1-SD increase in DHA+EPA and PD risk was stronger during the initial WHIMS follow-up period (HR = 0.85, 95% CI: 0.73, 1.00) than during the post-trial WHIMS-ECHO period (HR = 0.95, 95% CI: 0.86, 1.05; see Table 2).
Secondary Analyses: Probable Dementia and Mild Cognitive Impairment
In additional secondary analyses, we evaluated the relationship between baseline RBC DHA+EPA levels and incident MCI and a composite PD/MCI endpoint. There was no significant relationship between higher DHA+EPA levels and the risk of MCI or PD/MCI (Table 2). When follow-up was restricted to the initial WHIMS period, marginally significant protective associations were observed for MCI (HR = 0.90, 95% CI: 0.81, 1.01) and PD/MCI (HR = 0.91, 95% CI: 0.83, 1.01).
Secondary Analyses: RBC DHA and EPA Analyzed Separately
We also evaluated RBC DHA and EPA, considered separately, as predictors of incident PD, MCI and PD/MCI. Relationships between DHA and EPA and the study endpoints were similar to the findings for RBC DHA+EPA, although the standard errors for the HRs were larger when DHA and EPA were analyzed separately (Table 2).
Secondary Analyses: 3MS Scores
The 100-point Modified Mini-Mental State (3MS) exam was administered annually to WHIMS participants. Scores through the 11th year of follow-up were included in the present study. Results from our mixed effects linear regression models indicated that higher levels of RBC DHA+EPA were associated with lower 3MS scores at baseline (−0.109, 95% CI: −0.187, −0.032, per 1-SD increase), and with a slower rate of decline in 3MS scores over the follow-up period (0.045, 95% CI: 0.022, 0.067, 3MS points per year, per 1-SD increase). Full results for the 3MS outcomes are provided in Table 3.
Table 3.
Associations between red blood cell docosahexaenoic acid and eicosapentaenoic acid (RBC DHA+EPA) content at baseline and performance on the 100-point Modified Mini Mental State Exam (3MS).
| Model | Baseline cross-sectional effect estimates: mean difference in 3MS score per one standard deviation increase in RBC DHA+EPA. | Longitudinal effect estimates: mean difference in the rate of change in 3MS score per one standard deviation increase in RBC DHA+EPA. | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Estimate | 95% CI | P value | Estimate | 95% CI | P value | |||
| 1 | −0.089 | −0.165 | −0.013 | 0.022 | 0.043 | 0.021 | 0.066 | <0.001 |
| 2 | −0.094 | −0.170 | −0.018 | 0.016 | 0.043 | 0.021 | 0.066 | <0.001 |
| 3 | −0.109 | −0.187 | −0.032 | 0.006 | 0.045 | 0.022 | 0.067 | <0.001 |
Positive mean differences an association between higher DHA+EPA levels and improved cognitive performance, negative mean differences an associated between higher DHA+EPA levels and worse cognitive performance.
Model 1 covariates: RBC DHA+EPA FA content, HT trial arm, age at baseline, education, race/ethnicity, time (linear effect and indicator variable for each annual follow-up wave). Educational attainment was analyzed as an 11-level ordinal variable, ranging from 1 (no formal education) to 11 (doctoral degree). For simplicity, these 11 levels were collapsed to three broader categories in Table 1. Cubic spline transformations of age and education with knots at the 10th, 50th and 90th percentiles were used to allow for non-linear relationships with the study outcomes.
Model 2 covariates: Model 1 covariates + cardiovascular disease, hypertension, diabetes
Model 3 covariates: Model 2 covariates + BMI (cubic spline transformation), current smoking status, status as prior alcohol user, current average servings of alcohol per week (cubic spline transformation)
Covariates were included as both main effects (cross-sectional effects) and interactions with time (effects on rate of change). To account for within-person correlations in test scores, person-level random intercept and time effects were included.
DISCUSSION
In this cohort of 6706 older and relatively healthy women who participated in WHIMS, we found that higher baseline levels of RBC DHA+EPA were associated with a lower risk of incident PD and a slower rate of cognitive decline over a median follow-up period of 10 years. This relationship was independent of a variety of known risk factors and predictors for dementia. Our findings add to a growing literature supporting the hypothesis that long-chain omega-3 FAs may be protective against cognitive deterioration.
Strengths of our study include the fact that it is the largest cohort to date in which the association between AD/D and biomarkers of omega-3 FA status has been assessed (as opposed to estimated fish or omega-3 FA intake based on self-reported food intake data). With a maximum length of follow-up of 15 years, our study is also one of the longest cognitive outcome studies to date that has assessed biomarkers of omega-3 FA status. In addition, the well-characterized WHIMS cohort includes data on many important demographic and clinical characteristics, allowing us to adjust for key AD/D risk factors.
The protective association between RBC DHA+EPA and the HR for PD was more pronounced earlier in the follow-up period (WHIMS HR: 0.85) compared to later (WHIMS-ECHO HR: 0.95). Beyond chance, there are several possible explanations for this. It could be that higher DHA+EPA levels delayed dementia onset, but eventually, predisposed individuals did go on to develop the disease. In a time-to-event analysis of a given risk factor, HRs for later periods can become attenuated as the individuals most susceptible to the outcome event are “nudged” into having an earlier event by the risk factor, while susceptible individuals lacking that risk factor remain in the cohort for continued follow-up.[28] Secondly, because RBC omega-3 FAs were assessed only at baseline, it is possible that the association with cognitive outcomes was attenuated over time due to exposure misclassification, as participants’ dietary practices drifted from baseline.
Because of the suggestion of an interaction between DHA+EPA and length of follow-up, and the likelihood that study drop-out could vary by factors related to cognitive function, we explored how the composition of the study cohort changed over time. Baseline cohort characteristics are provided in Appendix Table A2 for the entire eligible WHIMS cohort (N = 6706) and the subset of participants with continued follow-up in WHIMS-ECHO (N = 2676). Compared to the cohort as a whole, those who remained in the study for WHIMS-ECHO were more educated, healthier at baseline, and had somewhat higher RBC DHA+EPA levels. Our fully adjusted Cox regression models, which included as predictors a larger set of demographic and health covariates related to both the risk of incident PD and the probability of censoring, are likely to be more robust against possible bias due to covariate-dependent censoring.[29]
Our results are consistent with evidence from prior epidemiologic studies supporting a protective association between omega-3 FAs and dementia. Two recent meta-analyses of observational studies found significant inverse relations between fish intake and incident dementia.[7, 8] In nine studies summarized by Lin et al.[30] and in other cross-sectional studies,[22, 31–33] DHA and EPA blood levels were inversely related to dementia, other adverse cognitive outcomes, and ischemic white matter lesions. In longitudinal cohort studies, the most (but not all) have found that biomarker measures of omega-3 FA exposure were associated with a reduced risk of incident dementia.[26, 34–39] These prior cohort studies, ranging from 246 to 2251 subjects, and 4–10 years of follow-up, were considerably smaller than the present study. Thus, our findings provide strong confirmation of these earlier reports. In prior studies, there have been findings that the relationship between omega-3 FAs and dementia pathology may vary by APOE genotype;[40, 41] while we did not find statistically significant evidence for interaction (p = 0.32), APOE data were only available for a subset of participants, and the possibility of clinically meaningful effect modification by APOE status cannot be ruled out.
Possible mechanisms to explain the apparent benefit of higher levels of the omega-3 FAs on the development of AD/D have focused on at least two pathways. First, the systemic anti-inflammatory effects of omega-3 FA[5] which are based in part on the downregulation of nuclear factor κB[42] and in part by the production of oxygenated metabolites of EPA and DHA that actively promote the resolution of inflammation.[43] Secondly, omega-3 FAs alter membrane biophysical properties that result in alterations in ion channels, receptors, and binding between phospholipids and proteins.[44] This may have particular relevance as regards neuronal cell membranes and their interactions with Aβ peptides, the hallmark molecules of AD pathology. Agents or interventions that increase the binding of soluble Aβ oligomers (which are believed to be the cytotoxic agents rather than the fully formed fibrils[45]) to membrane phospholipids slow fibril formation and are thus considered to be a beneficial.[46] DHA has recently been shown to have this effect.[47] Hence higher membrane levels of EPA and/or DHA may favorably affect the metabolism of Aβ peptides and thereby reduce risk for AD/D progression.
This study had several important limitations. Unmeasured confounding cannot be ruled out as an explanation for the associations observed in our study. As shown in Table 1, RBC DHA+EPA levels were associated strongly with baseline demographic characteristics associated with cognitive outcomes: compared to women in the lowest DHA+EPA tertile, women in the highest tertile were twice as likely to have graduated from college (40% vs. 22%), and were more likely to be non-white (19% vs. 8%). We sought to adjust for these observed differences in AD/D risk factors with Cox regression, and found a significant association between RBC DHA+EPA levels and a reduced risk of dementia—independent of demographics, major risk factors for dementia and cardiovascular disease, and cognitive status at baseline (as measured by the 3MSE). However, our study was observational rather than experimental, and our results should not be interpreted as definitive evidence of a causal relationship.
The fact that our findings were not consistent across all study endpoints is another reason to interpret our results circumspectly. Though we found a significant protective association between RBC DHA+EPA and the risk of incident AD/D and longitudinal changes in 3MSE over time, we did not find a protective association with incident MCI or 3MSE scores at baseline. A possible explanation for the lack of association with MCI is misclassification bias, as diagnostic criteria for MCI are less well defined.
In regards to randomized controlled trials (RCTs) that have tested the effects of omega-3 FA supplementation on cognitive function, a 2012 meta-analysis found no overall benefit in healthy subjects or those with frank dementia, but improvements in attention processing speed in those with CIND.[48] While most RCTs have not found a protective association, an important limitation of these studies is their short length of follow-up (≤2 years; typically ≤6 months), which may be too short to detect a measurable effect. Another hypothesis for the divergence between the RCTs and observational studies such as ours is that RBC DHA+EPA may serve as a marker not just for increased intake of DHA and EPA, but more generally for healthier dietary practices (e.g., lower intake of red meat and saturated fats, Mediterranean-style diet). To explore this hypothesis, we performed a post hoc sensitivity analysis in which we additionally adjusted for red meat intake based on food frequency questionnaire data[49] and RBC oleic acid content[24]—a proxy for consumption of healthy vegetable oils such as olive oil.[50] Our DHA+EPA effect estimates did not change meaningfully, suggesting that the protective association between RBC DHA+EPA and dementia risk may not simply be a substitution effect.
In conclusion, higher levels of RBC DHA+EPA were associated with a modestly lower risk of AD/D among older women. After scaling the effect estimates to reflect the contrast between having RBC DHA+EPA content 1-SD above versus 1-SD below the mean, the estimated HR and 15-year absolute risk reduction were 0.84 and 2.05%, respectively. In our view, these differences are large enough to be meaningful at the population level. Results from RCTs of omega-3 FA supplementation with extended participant follow-up are needed to help clarify whether this association is causal.
Supplementary Material
SUMMARY.
This study examined the association between red blood cell (RBC) docosahexaenoic acid and eicosapentaenoic acid (DHA+EPA) levels and risk of dementia in older women. RBC DHA+EPA levels were assessed at baseline, and cognitive status was evaluated annually in a cohort of 6706 women aged ≥65 years who participated in the Women’s Health Initiative Memory Study (WHIMS). During a median follow-up period of 9.8 years, 587 incident cases of probable dementia were identified. In the adjusted Cox regression analysis, a one standard deviation (SD) increase in DHA+EPA was associated with a significantly lower risk of dementia (HR = 0.92, 95% CI: 0.84, 1.00; p < 0.05). For women with high DHA+EPA exposure (1 SD above mean) compared to low exposure (1 SD below mean), the adjusted 15-year absolute risk difference for dementia was 2.1% (95% CI: 0.2%, 4.0%). Higher levels of DHA+EPA may help protect against the development of dementia.
Highlights.
We examined the association between erythrocyte EPA+DHA and risk for incident dementia in 6706 women in the USA.
After about 10 years of follow-up and after appropriate adjustments, we found a significant, 8% decreased risk for probable dementia associated with a 1-SD increase in EPA+DHA.
This large study confirms previous research suggesting that higher EPA+DHA levels may be protective against dementia.
Acknowledgments
Funding was provided by the National Heart Lung and Blood Institute through a Broad Agency Announcement for Women’s Health Initiative research proposals (BAA 19). The sponsor had no role in study design, study conduct, data analysis or manuscript preparation.
The authors thank the WHI study participants, investigators, and personnel for their contributions to the WHI and WHIMS studies. The authors also thank Jason Polreis (OmegaQuant Analytics, LLC), for performing the RBC fatty acid analyses.
Acknowledgement of WHI Investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY)
Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland
Study funding:
Funding was provided by the National Heart, Lung, and Blood Institute through a Broad Agency Announcement for Women’s Health Initiative research proposals (BAA 19). The sponsor had no role in study design, study conduct, data analysis or manuscript preparation.
Abbreviations
- 3MS
Modified Mini-Mental State
- CEE
Conjugated equine estrogens
- CEE+P
(CEE) + Progestin
- CI
Confidence interval
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FA
fatty acid
- HT
hormone therapy
- HR
hazard ratio
- MCI
mild cognitive impairment
- PD
Probably dementia
- RBC
red blood cell
- SD
standard deviation
- WHIMS
Women’s Health Initiative Memory Study
- WHIMS-ECHO
(WHIMS) Epidemiology of Cognitive Health Outcomes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
E.M.A. contributed to the statistical analysis and interpretation of the data, and drafting and revising the manuscript.
J.V.P. contributed to the design of the study, the acquisition of the data, and revising the manuscript.
J.G.R. contributed to the design of the study, and revising the manuscript.
M.A.E. contributed to the design of the study, the acquisition of the data, and revising the manuscript.
W.S.H. contributed to the design of the study, the acquisition of the data, and drafting and revising the manuscript.
Author disclosures:
E.M.A. reports no relevant disclosures.
J.V.P reports no relevant disclosures.
J.G.R. has received research grants from Amarin, Amgen, Astra-Zeneca, Eli Lilly, Esai, Glaxo-Smith Kline, Merck, Pfizer, Regeneron/Sanofi, and Takeda; in addition, she has consulted for Akcea/Ionis, Amgen, Eli Lilly, Esperion, Merck, Pfizer, and Regeneron/Sanofi.
M.A.E. reports no relevant disclosures.
W.S.H. is the founder and president of Omegaquant Analytics, LLC, a laboratory testing company that offers lipid testing services.
For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Supplemental data: WHIMS omega3 FAs and dementia appendix.docx
References
- 1.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D’Agostino RB. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 2.Waite LM. Treatment for Alzheimer’s disease: has anything changed? Australian prescriber. 2015;38:60–63. doi: 10.18773/austprescr.2015.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochemical pharmacology. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 5.Thomas J, Thomas CJ, Radcliffe J, Itsiopoulos C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed research international. 2015;2015:172801. doi: 10.1155/2015/172801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiala M, Halder RC, Sagong B, Ross O, Sayre J, Porter V, Bredesen DE. omega-3 Supplementation increases amyloid-beta phagocytosis and resolvin D1 in patients with minor cognitive impairment. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:2681–2689. doi: 10.1096/fj.14-264218. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Ding Y, Wu F, Li R, Hou J, Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neuroscience and biobehavioral reviews. 2015;48:1–9. doi: 10.1016/j.neubiorev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2016;103:330–340. doi: 10.3945/ajcn.115.124081. [DOI] [PubMed] [Google Scholar]
- 9.Morris MC, Brockman J, Schneider JA, Wang Y, Bennett DA, Tangney CC, van de Rest O. Association of Seafood Consumption, Brain Mercury Level, and APOE epsilon4 Status With Brain Neuropathology in Older Adults. JAMA. 2016;315:489–497. doi: 10.1001/jama.2015.19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 11.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 12.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, I. Writing Group for the Women’s Health Initiative Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Horn L Van, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S, C. Women’s Health Initiative Steering Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 16.Espeland MA, Brinton RD, Hugenschmidt C, Manson JE, Craft S, Yaffe K, Weitlauf J, Vaughan L, Johnson KC, Padula CB, Jackson RD, Resnick SM, W.S. Group Impact of Type 2 Diabetes and Postmenopausal Hormone Therapy on Incidence of Cognitive Impairment in Older Women. Diabetes Care. 2015;38:2316–2324. doi: 10.2337/dc15-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh KA, Breitner JCS, Magruderhabib KM. Detection of Dementia in the Elderly Using Telephone Screening of Cognitive Status. Neuropsy Neuropsy Be. 1993;6:103–110. [Google Scholar]
- 18.Ellis RJ, Jan K, Kawas C, Koller WC, Lyons KE, Jeste DV, Hansen LA, Thal LJ. Diagnostic validity of the dementia questionnaire for Alzheimer disease. Archives of neurology. 1998;55:360–365. doi: 10.1001/archneur.55.3.360. [DOI] [PubMed] [Google Scholar]
- 19.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive medicine. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2012;29:691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RS, Robins SJ, Seshadri S. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78:658–664. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI Study. Neurology. 2014;82:435–442. doi: 10.1212/WNL.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whalley LJ, Deary IJ, Starr JM, Wahle KW, Rance KA, Bourne VJ, Fox HC. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. The American journal of clinical nutrition. 2008;87:449–454. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- 24.Pottala JV, Espeland MA, Polreis J, Robinson J, Harris WS. Correcting the effects of −20 degrees C storage and aliquot size on erythrocyte fatty acid content in the Women’s Health Initiative. Lipids. 2012;47:835–846. doi: 10.1007/s11745-012-3693-y. [DOI] [PubMed] [Google Scholar]
- 25.Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, Hu FB. Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr. 2008;88:216–223. doi: 10.1093/ajcn/88.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ammann EM, Pottala JV, Harris WS, Espeland MA, Wallace R, Denburg NL, Carnahan RM, Robinson JG. Omega-3 fatty acids and domain-specific cognitive aging: Secondary analyses of data from WHISCA. Neurology. 2013;81:1484–1491. doi: 10.1212/WNL.0b013e3182a9584c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 28.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker SG, Fitzmaurice GM, Freedman LS, Kramer BS. Simple adjustments for randomized trials with nonrandomly missing or censored outcomes arising from informative covariates. Biostatistics. 2006;7:29–40. doi: 10.1093/biostatistics/kxi038. [DOI] [PubMed] [Google Scholar]
- 30.Lin PY, Chiu CC, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in dementia. J Clin Psychiatry. 2012;68:140–147. doi: 10.4088/JCP.11r07546. [DOI] [PubMed] [Google Scholar]
- 31.Lopez LB, Kritz-Silverstein D, Barrett Connor E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: the Rancho Bernardo study. The journal of nutrition, health & aging. 2011;15:25–31. doi: 10.1007/s12603-011-0009-5. [DOI] [PubMed] [Google Scholar]
- 32.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 33.Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RS, Robins SJ, Seshadri S. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78:658–664. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes-The EVA Study. Am J Clin Nutr. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 35.Samieri C, Feart C, Letenneur L, Dartigues JF, Peres K, Auriacombe S, Peuchant E, Delcourt C, Barberger-Gateau P. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008 Sep;88(3):714–21. doi: 10.1093/ajcn/88.3.714. 88 (2008) 714–721. [DOI] [PubMed] [Google Scholar]
- 36.Otsuka R, Tange C, Nishita Y, Kato Y, Imai T, Ando F, Shimokata H. Serum docosahexaenoic and eicosapentaenoic acid and risk of cognitive decline over 10 years among elderly Japanese. European journal of clinical nutrition. 2014;68:503–509. doi: 10.1038/ejcn.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–1111. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Archives of neurology. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 39.Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ. Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis. 2003;5:315–322. doi: 10.3233/jad-2003-5407. [DOI] [PubMed] [Google Scholar]
- 40.Yassine HN, Rawat V, Mack WJ, Quinn JF, Yurko-Mauro K, Bailey-Hall E, Aisen PS, Chui HC, Schneider LS. The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer’s disease. Alzheimer’s research & therapy. 2016;8:25. doi: 10.1186/s13195-016-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang S, Steffen LM, Steffen BT, Guan W, Weir NL, Rich SS, Manichaikul A, Vargas JD, Tsai MY. APOE genotype modifies the association between plasma omega-3 fatty acids and plasma lipids in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2013;228:181–187. doi: 10.1016/j.atherosclerosis.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massaro M, Scoditti E, Carluccio MA, Campana MC, De Caterina R. Omega-3 fatty acids, inflammation and angiogenesis: basic mechanisms behind the cardioprotective effects of fish and fish oils. Cell Mol Biol (Noisy-le-grand) 2010;56:59–82. [PubMed] [Google Scholar]
- 43.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochimica et biophysica acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochimica et biophysica acta. 2015;1848:211–219. doi: 10.1016/j.bbamem.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nature neuroscience. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 46.Williams TL, Serpell LC. Membrane and surface interactions of Alzheimer’s Abeta peptide— insights into the mechanism of cytotoxicity. The FEBS journal. 2011;278:3905–3917. doi: 10.1111/j.1742-4658.2011.08228.x. [DOI] [PubMed] [Google Scholar]
- 47.Emendato A, Spadaccini R, Santis A De, Guerrini R, D’Errico G, Picone D. Preferential interaction of the Alzheimer peptide Abeta-(1–42) with Omega-3-containing lipid bilayers: structure and interaction studies. FEBS letters. 2016;590:582–591. doi: 10.1002/1873-3468.12082. [DOI] [PubMed] [Google Scholar]
- 48.Mazereeuw G, Lanctot KL, Chau SA, Swardfager W, Herrmann N. Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiology of aging. 2012;33:1482 e1417–1429. doi: 10.1016/j.neurobiolaging.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 50.Harris WS, Masson S, Barlera S, Milani V, Pileggi S, Franzosi MG, Marchioli R, Tognoni G, Tavazzi L, Latini R, Investigators GH. Red blood cell oleic acid levels reflect olive oil intake while omega-3 levels reflect fish intake and the use of omega-3 acid ethyl esters: The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico-Heart Failure trial. Nutr Res. 2016;36:989–994. doi: 10.1016/j.nutres.2016.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


