Summary
The participation of transfer RNAs (tRNAs) in fundamental aspects of biology and disease necessitates an accurate, experimentally confirmed annotation of tRNA genes, and curation of tRNA sequences. This has been challenging, because RNA secondary structure, nucleotide modifications and tRNA gene multiplicity, complicate sequencing and mapping efforts. To address these issues, we developed hydro-tRNAseq, a method based on partial alkaline RNA hydrolysis that generates fragments amenable for sequencing. To identify transcribed tRNA genes, we further complemented this approach with Photoactivatable Crosslinking and Immunoprecipitation (PAR-CLIP) of SSB/La, a conserved protein involved in pre-tRNA processing. Our results show that approximately half of all predicted tRNA genes are transcribed in human cells. We also report nucleotide modification sites, their order of introduction, and identify tRNA leaders, trailers and introns. By using complementary sequencing-based methodologies we present a human tRNA atlas, and determine expression levels of mature and processing intermediates of tRNAs in human cells.
Graphical abstract
Gogakos et al. describe two complementary high-throughput techniques for characterization of human tRNAs. They combine hydro-tRNAseq and PAR-CLIP of SSB/La to curate and quantify mature tRNAs, annotation of pre-tRNAs, and report characteristics of POLR3 transcription. The study expands the resources and tools available for study of tRNAs.

Introduction
tRNAs have been among the earliest studied non-coding RNA molecules (Woese C.W., 1967). Yet, in recent years tRNAs received new attention in the context of codon-resolved translational control (Dana and Tuller, 2012; 2014; Mahlab et al., 2012; Plotkin and Kudla, 2011; Tuller et al., 2010; Weinberg et al., 2016), and due to the involvement of their metabolic byproducts in regulation and cross-talk with processing and effector functions of other classes of non-coding RNAs (ncRNAs) (Hasler et al., 2016; Ivanov et al., 2011; Lee et al., 2009). Nevertheless, the lack of reliable methods for tRNA quantification has hampered such analyses, and necessitated the use of predicted tRNA gene copy number as a surrogate index of expression (Iben and Maraia, 2014; Pechmann and Frydman, 2012; Tuller et al., 2010). This hinged on the assumption that predicted tRNA gene loci are all expressed constitutively and equally, even though there has been experimental evidence against it (Gingold et al., 2014). Similarly, experimental tRNA gene annotation in the past had to focus on RNA polymerase III (POLR3) ChIP-seq (Kutter et al., 2011; Moqtaderi et al., 2010; Oler et al., 2010) or hybridization-based approaches (Dittmar et al., 2004; Goodarzi et al., 2016). The former, however, were impeded by their restricted genomic resolution and the assumption that POLR3 binding always leads to productive tRNA expression followed by complete processing, while the latter fell short of providing absolute counts and did not address the discovery of new transcripts and genes, assuming also normal hybridization rules for modified nucleosides.
An improvement in tRNA quantification has arisen from recent efforts that employed modification-reverting enzymes prior to sequencing, in order to minimize stalling of reverse transcriptase at modified sites (Cozen et al., 2015; Zheng et al., 2015). However, an extensive annotation of human genes and transcripts was foregone because the focus was either on mature tRNAs only (Zheng et al., 2015) or on tRNA fragments not inclusive of full-length precursor tRNA (pre-tRNA) transcripts (Cozen et al., 2015). Thus, to-date an experimentally validated list of curated mature and pre-tRNA sequences and annotating tRNA genes in human is still missing.
We have combined complementary high-throughput techniques for obtaining the sequence composition and abundance of tRNAs in human embryonic kidney cells (HEK293). We developed hydro-tRNAseq, a modified small RNA sequencing protocol based on partial alkaline hydrolysis of input RNA, in order to identify and quantify tRNAs, and provided evidence for the validity of this approach when determining the accumulation of disease-associated tRNA intron fragments caused by mutations in the tRNA splicing machinery (Karaca et al., 2014). Similar efforts, but without a comprehensive transcriptome-wide analysis or pre-tRNA annotation, have also been applied towards the study of yeast tRNA modifications (Arimbasseri et al., 2015; 2016). Here we extend this approach by applying it to tRNA-enriched size fragments with the aim to annotate and curate all tRNAs. Since tRNA processing, such as precursor trimming and intron removal, is a fast process (Foretek et al., 2016), we also aimed to enrich specifically for pre-tRNAs in order to identify and annotate the corresponding unique tRNA gene template. Thus, we performed PAR-CLIP on SSB, a conserved and ubiquitous protein involved in 3′ tRNA processing (Bayfield and Maraia, 2009; Bayfield et al., 2010; Stefano, 1984).
Results
Hydrolysis-based tRNA sequencing (hydro-tRNAseq)
To curate and quantify human tRNAs, we isolated 60-100 nucleotide- (nt-) sized total RNA from HEK293 cells comprising both precursor and mature tRNAs, but being devoid of most other abundant RNAs and short tRNA turnover products (Lee et al., 2009). Full-length tRNAs have thermodynamically stable secondary and tertiary structures and are heavily modified by RNA editing, all of which compromise reverse transcription (RT) and RNAseq analysis, resulting in ∼2% tRNA read content in our RNAseq datasets (data not shown). To overcome these problems, we implemented a limited alkaline hydrolysis step, which generates shorter RNA fragments with less structure and fewer modifications per fragment, and consequently more amenable to small RNA cDNA library preparation and deep sequencing. Furthermore, basic conditions also cleave the aminoacyl-tRNA bond, freeing the 3′ terminal hydroxyl group required for 3′ adapter ligation during RNA cDNA library preparation. This approach increased the tRNA read content to >40% in our deepest dataset (Table S1). We named this procedure hydro-tRNAseq (Fig. 1A). In summary, partial hydrolysis of tRNAs overcame technical limitations of suboptimal adapter ligation and RT and albeit this resulted in shorter reads, it also resulted in fewer errors at sites of modification per sequenced read, which ultimately improved the performance of the mapping algorithm.
Figure 1. Experimental and bioinformatic pipeline for tRNA annotation and reference transcript curation by hydro-tRNAseq.
(A) tRNAs and pre-tRNAs were size-selected from HEK293 total RNA and subjected to limited alkaline hydrolysis, followed by dephosphorylation, rephosphorylation and conventional small RNA sequencing as described previously (Hafner et al., 2012b). (B) An iterative mapping and annotation protocol was used to first annotate and curate fully processed and nucleotide-modified mature tRNAs. Leftover reads that spanned the mature-precursor junctions were used to identify transcribed tRNA genes. See also Fig. S1, S2, S8 and Tables S1 and S2.
Hierarchical sequence read mapping followed by iterative manual tRNA curation
We mapped the reads to reference tRNA genes (hg19, http://gtrnadb.ucsc.edu/) using an iterative and hierarchical protocol (Fig. 1B). We started by mapping only to mature tRNAs, which included the 3′ CCA aminoacyl acceptor terminus introduced posttranscriptionally by tRNA nucleotidyl transferase, and the G-1 nucleotide added posttranscriptionally to histidine tRNAs (Gu et al., 2003; Juhling et al., 2009), but excluded tRNA introns. Starting with two most abundant tRNA transcripts per isotype (tRNAs encoding the same amino acid) as indicated after the first mapping round, except for selenocysteine, where only one mature tRNA sequence could be identified, we performed iterative rounds of mapping and manual reference transcript selection, focusing in every step on transcripts that collected more reads with an error distance of 1-2 than 0. If these reads with mismatches could be assigned to other tRNA isoacceptors (tRNA accepting the same amino acid), these were included in our candidate reference set. Otherwise, we reasoned that the mismatches were the results of nucleotide-modification-induced errors of RT. In those cases, we accounted for the modified nucleoside signatures by introducing a new, edited reference transcript in our set. For tRNAs that exhibited multiple positions with high modification rates (>10% compared to reference), we compiled reference sequences with all possible combinations of modified signatures at all detectably modified positions, aiming to account for the maximum possible number of mapped sequence reads. We ended the curation cycles when there was no observed modified position that exhibited a mismatch frequency greater than or equal to 10% compared to the reference. By performing this iterative process of curation, we obtained an experimentally validated reference set of mature tRNAs accounting for modified-nucleotide-induced sequence variation upon reverse transcription (Table S2).
In order to identify possible tRNA gene loci, we mapped the curated tRNA sequences back to the genome, allowing for gaps to accommodate tRNA introns, as well as up to 7 mismatches to accommodate terminal and internal RNA editing events. By appending 40 nt 5′ and 3′ of the location of genomic mapping, we obtained a candidate pre-tRNA gene set. We mapped non-annotated residual reads to these candidates to identify 5′ leader- and 3′ trailer-comprising pre-tRNA reads, which also distinguished actively transcribed tRNA genes from silent ones or pseudogenes. Leader- and trailer-comprising tRNA genes show higher sequence variation, as evidence by higher information entropy values, across the leader and trailer nucleotides than internal sequences within the mature tRNA suggesting that even short precursor sequences with read coverage are sufficient for the annotation of non-redundant tRNA genes (Fig. S1). At the end of our analysis we accounted for 93% of the 114,367,140 reads in our deepest library (Table S1). Given the depth of sequencing, we are confident that we accounted for the vast majority of precursor and mature tRNAs. Indeed, a posteriori we looked for genomic regions that collected at least 50 overlapping reads throughout their whole length, fell within the 60- to 100-nt size window, and adopted a cloverleaf structure, in an effort to detect any tRNAs that might have been overlooked by our approach or in prior literature. The only sequences that we identified were U1 snRNA (pseudo)genes (Fig. S2), suggesting that our analysis was exhaustive, at least for tRNAs in HEK293 cells.
Curation of pre-tRNAs by SSB PAR-CLIP
Even though the majority of our reads obtained from 60-100 nt size-fractionated total RNA were assigned to mature tRNAs, only 1% of the reads comprised sequences overlapping with pre-tRNA leader or trailer sequences (Fig 2A, Table S1). This raised the possibility that we might have missed reads corresponding to lowly expressed or very rapidly processed pre-tRNAs. Therefore, we decided to complement our efforts with PAR-CLIP-sequencing of SSB (Fig. 2B), a ubiquitous pre-tRNA binding protein (Bayfield and Maraia, 2009; Maraia and Lamichhane, 2010; Stefano, 1984). All tRNA genes are transcribed by POLR3, which terminates upon decoding an oligo-uridine (oligoU) region (Maraia and Lamichhane, 2010). SSB binds to the short pre-tRNA 3′ oligoU tail (Stefano, 1984) prior to removal of the entire 3′ trailer sequence. Therefore, we reasoned that SSB should bind all tRNA precursors, and that if we could isolate its targets, we would be able to reliably identify transcribed tRNA loci.
Figure 2. SSB PAR-CLIP.
(A) Total RNA composition of the 60-100 nt size fraction from hydro-tRNAseq according to RNA classes; data from deepest and richest in tRNA library (replicate 1) shown. (B) Phosphorimage of SSB-crosslinked to radiolabeled RNA. PAR-CLIP was performed using RNase A or RNase T1, at two different concentrations to account for possible biases of RNase treatment conditions. Libraries from PAR-CLIP using 1 U/μL of RNase A and RNase T1 were prepared and submitted for sequencing. Western blot against HA, shown in the bottom, confirmed the immunoprecipitation of SSB. (C) Assignment of reads from SSB PAR-CLIP (RNase T1, 1 U/μL) to RNA classes. (D) SSB PAR-CLIP reads mapping to tRNA precursors with 0, 1 or 2 mismatches (d0, d1, d2); reads with T-to-C mismatches are separated (red) from the rest of the reads with one mismatch (gray). Read length and number of reads are represented on the x- and y-axis, respectively. (E) Positional preference of SSB crosslinking by metagene analysis of all crosslinking events to precursor tRNAs. The incidence of T-to-C transitions is indicated by color intensity. (F) Positional preference of SSB crosslinking by metagene analysis of reads mapped hierarchically; first to mature and then to precursor tRNAs. For each class, PAR-CLIP signal (reads containing T-to-C) and background (reads with no mismatches) are shown. The normalized boundaries of pre-tRNAs (labeled as 0,1) and mature tRNAs (labeled as 5′ end, 3′ end), and read count are shown on the x- and y-axis, respectively. See also Fig. S3, and Tables S1 and S3.
SSB exhibited a striking binding preference for pre-tRNAs and showed a drastic enrichment in precursor tRNAs compared to hydro-tRNAseq (Fig. 2C), which confirmed our hypothesis, as well as previous observations (Bayfield and Maraia, 2009). We performed PAR-CLIP using two different nucleases to control for sequence biases at the nuclease digestion step. RNase T1 resulted in longer precursor tRNA trailer sequences than RNase A, due the latter's preference for cleaving 3′ to pyrimidines, which are highly abundant in the 3′ trailer sequences. Overall, 46% of all PAR-CLIP reads mapped to pre-tRNAs (Fig. 2C), the overwhelming majority of which showed the characteristic T-to-C transition, indicative of crosslinking (Fig 2D; Table S3). The vast majority of crosslinking sites in pre-tRNAs were concentrated, as expected, in the oligoU tract of the 3′ trailer sequence (Fig. 2E, F). We also found that SSB crosslinked to the 5′ segment of the mature tRNA body at conserved sites in the D-stemloop (Fig. 2F), which had been hinted at by a report proposing that the affinity of SSB for a full-length pre-tRNA cannot be explained solely by its binding to the 3′ oligoU tract (Bayfield and Maraia, 2009). The other major target of SSB was 5S ribosomal RNA (rRNA), which is the only POLR3-transcribed rRNA, and as such also terminates with an oligoU stretch to which SSB crosslinked (Fig. S3).
tRNA gene annotation
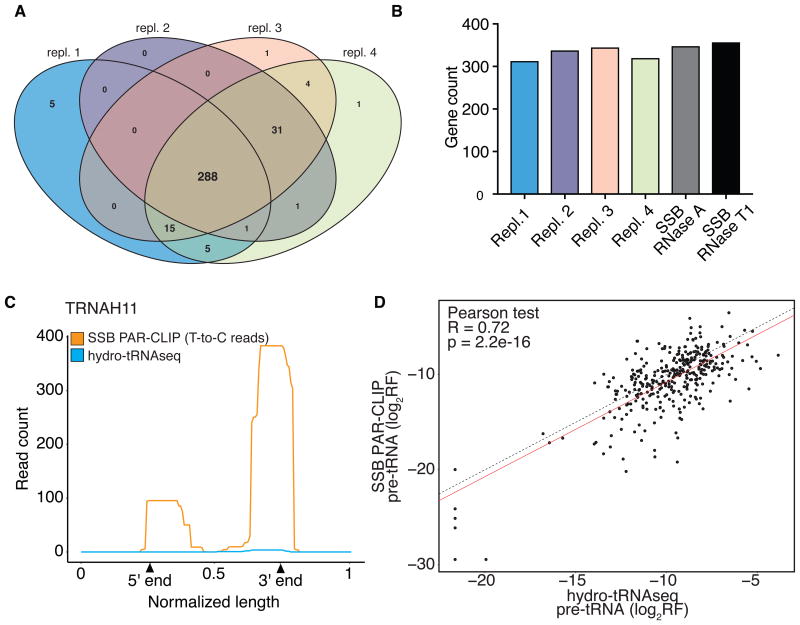
We combined hydro-tRNAseq and SSB PAR-CLIP to identify actively transcribed tRNA genes (genomic locations that give rise to supported pre-tRNAs). We confidently identified 288 tRNA genes as the intersection of 4 replicates of hydro-tRNAseq (Fig. 3A), and 349 tRNA genes as the intersection of two SSB PAR-CLIP experiments. Of note, SSB PAR-CLIP confirmed the expression of an additional 7 tRNA genes that were not supported in hydro-tRNAseq replicate (e.g. Fig. 3B,C and Table S4), further showcasing the complementarity of the two approaches. Notably, all seven of them show mature read evidence, so we are confident they do make it to maturity. We observed a strong correlation of pre-tRNA abundances between SSB PAR-CLIP and hydro-tRNAseq (Pearson R = 0.72; Fig. 3D), providing confidence that SSB PAR-CLIP quantitatively detected pre-tRNAs, without introducing biases (e.g. artificially enriching for lowly expressed pre-tRNAs). Instead, we observed no strong correlation between precursor and mature tRNA read counts in either of the two techniques (R < 0.2; Fig. S4). This suggests that pre-tRNA abundance cannot be used as a proxy for mature tRNA abundance and vice versa, likely due to different kinetics of processing and turnover between pre-tRNA fragments and mature tRNAs.
Figure 3. tRNA gene annotation.
(A) Venn diagram of expressed tRNA genes detected by hydro-tRNAseq in HEK293 cells. Genes with read evidence in both the 5′ leader and 3′ trailer of the pre-tRNA were counted. (B) Bar chart showing the number of pre-tRNAs detected in each replicate of hydro-tRNAseq and SSB PAR-CLIP. (C) Example of one out of seven pre-tRNAs that were detected by SSB PAR-CLIP, but were not detected in any of four hydro-tRNAseq replicates. (D) Correlation of relative read frequencies (log2-transformed) of precursor tRNAs between hydro-tRNAseq (x-axis) and SSB PAR-CLIP (y-axis). Correlation was calculated using the Pearson test. Linear fit is shown in red. The y=x line (dotted) is shown for comparison. See also Fig. S4, S5 and S6, and Table S2, S3 and S4.
Although tRNA isotypes with higher relative abundances generally tend to have higher tRNA gene numbers, we did not observe a clear linear correlation between read frequency and gene count (R = 0.12; Fig. 4A), like it has been reported before (Tuller et al., 2010). We then focused on the number of tRNA isoacceptors per amino acid, and isodecoders (tRNAs with the same anticodon sequence) per anticodon. We noticed a wide range of pre-tRNA counts per isoacceptor (Fig. 4B), with our data providing read evidence for 47 out of 62 coding codons (61 canonical and 1 selenocysteine TGA). The correlation of identified isoacceptor counts between SSB PAR-CLIP and hydro-tRNAseq was virtually perfect (Pearson R = 0.99; Fig. 4C), ruling out the introduction of a pronounced systematic bias from our hydrolysis-based protocol. Some anticodons seemed to be served by multiple isodecoders (e.g. 19 isodecoders for tRNACysGCA), while others only from one (e.g. tRNASerACT; Fig. 4B, D). Selenocysteine was the only amino acid that, in our data, was decoded by only one tRNA gene.
Figure 4. Number and relative abundance of tRNA genes per isotype and isoacceptor.
(A) Correlation of relative read frequency (y-axis) of mature tRNAs with number of genes (x-axis) per tRNA isotype. Hydro-tRNAseq data; four replicates; mean values shown; error bars represent standard deviation. (B) Number of tRNA genes for each anticodon and isotype. Hydro-tRNAseq data are shown in black (mean of 4 replicates; error bars represent standard deviation), SSB PAR-CLIP (RNase T1 treated) in red. (C) Correlation of gene counts for each anticodon detected by hydro-tRNAseq (y-axis) and SSB PAR-CLIP (x-axis). Each dot represents an anticodon shown in (B). Correlation was calculated using the Pearson test. (D) Average gene count and relative frequency for each anticodon. The gene count (mean of 4 replicates) is shown on the y-axis, anticodons on the x-axis; isotypes are indicated on the top. The area of each black disc is proportional to the fraction of reads mapping to all mature tRNAs for a given anticodon, normalized over all mature tRNAs for all anticodons. See also Fig. S5, S8, and Table S5.
Different isodecoders had varying degrees of contribution to the total read count per anticodon, with some showing highly skewed (e.g. tRNAArgCCG), and others relatively equal contribution (e.g. tRNALeuCAA; Table S5). The lack of linear correlation between tRNA gene count and relative read frequency that we noted previously on the isotype level, was also evident on the isoacceptor level. For example, the tRNAProTGG isoacceptors were collectively more abundant than tRNAProCGG isoacceptors, even though they comprise a smaller gene set (Figure 4D). Consistently, tRNAGlyGCC was found as the most abundant isoacceptor across experiments.
In terms of genomic organization of tRNA genes, more than 50% of pre-tRNAs with expression evidence are on chromosomes 1 and 6, grouped mostly in two densely populated clusters (Fig. S5), which are not restricted to tRNAs of a specific isotype (Table S2). In contrast, several chromosomes harbor no or very few tRNA genes (e.g. chr. 20 and 4) and chromosomes with intermediate number of tRNA genes (e.g. chr. 17) show a broad pattern of distribution.
Compared to POLR3 ChIP-seq, our approach provided much higher accuracy and precision in detection of pre-tRNAs (Fig. S6). The large size of genomic regions in such studies is responsible for low resolution and renders closely located tRNA genes indistinguishable. Our techniques provided nucleotide-level resolution, and were still capable of detecting more pre-tRNAs on average than POLR3 ChIP-seq.
Annotation of intron-containing tRNA genes
Intron-containing tRNAs represent a particularly interesting set of tRNA genes, as mutations in their evolutionarily conserved, yet distinct, processing machinery have emerged recently as causes of severe neurodevelopmental syndromes, such as pontocerebellar hypoplasia (Karaca et al., 2014; Namavar et al., 2011; Schaffer et al., 2014). Therefore, there is documented need for a comprehensive annotation of human intron-containing tRNAs, which should be revisited as markers or disease-causing candidates in phenotypically similar conditions. We confirmed 26 out of 32 predicted intron-containing tRNAs by hydro-tRNAseq (Fig. 5A). Intriguingly, we could not confirm expression of any predicted intronless tyrosine pre-tRNA gene or isoleucine-TAT and leucine-CAA isodecoders. Excluding any unknown biologically redundant mechanism, this suggests that the integrity of the tRNA splicing complex is essential for survival, a conclusion that could not have been reached with confidence in the absence of detailed curation and expression levels of pre-tRNA transcripts. To further confirm our observations, we coupled hydro-tRNAseq results with previously published PAR-CLIP data on the human tRNA ligase, RTCB (Baltz et al., 2012). Despite the shallow read depth of the dataset, we identified a crosslinked read peak at the anticodon loop of all intron-containing tRNAs annotated by our approaches (Fig. 5B,C).
Figure 5. Annotation of intron-containing tRNA genes.
(A) Number of validated intron-containing (orange) and intronless (blue) tRNA genes for all tRNA isotypes with possible intron-containing tRNAs. (B), (C) Analysis of PAR-CLIP for the tRNA ligase, RTCB (previously published in (Baltz et al., 2012)). Positional preference of crosslinking by metagene analysis of all crosslinking events (B) and all crosslinked reads (C) to mature tRNAs. The incidence of T-to-C transitions is indicated by color intensity in (B). PAR-CLIP signal (reads containing T-to-C), background (reads with no mismatches), normalized boundaries of the pre-tRNAs (labeled as 0, 1) and mature tRNAs (labeled as 5′ end, 3′ end) are shown in (C). See also Table S2.
tRNA transcription initiation and termination
Besides tRNA gene annotation and quantification, our approach yielded insights about pre-tRNA 3′ trailer sequences. Based on hydro-tRNAseq, we determined the median 5′ leader and 3′ trailer lengths to be 6 and 10 nt, respectively, with the trailer lengths showing a broader distribution (Fig. 6A,B). Interestingly, SSB PAR-CLIP revealed a subset of much longer trailers (Fig. 6C), suggesting that SSB PAR-CLIP captured the very initial steps of precursor tRNA processing, and accordingly that hydro-tRNAseq captures pre-tRNAs partially trimmed, either by ELAC2 (tRNase Z) or some other nuclease (Phizicky and Hopper, 2010).
Figure 6. Boundaries of tRNA transcription initiation and termination.
(A-C) Histogram of the length distribution of precursor tRNA 5′ leaders (A) and 3′ trailers (B) detected by hydro-tRNAseq, and trailers detected by SSB PAR-CLIP (C). (D,E) Histogram of the length distribution for the longest oligoU tract per precursor tRNA 3′ trailer detected by hydro-tRNAseq (D) (aggregate gene total of all replicates shown on the y-axis), and by SSB PAR-CLIP (E). Mean of 4 hydro-tRNAseq replicates ± SD. RNase T1-treated replicate is shown for SSB PAR-CLIP. See also Fig. S7.
We next focused on the POLR3 oligoU termination signals. Various reports in the past have focused on the oligoU requirements for transcription termination in different species (Arimbasseri et al., 2014; Nielsen et al., 2013). SSB protected consistently a 3′ 4 to 5 nt oligoU stretch, which was also confirmed by hydro-tRNAseq (Fig. 6D,E). This is in agreement with previous in vitro results (Bayfield and Maraia, 2009; Stefano, 1984; Teplova et al., 2006). We also addressed the proposed requirement for a stem-loop immediately upstream of the oligoU termination signal (Nielsen et al., 2013). Secondary structure predictions for the trailer sequences with documented sequence evidence in hydro-tRNAseq and SSB PAR-CLIP did not detect predicted stable stem-loop structures for approximately half of all pre-tRNAs (Fig. S7). This argued against a formal requirement for a stem-loop in the termination process of POLR3, at least on tRNA genes, in accordance with previous biochemical evidence (Arimbasseri et al., 2014).
Ribonucleotide modifications
RT across modified nucleotide-containing RNA leads to errors in cognate deoxynucleotide incorporation, revealed by mismatches in sequence reads upon mapping to reference genomic sequence. Read coverage across regions with a high degree of modifications may result in incomplete or largely uneven coverage. Therefore, we included in our mature tRNA reference the combination of all frequent mismatch signatures in all heavily modified positions. We reported the most frequently modified positions per tRNA gene (Table S6), and computed the frequencies of every nucleoside change per position across all tRNA genes (Fig. 7A). The majority of editing events were A-to-G transitions at the first position of the anticodon and at the position 3′ to the anticodon (usually position 37). Both positions are known to be heavily modified, the former being deaminated to inosine, and the latter further modified (e.g. 1-methylinosine) (Machnicka et al., 2013). In our data the majority of the reads that mapped to the anticodon of the modified tRNAs contained the mismatches. To a lesser extent we could also detect 1-methyladenosine in the pseudouridine loop (returned as A-to-T or A-to-G), and various guanosine modifications at positions 9, 26, and 45, which most likely correspond to 1-methyl-, N2,N2-dimethyl-, and 7-methyl-guanosine, respectively (Machnicka et al., 2013).
Figure 7. tRNA modifications.
(A) The positions that resulted in the most common mismatches over all tRNAs, along with the reference nucleotide and the most likely modification are indicated in the center of each ring. The relative frequency of each returned nucleoside is proportional to the corresponding color-coded area of the ring (m1G: 1-methylguanosine; m2,2G: N2,N2-dimethylguanosine; I: inosine; m1I: 1-methylinosine; m7G: 7-methylguanosine; m1A: 1-methyladenosine). (B) Read alignments for precursor TRNAA6 with one (top) and two mismatches (bottom). The position of the anticodon is marked by the black rectangle at the top of the alignments. Mismatches of the heavily modified adenosines in the anticodon loop are indicated by red lowercase letters. Read counts and mapping locations for each read are shown on the right side. Vertical bars represent binned, log4-transformed, and normalized read counts for each alignment. See also Table S6.
The temporal resolution of tRNA modifications by RNAseq has begun to be addressed recently (Torres et al., 2015), however at a single modification level (inosine 34), and by using libraries relative poor in tRNA reads (<1% of total reads). We were appropriately poised to address this issue since our very deep sequencing set, in combination with our hierarchical annotation pipeline, offered the advantage of dissecting multiple modifications simultaneously. We focused on the inosine modifications, since they represented the majority of modified nucleosides. By inspecting read alignments with error distance 1 to the reference pre-tRNA, we noticed A-to-G transition mismatches at position 34 in reads that retained the leader and trailer sequences of the precursor tRNA (Fig. 7B, top). This confirmed that A34 deamination takes place at the precursor level, and therefore is a nuclear modification, as it has been previously reported (Torres et al., 2015). Next, we noticed that 1-methyl-inosine at position 37 also appears at the precursor stage. Of note the A37 modification became apparent prior to A34, as the majority of the error distance 1 reads contained a mismatch at A37. Reads with two mismatches contained both modifications (Fig. 7B, bottom).
Discussion
We have combined two complementary transcriptome-wide approaches to provide experimentally validated annotation for mature and pre-tRNA transcripts and their respective genes, in addition to furnishing an accurate quantification of tRNA abundance in human cells.
First, we developed hydro-tRNAseq, a fragmentation-based protocol for overcoming hurdles of tRNA sequencing, and obtained deep sequencing sets that enabled the annotation of tRNA genes and derivation of mature tRNA reference sequences for accurately assigning sequence reads to the otherwise edited and nucleotide-modified original tRNA. Alkaline hydrolysis of the tRNA-containing pool relieved thermodynamically stable structural constraints that impair ligation steps in the cDNA library preparations, reduced the number of modified nucleosides per sequenced fragment, resulting in high read-through in the RT step, and release of the 3′ hydroxyl group of the otherwise aminoacylated tRNA 3′ end.
Then, we took advantage of the pre-tRNA binding properties of SSB protein, which coordinates posttranscriptional processing and maturation of tRNAs (Maraia and Lamichhane, 2010), to enrich for tRNA precursors and allow for a comprehensive curation of pre-tRNA transcripts and annotation of tRNA genes. Of note, since SSB interacts with pre-tRNAs and other small nuclear RNA U-rich 3′ ends in all organisms examined so far (Maraia and Bayfield, 2006; Teplova et al., 2006), our approach can be adapted towards tRNA annotation in other species.
Our data suggest that, at least in our experimental system, the tRNA gene space is considerably more contracted than it has been previously predicted by bioinformatics, evidenced by the fact that almost half of the predicted tRNA loci were transcriptionally silent, presumably representing retrointegrated tRNA pseudogenes. It would be interesting to examine whether such an observation holds for various cell types and at different stages of development or disease, in order to confirm the differential expression and regulation of tRNA gene expression that has been reported before (Gingold et al., 2014; Goodarzi et al., 2016). Our analysis shows that selenocysteine seems to be the only monogenic tRNA species, suggesting an increased sensitivity to mutations due to the lack of functional redundancy.
Our approach allowed the elucidation of relevant issues regarding POLR3 transcription such as the length of pre-tRNA leaders and trailers, the length of oligoU required for recognition by SSB, both of which have shown species specificity (Arimbasseri and Maraia, 2015). We also detected that 4 sequential Us act as the transcription termination signal for POLR3, confirming similar predictions based on genomic sequences that suggested a requirement for 4 Us for efficient termination in vertebrates (Arimbasseri and Maraia, 2013; Iben and Maraia, 2012) as well as structural data documenting the capacity of SSB to accommodate 4 Us in its binding site (Stefano, 1984; Teplova et al., 2006). At the same time, the length distribution of the oligoU tract identified in our experiments reflects the heterogeneity of termination signal lengths that has been noted as an intrinsic property of pol III in vitro (Arimbasseri and Maraia, 2015).
We could also confirm a second binding site of SSB in the 5′ half of the mature tRNA sequence, in support of previous observations proposing the presence of additional pre-tRNA binding sites besides the 3′ tail (Bayfield and Maraia, 2009; Stefano, 1984). It has been previously noted that the binding mode of SSB to tRNA is more complex than the recognition alone of the 3′ tails, and that one of the RNA recognition motifs present in SSB, RRM1, could bind elsewhere in the tRNA. This stems from two observations: 1) SSB has a higher affinity for precursor over mature tRNAs, and that 2) structural data show that RRM1 is unoccupied when SSB is bound to UUU-3′-OH substrate. Our data seem to validate this observation, and could shed light into new modes of SSB-mediated processing of pre-tRNAs into either mature tRNAs or other kinds of ncRNAs (Hasler et al., 2016).
Recently tRNA sequencing methods have been developed that employ dealkylating enzymes and/or highly thermostable reverse transcriptase to overcome respectively the hurdles of modifications and stable structures that impede tRNA sequencing (Cozen et al., 2015; Zheng et al., 2015). However, they both have specific limitations that we tried to address. We size-selected at a higher size window to avoid contamination by tRNA-derived fragments (as compared to Cozen et al., 2015). Also, we used two sequential adapter ligation methods to make sure that only full-length fragments were sequenced and the sequencing reads were not results of blocks during RT (as compared to Zheng et al., 2015), which allowed us to differentiate RT stops from fragment ends. Additionally, we did not bias our sequencing protocol towards mature tRNAs (as compared (Zheng et al., 2015) and also (Shigematsu et al., 2017)), but instead we captured more precursors by both RNAseq and more importantly PAR-CLIP methods, allowing us to perform a deeper precursor tRNA curation. Importantly, despite the reportedly high processivity conferred by dealkylating methyl modifications, in the previous studies only a small fraction of reads mapped at a given transcript were full-length reads (<1% of all reads), with a marginal increase compared to untreated controls (Fig. S8A). In contrast, hydro-tRNAseq yielded a higher cumulative fraction of mature tRNAs with read evidence across their whole length with a mean read coverage of 0.99 of the full length (compared to 0.95 and 0.87 in previous studies; (Fig. S8B). Also, SSB PAR-CLIP was more sensitive in identifying tRNA genes, detecting 349 genes as compared to ∼159 and ∼212 in the other methods.
Moreover, we were able to carry out a careful overview and tRNA modifications that result in characteristic mismatch signatures. We introduced all possible combinations of all “mutated” nucleotides at the most prominently modified positions in every tRNA in order to collect as many reads that could be having RT misincorporations at the modified positions. This created a large number of similar tRNA sequences, and therefore we allowed for extensive multimapping, but split the read counts in order to avoid artificial read count inflation. By making use of our hierarchical annotation pipeline, we were able to dissect the temporal order of inosine modifications at the tRNA anticodon, confirming that A34 deamination occurs in the nucleus prior to the nucleolytic processing of the pre-tRNA, and establishing that the same holds true for A37 modifications, which in fact precede A34 deamination.
Accounting for modification signatures was also important for the reason that CLIP-seq, and especially PAR-CLIP, depends on apparent mismatches (in the case of PAR-CLIP, T-to-C conversions) for the identification of RBP binding sites on target RNAs. Since we used PAR-CLIP of SSB for the annotation of pre-tRNAs and tRNA genes, we first examined uridine modifications that result in T-to-C conversions. Only a small minority of modification signatures were T-to-C transitions, suggesting that it is highly unlikely that our PAR-CLIP data were artificially inflated.
Collectively, our results give a census of the tRNA transcriptome in human cells. We provide a method and computational flowchart for the analysis of tRNAs. We expect that these results will inform studies of tRNA-related human disease.
Experimental Procedures
Hydro-tRNAseq
Total RNA from HEK293 (Flp-In T-REx, Invitrogen) was isolated using TRIzol (Invitrogen). For each sample 20 μg of total RNA were resolved on 12% urea-polyacrylamide gels and recovered within a size window of 60-100 nt. The eluted fraction was subjected to limited alkaline hydrolysis in a 15 μL buffer of 10 mM Na2CO3 and 10 mM NaHCO3 at pH 9.7 either at 65 °C for 10 min (replicate 1) or 1 h (replicates 2-4).
The partially hydrolyzed RNA was dephosphorylated with 10 U of calf intestinal phosphatase (NEB) in a 50 μL reaction of 100 mM NaCl, 50 mM Tris-HCl, pH 7.9 at 25 °C, 10 mM MgCl2, 1 mM DTT, 3 mM Na2CO3 and 3 mM NaHCO3, at 37 °C for 1 h. The resulting RNA was re-phosphorylated with 10 U of T4 polynucleotide kinase (NEB) in a 20 μL reaction of 70 mM Tris-HCl, pH 7.6, 10 mM MgCl2, 5 mM DTT and 1 mM ATP, at 37 °C for 1 h. Fragments of 19-35 nt were converted into barcoded small RNA cDNA libraries, as previously described (Hafner et al., 2012a), and sequenced on an Illumina HiSeq 2500 instrument. Adapters were trimmed using cutadapt (http://journal.embnet.org/index.php/embnetjournal/article/view/200/458). Sequencing read alignments were performed using the Burrows-Wheeler aligner against an in-house curated and annotated list of mature and precursor tRNAs containing predicted tRNA sequences for human genome version hg19 (http://gtrnadb.ucsc.edu). Sequencing reads were first mapped against mature tRNAs. Remaining reads were mapped against genomic tRNA sequences that included 5′ leader and 3′ trailer sequences, as well as tRNA introns.
SSB PAR-CLIP
Flp-In T-REx HEK293 cells (Invitrogen) were grown in high glucose DMEM supplemented with 10% (v/v) FBS, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL zeocin and 15 μg/mL blasticidin. Cell lines stably expressing FLAG/HA- (FH-) SSB were generated as described previously (Spitzer et al., 2013). Expression of FH-SSB was induced by addition of 1 μg /mL doxycycline for 24 h. 4SU PAR-CLIP was performed as described previously, using either RNase T1 or RNase A (Garzia et al., 2016). PAR-CLIP cDNA libraries were sequenced on an Illumina HiSeq 2500 instrument. Adapter extracted reads were aligned against an in-house curated and annotated list of mature and precursor mRNAs and ncRNAs. Bioinformatic analysis was performed using a analysis pipeline based on a curated and annotated reference RNA collection, which we organized into categories, such as rRNA, tRNA, snoRNA, mRNAs, etc. This pipeline is available at https://rnaworld.rockefeller.edu/PARCLIP_suite/. T-to-C conversion frequency, indicative of binding, was calculated for each annotated category of RNA.
Bioinformatic analysis
Reads were mapped to our transcriptomic database with error distance 0 (d0), 1 (d1) or 2 (d2), allowing mismatches, insertions and deletions. Assignment of reads with more than one mapping locations that belong to different RNA classes followed a hierarchical procedure reflective of the cellular abundance of each RNA class. Mature RNA sequences (e.g. fully processed tRNAs) received priority compared to precursors (e.g. pre-tRNAs), thus minimizing multimapping events. A tRNA gene was considered to be expressed when there were reads spanning the precursor/mature junctions, including exon/intron junctions for intron-containing tRNAs. For abundance reports, multimapping reads were split equally over the number of their mapping locations, and all reads mapping to edited and non-edited versions of the same tRNA transcript were summed for quantification of a given tRNA. Naming of tRNAs followed HUGO guidelines, with edited variants of reference tRNAs exhibiting the edited position and the identity of induced mismatch in their naming. The same analysis pipeline was applied to mitochondrial tRNAs, with the exception that no tRNA precursors were annotated due to continuous transcription of the mitochondrial genome. Remaining reads that did not map to any annotated transcript were mapped to the human genome. The mapped read annotation process was based on a hierarchical procedure that assigned priority to reads mapping in their entirety to mature sequences, followed by reads that spanned the precursor-mature junctions. Bioinformatic analysis was performed by custom Perl and Python scripts, all available upon request. Graphs were created in R and Prism (Graphpad).
Statistical Methods
Correlation for bivariate plots of continuous variables was identified by calculating Pearson's correlation coefficient in Prism (Graphpad) or R. Correlation coefficients (R) and associated p-values are reported. Linear regressions were carried out in Prism. When multiple replicates were used, the number of replicates is indicated in the relevant figure legends. Arithmetic mean values and standard deviations are used for plotting in such cases.
Accession codes
The RNAseq and PAR-CLIP sequence data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive under accession numbers GSE95683.
Supplementary Material
Table S1. Hydro-tRNAseq reads per RNA type, following hierarchical annotation. Related to Fig. 1 and 2
Table S2. Master table of mature, pre-tRNA, and intron-containing tRNA sequences, genomic coordinates of pre-tRNAs, and the comparison of confirmed and predicted tRNA gene counts. Related to Fig. 1, 3 and 5.
Table S3. SSB PAR-CLIP reads per RNA type, following hierarchical annotation. Related to Fig. 2 and 3.
Table S4. Leader and trailer sequences detected by hydro-tRNAseq and SSB PAR-CLIP. Related to Fig. 3.
Table S5. Mature tRNA abundance per isotype, pre-tRNA count, and anticodon. Related to Fig. 4.
Table S6. Positions resulting to modifications-induced mismatches. Related to Fig. 7.
Highlights.
Hydro-tRNAseq is a facile and efficient method for sequencing tRNAs
PAR-CLIP of SSB/La informs the annotation tRNA genes and curation of pre-tRNAs
Combined, they facilitate the study of tRNA processing and POLR3 transcription
Acknowledgments
We would like to thanks Connie Zhao and the Rockefeller Genomics Resource Center. This work was supported by NIH TRA R01CA159227 (T.T.). T.G. is supported by the Weill Cornell/Rockefeller/Sloan Kettering MD/PhD Program. We would like to thank Masashi Yamaji and Kemal Akat for scientific input on discussions, and Dr. Pavel Morozov for bioinformatics advice. Thomas Tuschl is cofounder and advisor to Alnylam Pharmaceuticals.
Footnotes
Author Contributions: T.G. and T.T. conceived the project. T.G. and M.H. designed and performed experiments. T.G. and M.B. performed bioinformatic analysis. A.G. performed PAR-CLIP experiments and analysis. C.M. created the SSB cell lines. T.G. and T.T. analyzed data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arimbasseri AG, Maraia RJ. Distinguishing Core and Holoenzyme Mechanisms of Transcription Termination by RNA Polymerase III. Molecular and Cellular Biology. 2013;33:1571–1581. doi: 10.1128/MCB.01733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimbasseri AG, Maraia RJ. Mechanism of Transcription Termination by RNA Polymerase III Utilizes a Non-template Strand Sequence-Specific Signal Element. Molecular Cell. 2015;58:1124–1132. doi: 10.1016/j.molcel.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimbasseri AG, Blewett NH, Iben JR, Lamichhane TN, Cherkasova V, Hafner M, Maraia RJ. RNA Polymerase III Output Is Functionally Linked to tRNA Dimethyl-G26 Modification. PLoS Genet. 2015;11:e1005671–22. doi: 10.1371/journal.pgen.1005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimbasseri AG, Iben J, Wei FY, Rijal K, Tomizawa K, Hafner M, Maraia RJ. Evolving specificity of tRNA 3-methyl-cytidine-32 (m 3C32) modification: a subset of tRNAs Serrequires N6-isopentenylation of A37. Rna. 2016;22:1400–1410. doi: 10.1261/rna.056259.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimbasseri AG, Kassavetis GA, Maraia RJ. Transcription. Comment on “Mechanism of eukaryotic RNA polymerase III transcription termination”. Science. 2014;345:524–524. doi: 10.1126/science.1253783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz AG, Munschauer M, Schwanhäusser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, et al. The mRNA-Bound Proteome and Its Global Occupancy Profile on Protein-Coding Transcripts. Molecular Cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Bayfield MA, Maraia RJ. Precursor-product discrimination by La protein during tRNA metabolism. Nature Structural & Molecular Biology. 2009;16:430–437. doi: 10.1038/nsmb.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayfield MA, Yang R, Maraia RJ. Conserved and divergent features of the structure and function of La and La-related proteins (LARPs) BBA - Gene Regulatory Mechanisms. 2010;1799:365–378. doi: 10.1016/j.bbagrm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozen AE, Quartley E, Holmes AD, Hrabeta-Robinson E, Phizicky EM, Lowe TM. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat Meth. 2015;12:879–884. doi: 10.1038/nmeth.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana A, Tuller T. Determinants of Translation Elongation Speed and Ribosomal Profiling Biases in Mouse Embryonic Stem Cells. PLoS Comput Biol. 2012;8:e1002755–11. doi: 10.1371/journal.pcbi.1002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana A, Tuller T. Mean of the typical decoding rates: a new translation efficiency index based on the analysis of ribosome profiling data. G3 (Bethesda) 2014;5:73–80. doi: 10.1534/g3.114.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KA, Mobley EM, Radek AJ, Pan T. Exploring the Regulation of tRNA Distribution on the Genomic Scale. Journal of Molecular Biology. 2004;337:31–47. doi: 10.1016/j.jmb.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Foretek D, Wu J, Hopper AK, Boguta M. Control of Saccharomyces cerevisiae pre-tRNA processing by environmental conditions. RNA. 2016;22:339–349. doi: 10.1261/rna.054973.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzia A, Meyer C, Morozov P, Sajek M, Tuschl T. Optimization of PAR-CLIP for transcriptome-wide identification of binding sites of RNA-binding proteins. Methods. 2016:1–17. doi: 10.1016/j.ymeth.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold H, Tehler D, Christoffersen NR, Nielsen MM, Asmar F, Kooistra SM, Christophersen NS, Christensen LL, Borre M, Sørensen KD, et al. A Dual Programfor Translation Regulationin Cellular Proliferation and Differentiation. Cell. 2014;158:1281–1292. doi: 10.1016/j.cell.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Goodarzi H, Nguyen HCB, Zhang S, Dill BD, Molina H, Tavazoie SF. Modulated Expression of Specific tRNAs Drives Gene Expression and Cancer Progression. Cell. 2016;165:1416–1427. doi: 10.1016/j.cell.2016.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Jackman JE, Lohan AJ, Gray MW, Phizicky EM. tRNAHis maturation: an essential yeast protein catalyzes addition of a guanine nucleotide to the 5' end of tRNAHis. Genes & Development. 2003;17:2889–2901. doi: 10.1101/gad.1148603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Renwick N, Farazi TA, Mihailović A, Pena JTG, Tuschl T. Barcoded cDNA library preparation for small RNA profiling by next-generation sequencing. Methods. 2012a;58:164–170. doi: 10.1016/j.ymeth.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Renwick N, Farazi TA, Mihailović A, Pena JTG, Tuschl T. Barcoded cDNA library preparation for small RNA profiling by next-generation sequencing. Methods. 2012b;58:164–170. doi: 10.1016/j.ymeth.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler D, Lehmann G, Murakawa Y, Klironomos F, Jakob L, Grässer FA, Rajewsky N, Landthaler M, Meister G. The Lupus Autoantigen La Prevents Mis-channeling of tRNA Fragments into the Human MicroRNA Pathway. Molecular Cell. 2016;63:110–124. doi: 10.1016/j.molcel.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Iben JR, Maraia RJ. tRNAomics: tRNA gene copy number variation and codon use provide bioinformatic evidence of a new anticodon:codon wobble pair in a eukaryote. Rna. 2012;18:1358–1372. doi: 10.1261/rna.032151.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iben JR, Maraia RJ. tRNA gene copy number variation in humans. Gene. 2014;536:376–384. doi: 10.1016/j.gene.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-Induced tRNA Fragments Inhibit Translation Initiation. Molecular Cell. 2011;43:613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Research. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca E, Weitzer S, Pehlivan D, Shiraishi H, Gogakos T, Hanada T, Jhangiani SN, Wiszniewski W, Withers M, Campbell IM, et al. Human CLP1 Mutations Alter tRNA Biogenesis, Affecting Both Peripheral and Central Nervous System Function. Cell. 2014;157:636–650. doi: 10.1016/j.cell.2014.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter C, Brown GD, Gonçalves Â, Wilson MD, Watt S, Brazma A, White RJ, Odom DT. Pol III binding in six mammals shows conservation among amino acid isotypes despite divergence among tRNA genes. Nat Genet. 2011;43:948–955. doi: 10.1038/ng.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes & Development. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Research. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlab S, Tuller T, Linial M. Conservation of the relative tRNA composition in healthy and cancerous tissues. Rna. 2012;18:640–652. doi: 10.1261/rna.030775.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraia RJ, Bayfield MA. The La Protein-RNA Complex Surfaces. Molecular Cell. 2006;21:149–152. doi: 10.1016/j.molcel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Maraia RJ, Lamichhane TN. 3′ processing of eukaryotic precursor tRNAs. WIREs RNA. 2010;2:362–375. doi: 10.1002/wrna.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqtaderi Z, Wang J, Raha D, White RJ, Snyder M, Weng Z, Struhl K. Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nature Structural & Molecular Biology. 2010;17:635–640. doi: 10.1038/nsmb.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namavar Y, Barth PG, Poll-The BT, Baas F. Classification, diagnosis and potential mechanisms in Pontocerebellar Hypoplasia. Orphanet Journal of Rare Diseases. 2011;6:50. doi: 10.1186/1750-1172-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Yuzenkova Y, Zenkin N. Mechanism of eukaryotic RNA polymerase III transcription termination. Science. 2013;340:1577–1580. doi: 10.1126/science.1237934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler AJ, Alla RK, Roberts DN, Wong A, Hollenhorst PC, Chandler KJ, Cassiday PA, Nelson CA, Hagedorn CH, Graves BJ, et al. Nature Structural & Molecular Biology. 2010;17:620–628. doi: 10.1038/nsmb.1801. nsmb.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann S, Frydman J. Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nature Publishing Group. 2012;20:237–243. doi: 10.1038/nsmb.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes & Development. 2010;24:1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet. 2011;12:32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Eggens VRC, Caglayan AO, Reuter MS, Scott E, Coufal NG, Silhavy JL, Xue Y, Kayserili H, Yasuno K, et al. CLP1 Founder Mutation Links tRNA Splicing and Maturation to Cerebellar Development and Neurodegeneration. Cell. 2014;157:651–663. doi: 10.1016/j.cell.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu M, Honda S, Loher P, Telonis AG, Rigoutsos I, Kirino Y. YAMAT-seq: an efficient method for high-throughput sequencing of mature transfer RNAs. Nucleic Acids Research. 2017 doi: 10.1093/nar/gkx005. gkx005–gkx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer J, Landthaler M, Tuschl T. Rapid Creation of Stable Mammalian Cell Lines for Regulated Expression of Proteins Using the Gateway® Recombination Cloning Technology and Flp-In T-REx® Lines. Elsevier Inc; 2013. [DOI] [PubMed] [Google Scholar]
- Stefano JE. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984;36:145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Teplova M, Yuan YR, Phan AT, Malinina L, Ilin S, Teplov A, Patel DJ. Structural Basis for Recognition and Sequestration of UUUOH 3′ Temini of Nascent RNA Polymerase III Transcripts by La, a Rheumatic Disease Autoantigen. Molecular Cell. 2006;21:75–85. doi: 10.1016/j.molcel.2005.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AG, Pineyro D, Rodriguez-Escriba M, Camacho N, Reina O, Saint-Leger A, Filonava L, Batlle E, Ribas de Pouplana L. Inosine modifications in human tRNAs are incorporated at the precursor tRNA level. Nucleic Acids Research. 2015:1–13. doi: 10.1093/nar/gkv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y. An Evolutionarily Conserved Mechanism for Controlling the Efficiency of Protein Translation. Cell. 2010;141:344–354. doi: 10.1016/j.cell.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Weinberg DE, Shah P, Eichhorn SW, Hussmann JA, Plotkin JB, Bartel DP. Improved Ribosome-Footprint and mRNA Measurements Provide Insights into Dynamics and Regulation of Yeast Translation. Cell Reports. 2016;14:1787–1799. doi: 10.1016/j.celrep.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. The Molecular basis for Genetic Expression. Harper; 1967. The Genetic Code. [Google Scholar]
- Zheng G, Qin Y, Clark WC, Dai Q, Yi C, He C, Lambowitz AM, Pan T. Efficient and quantitative high-throughput tRNA sequencing. Nat Meth. 2015:1–5. doi: 10.1038/nmeth.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hydro-tRNAseq reads per RNA type, following hierarchical annotation. Related to Fig. 1 and 2
Table S2. Master table of mature, pre-tRNA, and intron-containing tRNA sequences, genomic coordinates of pre-tRNAs, and the comparison of confirmed and predicted tRNA gene counts. Related to Fig. 1, 3 and 5.
Table S3. SSB PAR-CLIP reads per RNA type, following hierarchical annotation. Related to Fig. 2 and 3.
Table S4. Leader and trailer sequences detected by hydro-tRNAseq and SSB PAR-CLIP. Related to Fig. 3.
Table S5. Mature tRNA abundance per isotype, pre-tRNA count, and anticodon. Related to Fig. 4.
Table S6. Positions resulting to modifications-induced mismatches. Related to Fig. 7.