Abstract
Activated CD8+ T cells differentiate into cytotoxic effector (TEFF) cells that eliminate target cells. How TEFF cell identity is established and maintained remains less understood. Here we show Runx3 deficiency limits clonal expansion and impairs upregulation of cytotoxic molecules in TEFF cells. Runx3-deficient CD8+ TEFF cells aberrantly upregulate genes characteristic of follicular helper T (TFH) cell lineage, including Bcl6, Tcf7 and Cxcr5. Mechanistically, the Runx3-CBFβ complex deploys H3K27me3 to Bcl6 and Tcf7 genes to suppress the TFH program. Ablating Tcf7 in Runx3-deficient CD8+ TEFF cells prevents the upregulation of TFH genes and ameliorates their defective induction of cytotoxic genes. As such, Runx3-mediated Tcf7 repression coordinately enforces acquisition of cytotoxic functions and protects the cytotoxic lineage integrity by preventing TFH-lineage deviation.
Transcription factors play central roles in establishing and maintaining cell identity during development, homeostasis and response to environmental changes1. In the immune system, CD4+ and CD8+ T cells are functionally distinct helper and cytotoxic lineages whose identity is stipulated by distinct transcription factors2–4. ThPOK is essential for the CD4+ T lineage choice during development and for maintaining CD4+ T lineage integrity, largely through restraining activation of Runx-CBF complex-dependent transcriptional programs5,6. Tcf1 and Lef1, although not required for CD8+ T lineage decision, have critical roles in establishing CD8+ T cell identity through their intrinsic HDAC activity7,8. In response to acute infection by intracellular microbes, CD8+ T cells differentiate into dedicated cytotoxic effector cells that eliminate infected target cells in response to acute infection by intracellular pathogens9–11, while CD4+ T cells give rise to T helper 1 (TH1), TH2, TH17, and TFH cells depending on the nature of pathogens12,13.
Maintaining the identity of CD8+ T effector (TEFF) cells elicited by acute infections is essential for their cytotoxic capacity. The best-known transcriptional regulators in this regard include T-bet, Eomes and Blimp-1, which are potently induced upon CD8+ T cell activation14. Whereas deletion of either T-bet or Eomes alone does not have a pronounced effect, combined deletion of both factors causes aberrant activation of the TH17 program, including upregulation of Rorγt, along with IL-17A and IL-2115. Compound deletion T-bet and Blimp-1 leads to induction of Rorγt and IL-17A in CD8+ TEFF cells16. These IL-17-producing, T-bet-Eomes- or T-bet-Blimp-1-deficient CD8+ TEFF cells caused progressive inflammatory and wasting syndrome, highlighting an essential requirement for maintaining the cytotoxic lineage integrity. However, it remains unknown if other T helper subset plasticity is transcriptionally and/or epigenetically suppressed in CD8+ TEFF cells.
The Runx-CBF complex consists of unique DNA-binding α subunits (Runx1, 2 or 3) and the obligatory cofactor CBFβ, which does not bind DNA but stabilizes Runx-DNA interaction17,18. Runx1 and Runx3 are predominantly expressed in T lineage cells and have redundant functions in repressing ThPOK expression to ensure generation of CD8+ T cells and Cd4 gene silencing in CD8+ T cells during thymic development19,20. A role of Runx3 in inducing interferon-γ (IFN-γ), perforin and granzyme B expression in activated mature CD8+ T cells was suggested from studies utilizing germline-targeted Runx3-deficient CD8+ T cells responding to in vitro stimulation21,22. However, the in vivo role of the Runx-CBF complex in CD8+ T cell responses remains uncharted. We specifically targeted Runx3 in mature T cells and used in vivo infection models to reveal an essential role of Runx3 in guarding CD8+ TEFF cells from deviation to the TFH cell lineage, in addition to inducing the expression of cytotoxic mediators.
Results
Loss of Runx3 impairs CD8+ TEFF cell expansion and function
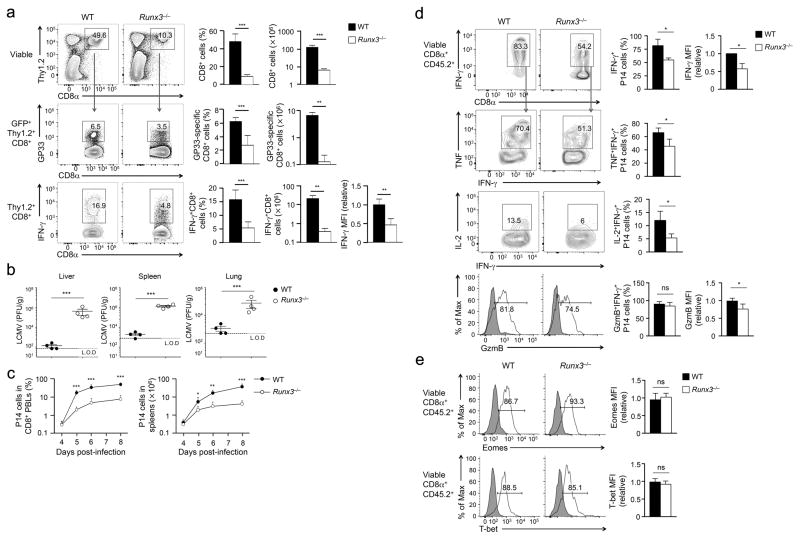
To address the role of Runx3 in CD8+ T cell responses in a physiological setting of in vivo infection, we generated hCD2-Cre+Rosa26GFPRunx3FL/FL (Runx3−/− hereafter) mice where hCD2-Cre specifically deleted floxed genes in mature T cells6,23. Thymic development was similar between Runx3−/− mice and littermate controls with the genotypes of Runx3+/+ or Runx3+/− (referred to as wild-type because CD8+ T cells in these genotypes behaved similarly in all assays, Supplementary Fig. 1a). hCD2-Cre-mediated deletion did not occur in natural killer cells, but was efficient in CD8+ T cells, as indicated by GFP expression (Supplementary Fig. 1b). Deletion of Runx3 protein in Runx3−/− CD8+ T cells was confirmed by intracellular staining (Supplementary Fig. 1c). The number of CD8+ T cells in the spleen of Runx3−/− mice was reduced by about 40% compared with wild-type mice but did not exhibit derepression of CD4 coreceptor or an aberrant activation phenotype (Supplementary Fig. 1d,e). On day 8 post-infection (8 dpi) with the Armstrong strain of lymphocytic choriomeningitis virus (LCMV-Arm), Runx3−/− mice had diminished frequency and numbers of LCMV glycoprotein 33–41 (GP33)-specific CD8+ TEFF cells compared with wild-type mice, as detected by GP33-tetramer or GP33 peptide-stimulated IFN-γ production (Fig. 1a). In contrast to effective clearance of the virus in wild-type mice on 8 dpi, high titers of LCMV were detected in the livers, spleens and lungs of Runx3−/− mice (Fig. 1b). These data suggest an essential requirement for Runx3 in mounting protective CD8+ T cell responses.
Figure 1. Runx3 is essential for clonal expansion and cytotoxic functions in CD8+ TEFF cells.
(a) Frequency and numbers of total (top) or antigen-specific (middle, bottom) Thy1.2+CD8+ TEFF cells determined by either GP33 tetramer (middle) or GP33 peptide-stimulated IFN-γ production (bottom) in splenocytes from Runx3−/− or wild-type (WT) mice infected with LCMV-Arm on day 8 post-infection. (b) Titers of LCMV in the liver, spleen and lung in Runx3−/− or WT mice infected with LCMV-Arm on day 8 post-infection. L.O.D., limit of detection. (c) Frequency and numbers of P14 CD8+ TEFF cells determined in the peripheral blood lymphocytes (PBLs, left) and the spleens (right), respectively, during days 4–8 post-infection after naïve Runx3−/− or WT P14 CD8+ T cells were adoptively transferred into congenic recipients followed by LCMV-Arm infection. (d) IFN-γ, TNF, IL-2 and granzyme B (GzmB) expression detected by intracellular staining in GP33 peptide-stimulated splenic Runx3−/− or WT P14 CD8+ TEFF cells on day 5 post-infection. (e) T-bet and Eomes expression detected by intranuclear staining in splenic Runx3−/− or WT P14 CD8+ TEFF cells on day 5 post-infection, with shaded areas denote corresponding isotype controls. Cumulative data in (a), (c–e) are means ± s.d. (n = 3–6) from 3 independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001 (Student’s t-test), and data in (b) are from 2 experiments.
We also generated hCD2-Cre+Rosa26GFPCbfbfl/fl (Cbfb−/− hereafter) mice to ablate CBFβ in peripheral CD8+ T cells (Supplementary Fig. 2a). Cbfb−/− mice showed greatly diminished CD8+ TEFF cells in response to LCMV-Arm infection compared with Cbfb+/+ or Cbfb+/− littermate controls, similar to Runx3−/− mice (Supplementary Fig. 2b), suggesting that the Runx3-CBFβ complex has a predominant role in CD8+ T cell responses to acute infections, without a strong contribution from the Runx1-CBFβ complex. In addition, in a bacterial infection model using Listeria monocytogenes expressing ovalbumin 257–264 (OVA257) and GP33 epitopes (LM-OVA-GP33), Runx3−/− mice showed a >4-fold reduction in both OVA- and GP33-specific CD8+ TEFF cells compared with wild-type mice (Supplementary Fig. 2c–e), indicating a requirement for Runx3 in CD8+ T cell responses independent of infection type or epitope.
To control for potential alterations in precursor frequency, we crossed Runx3−/− with an MHC I-restricted P14 transgenic TCR that is specific for the LCMV-GP33 epitope. We adoptively transferred 2×104 naïve Runx3−/− or wild-type (Runx3+/+ or Runx3+/−) P14 CD8+ T cells into congenic recipients, followed by infection with LCMV-Arm. Runx3−/− P14 CD8+ TEFF cells showed substantial lower clonal expansion than wild-type P14 CD8+ TEFF cells from 5 to 8 dpi in the blood and spleen of infected recipient mice (Fig. 1c). Functionally, Runx3−/− P14 CD8+ TEFF cells produced less IFN-γ, TNF and IL-2, and showed reduced expression of granzyme B compared with wild-type P14 CD8+ TEFF cells (Fig. 1d). Runx3−/− and wild-type P14 CD8+ TEFF cells showed similar capacity to upregulate T-bet and Eomes (Fig. 1e), in contrast to a previous study reporting impaired induction of Eomes in Runx3-deficient CD8+ T cells activated in vitro21. These data indicate an intrinsic requirement for Runx3 in the clonal expansion and the acquisition of cytotoxic functions in CD8+ TEFF cells.
Runx3 activates the cytotoxic program in CD8+ TEFF cells
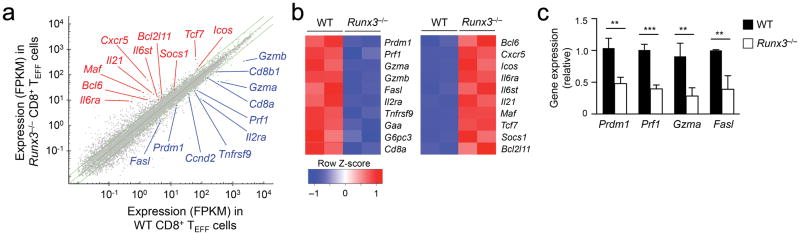
We detected more active caspase3 and caspase7 in Runx3−/− P14 CD8+ TEFF cells than wild-type cells on 6 and 8 dpi (Supplementary Fig. 3a), indicating Runx3-deficient CD8+ TEFF cells are more prone to apoptosis, whereas this effect was less pronounced on 4 dpi. To assess the early impact of Runx3 deficiency on CD8+ TEFF cells while avoiding including a high portion of apoptotic cells, we sort-purified CD45.2+ Runx3−/− P14 or wild-type P14 CD8+ TEFF cells on 4 dpi from CD45.1+ recipient spleens and performed RNA-Seq. Using the Cuffdiff algorithm at a setting of ≥ 2-fold expression changes and false discovery rate ≤ 0.01, we found 422 genes upregulated and 231 genes downregulated in Runx3−/− P14 CD8+ TEFF cells compared with wild-type P14 CD8+ TEFF cells (Fig. 2a,b), some of which (13.2% for down- and 11.4% for up-regulated genes) were previously identified in Runx3-deficient CD8+ T cells activated in vitro22(Supplementary Fig. 3b,c).
Figure 2. Runx3 activates the cytotoxic program in CD8+ TEFF cells.
(a) RNA-Seq analysis of Runx3−/− or WT P14 CD8+ TEFF cells sort-purified from the spleen on day 4 after adoptive transfer and LCMV-Arm infection. Shown are scatterplot of the average fragments per kilobase of transcripts per million mapped reads (FPKM) values of two replicates of WT vs. Runx3−/−P14 TEFF cells are shown, with green lines denoting gene expression changes by a factor of 2. (b) Heatmap showing select downregulated (left) or upregulated (right) genes in Runx3−/− P14 TEFF cells. (c) qRT-PCR analysis of Prdm1, Prf1, Fasl and Gzma expression (relative to the Hprt housekeeping gene) in WT or Runx3−/− P14 TEFF cells sorted from the recipient spleens on day 4 after adoptive transfer and LCMV-Arm infection. Data are means ± s.d. (n = 4) from 2 independent experiments. **, p<0.01; ***, p<0.001 (Student’s t-test).
Among the downregulated genes, Runx3−/− P14 CD8+ TEFF cells showed reduced expression in Prdm1, which encodes the transcription factor Blimp-1, a critical regulator of CD8+ TEFF cell differentiation24,25 (Fig. 2a,b), as well as genes encoding cytotoxic effector molecules, such as Gzma, Gzmb, Prf1 and Fasl, as validated by quantitative RT-PCR (Fig. 2c) or flow cytometry (Fig. 1d). Among the upregulated genes, Runx3−/− P14 CD8+ TEFF cells showed increased expression in Bcl2l11, which encodes the pro-apoptotic Bim protein, and many genes associated with the TFH lineage cells, including Bcl6, Maf and Tcf7 (encoding Bcl-6, Maf and Tcf1 transcription factors, respectively), Icos, Il6ra and Il6st (encoding ICOS, IL-6Rα and gp130 signaling receptors, respectively), Cxcr5 and Il2113 (Fig. 2a,b). These data indicate that Runx3 controls a broad transcriptional program including activation of the cytotoxic machinery in CD8+ TEFF cells.
Runx3 suppresses the TFH program in CD8+ TEFF cells
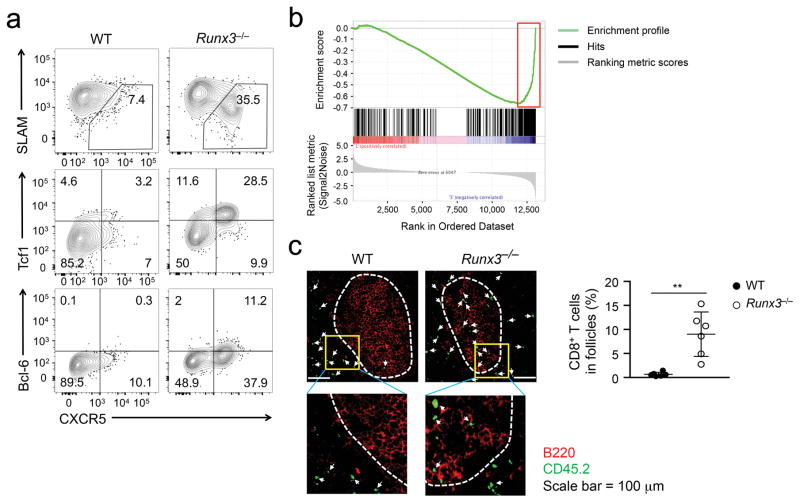
We next investigated the functional relevance of the induced TFH lineage-associated genes in Runx3−/− CD8+ TEFF cells during acute viral infection. During 4–8 days after LCMV-Arm infection following adoptive transfer of P14 CD8+ T cells, >35% Runx3−/− P14 CD8+ TEFF cells in the spleens were CXCR5+, and these Runx3−/− P14 CXCR5+CD8+ T cells were SLAMlo Tcf1hi Bcl-6+, whereas only very few wild-type P14 CD8+ TEFF cells were CXCR5+ (Fig. 3a). Runx3−/− P14 CD8+ TEFF cells generated in response to infection by LM-GP33 also contained a CXCR5+ subset (Supplementary Fig. 4a). A TFH-enriched gene set, which contains genes that are expressed ≥ 2 fold higher in wild-type CD4+ TFH cells than TH1 cells elicited by LCMV-Arm infection was previously defined23. GSEA revealed a broad upregulation of TFH-enriched genes in Runx3−/− CD8+ TEFF cells compared with wild-type cells (Fig. 3b, Supplementary Fig. 4b).
Figure 3. Runx3 represses TFH-associated genes in CD8+ TEFF cells.
(a) Expression of CXCR5, SLAM, Tcf1 and Bcl-6 detected by surface or intracellular staining in WT or Runx3−/− P14 CD8+ TEFF cells in spleen on day 6 after adoptive transfer and LCMV-Arm infection. Data are representative from 3 independent experiments. (b) GSEA enrichment plot showing the enrichment of TFH-associated gene set in Runx3−/− P14 TEFF cells, as highlighted in a red rectangle. (c) Immunofluorescence staining of distribution of CD45.2+ WT or Runx3−/− P14 TEFF cells (green) in B cell (red) follicles in spleen sections on day 8 after adoptive transfer and LCMV-Arm infection. Lower panels are 9-fold enlargement of highlighted areas outlined in the main images, and white dotted lines denote the boundary of B cell follicles and T cell zone. The frequency of CD45.2+CD8+ T cells in B cell follicles among all CD45.2+ cells in the scanned images is shown in dot plots, with each dot representing one recipient mouse (pooled from 2 independent experiments). **, p<0.01 (Student’s t-test).
CXCR5 expression guides CD4+ TFH cells into the B cell follicles. Among CD45.2+ CD8+ TEFF cells elicited by LCMV-Arm infection following adoptive transfer, <1% wild-type P14 CD8+ TEFF cells were detected in the B cell follicles of recipient spleens. In contrast, approximately 10% Runx3−/− P14 CD8+ TEFF cells localized in the B cell follicles, in spite of their greatly reduced numbers (Fig. 3c). P14 CD8+ T cells activated by LCMV clone 13 (LCMV-Cl13), which causes chronic infection in mice, became exhausted. Consistent with recent studies26–28, about 20% of wild-type exhausted P14 CD8+ T cells were CXCR5+ and detected in the B cell follicles; by comparison, Runx3−/− exhausted P14 CD8+ T cells showed 2–3 fold increase in the CXCR5+ subset and follicle localization (Supplementary Fig. 4c,d). These data indicate that Runx3 suppresses the induction of a TFH cell transcriptional program in CD8+ T cells in response to diverse activation signals.
Runx3−/− CXCR5+CD8+ T cells acquire B cell help function
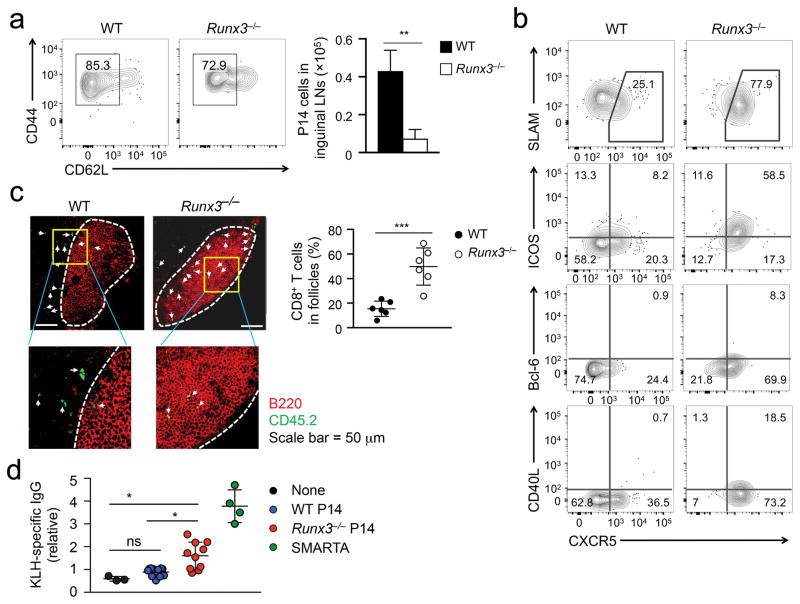
To test whether Runx3−/− CXCR5+CD8+ T cells have TFH-like B cell helper functions, we adoptively transferred P14 CD8+ T cells into wild-type congenic mice and immunized recipient mice with GP33 peptide conjugated with keyhole limpet hemocyanin (KLH-GP33) in the rear footpads. On day 7 post-immunization, both Runx3−/− and wild-type P14 CD8+ T cells were activated (Fig. 4a), and the resulting Runx3−/− P14 CD8+ TEFF cells were diminished in numbers compared with wild-type cells in the draining inguinal lymph node (LN) (Fig. 4b). Whereas about 25% wild-type P14 CD8+ TEFF cells in the inguinal LNs elicited by protein immunization were CXCR5+, >70% Runx3−/− P14 CD8+ TEFF cells exhibited a CXCR5+SLAMlo phenotype (Fig. 4b). Compared with wild-type P14 CXCR5+CD8+ TEFF cells, Runx3−/− P14 CXCR5+CD8+ TEFF cells had higher expression of ICOS and Bcl-6, as well as CD40L, which directly mediates T-B cell interaction (Fig. 4b). In addition, Runx3−/− P14 CD8+ TEFF cells were about 3-fold more frequently detected in the B cell follicles in inguinal LNs than wild-type P14 CD8+ TEFF cells (Fig. 4c).
Figure 4. Runx3-deficient CD8+ TEFF cells provide B cell help.
(a) Activation markers and numbers of WT or Runx3−/− P14 CD8+ TEFF cells determined in inguinal LNs on day 7 post-immunization after naïve WT or Runx3−/− P14 CD8+ T cells were adoptively transferred into congenic recipients followed by KLH-GP33 immunization. (b) Expression of CXCR5, SLAM, ICOS, CD40L and Bcl-6 determined by surface or intracellular staining in WT or Runx3−/− P14 TEFF cells on day 7 post-immunization after adoptive transfer and KLH-GP33 immunization. Data are from 2 independent experiments (n = 4–5). (c) Immunofluorescence staining of distribution of CD45.2+ WT or Runx3−/− P14 TEFF cells (green) in B cell (red) follicles in inguinal LN sections on day 21 after adoptive transfer and KLH-GP33 immunization. Lower panels are 9-fold enlargement of highlighted areas outlined in the main images, and white dotted lines denote the boundary of B cell follicles and T cell zone. The frequency of CD45.2+ cells in B cell follicles among all CD45.2+CD8+ cells in the scanned images is shown in dot plots, with each dot representing one recipient mouse (pooled from 2 independent experiments). ***, p<0.001 (Student’s t-test). (d) Serum KLH-specific IgG measured with ELISA on day 7 post-immunization after CD45.1+Bcl6−/− recipient mice were not transferred, or adoptively transferred with 1 × 105 sort-purified naïve CD45.2+ WT or Runx3−/− P14 CD8+ T cells and then immunized with KLH-GP33. KLH-specific IgG was also determined in another cohort of CD45.1+Bcl6−/− mice that were transferred with 1 × 105 naïve WT SMARTA CD4+ T cells and then immunized with KLH-GP61. The relative titers at 1:50 dilution, after normalizing to the average of WT P14 T cell recipient replicates, were pooled from 2–3 independent experiments. Statistical significance for the multi-group comparisons was performed using both unpaired t-test and post hoc tests, yielding similar outcomes. ns, not statistically significant; *, p<0.05 (Bonferroni’s test).
To specifically address if Runx3−/− CXCR5+CD8+ TEFF cells can provide B cell help, CD45.2+ wild-type or Runx3−/−P14 CD8+ T cells were sort-purified to eliminate contaminating CD4+ T cells and adoptively transferred into CD45.1+CD4-Cre+Bcl6fl/fl (hereafter Bcl6−/−) mice, in which the endogenous TFH response is abrogated but B cells are functional, followed by immunization with KLH-GP33. On day 7 post-immunization, serum KLH-specific IgG titers were not significantly different in Bcl6−/− mice that were not transferred or transferred with wild-type P14 CD8+ T cells (Fig. 4d). On the other hand, Bcl6−/− mice that received Runx3−/− P14 CD8+ T cells showed elevated titers of KLH-specific IgG on day 7 and 21 post-immunization (Fig. 4d, Supplementary Fig. 4e), suggesting Runx3−/− CXCR5+CD8+ effectors can function as B cell helpers. Of note, Bcl6−/− mice transferred with Runx3−/− P14 cells did not develop germinal centers (data not shown), indicating that KLH-specific IgG production in these mice was not dependent of germinal centers29–31. As a control, SMARTA CD4+ T cells, which express an MHC II-restricted TCR specific for LCMV GP61 epitope, adoptively transferred into Bcl6−/− recipients and immunized with GP61-conjugated KLH, induced higher titers of KLH-specific IgG in recipient mice than Bcl6−/− mice transferred with Runx3−/− P14 CD8+ T cells (Fig. 4d). To directly compare the expression of key TFH molecules in Runx3−/− CD8+ T cells and wild-type CD4+ TFH cells, we co-transferred Runx3−/− P14 CD8+ T cells and wild-type SMARTA CD4+ T cells into Bcl6−/− recipients, followed by immunization with both GP33-KLH and GP61-KLH. On day 7 post-immunization, wild-type CD4+ TFH cells showed similar ICOS and CD40L expression as Runx3−/− CXCR5+CD8+ TEFF cells, but expressed higher CXCR5, Bcl-6 and Tcf1 proteins than the latter (Supplementary Fig. 4f), possibly explaining the more efficient B cell help by wild-type CD4+ TFH cells. Together, these results indicate that loss of Runx3 expression confers TFH-like functions to CD8+ TEFF cells.
Runx3 deploys H3K27me3 to key TFH gene loci
To define the mechanisms by which the Runx3-CBFβ complex coordinates the repression of the TFH program and activation of the cytotoxic program in CD8+ TEFF cells in response to acute infection, we performed CBFβ ChIP-Seq using a CBFβ antiserum32 in wild-type KLRG1hiIL-7Rαlo P14 CD8+ TEFF cells sort-purified on day 8 after LCMV-Arm infection. Cbfb−/− naïve CD8+ T cells were used as a negative control. Using MACS algorithm with stringent settings (≥ 4 fold enrichment over the negative control, p<1×10–5 and FDR<0.05), we identified 12,981 high-confidence CBFβ binding peaks in CD8+ TEFF cells. Approximately 40% CBFβ peaks were detected in gene promoters, while 23% and 37% were distributed in the gene body or intergenic regions, respectively (Supplementary Fig. 5a). The CBFβ peaks partly overlapped with Runx3 binding peaks previously identified by Runx3 ChIP-Seq in CD8+ T cells activated in vitro22, and de novo motif discovery analysis identified a highly enriched Runx binding motif in the CBFβ peaks in both promoters and enhancer-overlapping regions (Supplementary Fig. 5b,c).
To define how the Runx3-CBFβ complex co-opts epigenetic mechanisms for target gene regulation, we performed ChIP-Seq of H3K4me1, H3K4me3, H3K27me3 and H3K27ac histone marks on wild-type and Runx3−/− P14 CD8+ TEFF cells sort-purified on day 4 after LCMV-Arm infection. We first mapped the direct association of CBFβ peaks with the Runx-CBF-repressed gene loci, i.e., genes upregulated in Runx3−/− over control CD8+ TEFF cells (Supplementary Table 1). The CBFβ peaks within the –5 kb to transcription end site (TES) regions were associated with strong H3K27me3 signals in wild-type P14 CD8+ TEFF cells, while the H3K27me3 marks at the same genomic locations were greatly diminished in Runx3−/− P14 CD8+ TEFF cells (Supplementary Fig. 5d). In contrast, CBFβ peaks outside these regions were not marked with strong H3K27me3 in wild-type P14 CD8+ TEFF cells, and the H3K27me3 marks were largely unaffected in Runx3−/− P14 CD8+ TEFF cells. On the other hand, H3K4me3 signals at the CBFβ peaks were similar between wild-type and Runx3−/− P14 CD8+ TEFF cells (Supplementary Fig. 5d).
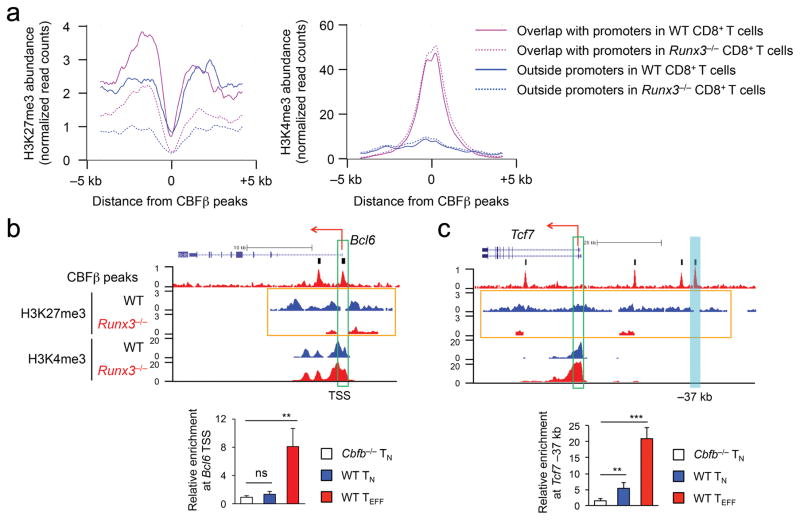
We further partitioned CBFβ peaks based on their association with the promoter regions, defined as –1 kb to +1 kb flanking the transcript start sites (TSS). Promoter-associated CBFβ peaks had strong signals of both H3K27me3 and H3K4me3 in wild-type CD8+ TEFF cells (Fig. 5a), indicating a bivalent state. In contrast, these CBFβ peaks showed substantial decrease in H3K27me3, while retaining H3K4me3 in Runx3−/− P14 CD8+ TEFF cells (Fig. 5a), suggesting that the poised, bivalent promoters may become actively transcribed without Runx3-mediated repression. Among the 422 Runx3-CBF-repressed genes, 219 promoters showed bivalency in wild-type P14 CD8+ TEFF cells. Forty-eight of these bivalent promoters were marked solely with H3K4me3 in Runx3−/− P14 CD8+ TEFF cells, including Bcl2l11 and key TFH genes such as Bcl6 and Tcf7 (Fig. 5b,c and Supplementary Fig. 5e). CBFβ did not bind to Bcl6 TSS but showed modest enriched binding at a –37 kb regulatory region upstream of Tcf7 in naïve CD8+ T cells; on the other hand, CBFβ bound strongly to both regions in wild-type P14 CD8+ TEFF cells (Fig. 5d,e). This observation suggests that Runx3-CBFβ can be pre-positioned at critical regulatory regions before antigen encounter and then further stabilize binding to these regions or acquire access to new regulatory elements during CD8+ TEFF cell differentiation. Our data indicate that Runx3-CBFβ deploys H3K27me3 mark to repress its target genes, either through promoters or distal regulatory regions.
Figure 5. The Runx-CBF complex deploys H3K27me3 to repress TFH genes during CD8+ TEFF cell differentiation.
(a) The profiles of H3K27me3 (left) and H3K4me3 (right) at CBFβ peaks overlapping with promoters of Runx-CBF-repressed genes (purple lines) or regions outside promoters but within – 5 kb to TES (blue lines) in WT (solid lines) or Runx3−/− (dotted lines) P14 CD8+ TEFF cells. CBFβ ChIP-Seq was performed on KLRG1hiIL-7Rαlo P14 CD8+ TEFF cells sort-purified from recipient spleens on day 8 after adoptive transfer of WT P14 CD8+ T cells followed by LCMV-Arm infection, and H3K27me3 and H3K4me3 ChIP-Seq was performed on all CD45.2+ WT or Runx3−/− P14 TEFF cells sort-purified from recipient spleens on day 4 after adoptive transfer and LCMV-Arm infection. (b–c) ChIP-Seq tracks of CBFβ binding peaks and histone modifications at the Bcl6 (b) and Tcf7 (c) gene loci (top panels), with vertical bars on top of CBFβ ChIP-Seq tracks (raw data) denoting the MACS-called high-confidence CBFβ peaks. Green rectangles mark the gene TSS, and orange rectangles highlight the widespread changes in H3K27me3 signals (island-filtered) between WT and Runx3−/− P14 TEFF cells. Shown in the bottom panels are relative enrichment of CBFβ binding to the Bcl6 TSS (b) and a Tcf7 –37 kb regulatory region (cyan filled rectangle, c) determined by ChIP-qPCR in Cbfb−/− or WT naïve CD8+ T (TN) cells, or WT P14 TEFF cells sort-purified from recipient spleens on day 5 after adoptive transfer and LCMV-Arm infection. Data are means ± s.d. (n = 4 to 6) from 3 independent experiments. ns, not statistically significant; **, p<0.01; ***, p<0.001 (Student’s t-test).
Runx3 activates promoters and enhancers of the cytotoxic genes
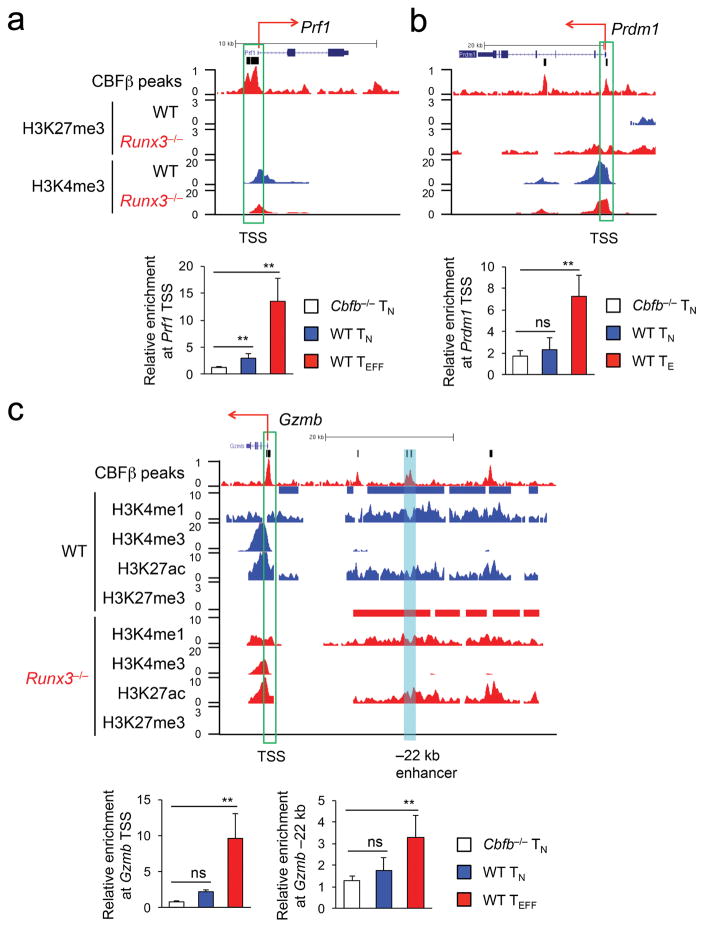
Runx3 binds to key cytotoxic genes such as Prf1 and Gzmb21,22. To validate and expand on the regulatory roles of the Runx3-CBFβ complex in activating the cytotoxic program, we focused on the 231 Runx-CBF-activated gene loci, i.e., genes downregulated in Runx3−/− over wild-type P14 CD8+ TEFF cells. The CBFβ ChIP-Seq peaks were found in 39 gene promoters in wild-type P14 CD8+ TEFF cells, including Prf1, Gzmb and Prdm1 (Fig. 6 and Supplementary Table 1). Using the H3K4me1hiH3K4me3neg/loH3K27achi H3K27me3neg/lo histone modification signature33, we mapped active enhancers in wild-type and Runx3−/− P14 CD8+ TEFF cells. Among the 321 CBFβ peaks found within 50 kb of the Runx-CBF-activated gene loci (but outside promoters) in wild-type P14 CD8+ TEFF cells, 72 were associated with 57 active enhancers, which were distributed in 35 genes including Gzmb, Ifng, Fasl and Gzma (Fig. 6c, Supplementary Fig. 6a–c and Supplementary Table 1). Clustering analysis showed that 43 active enhancers in wild-type P14 CD8+ TEFF cells, including those in Gzmb and Ifng, remained active in Runx3−/− P14 CD8+ TEFF cells (Supplementary Fig. 6d), while 14 active enhancers, including those in Fasl and Gzma, lost H3K27ac and/or gained H3K27me3 in Runx3−/− P14 CD8+ TEFF cells (Supplementary Fig. 6d), indicating they became inactive. These analyses suggest that Runx3 not only acts through pre-formed enhancers, but also contributes to enhancer activation in select target genes. By ChIP-qPCR Runx3-CBFβ complex was detected in the TSS of Prf1, Gzmb and Prdm1 genes, and a –22 kb enhancer upstream of Gzmb in wild-type P14 CD8+ TEFF cells (Fig. 6a–c). In naïve CD8+ T cells, however, CBFβ did not bind to the Gzmb TSS or –22 kb enhancer or Prdm1 TSS, and only showed modest enrichment at the Prf1 TSS (Fig. 6a–c), indicating that the Runx3-CBFβ complex can be pre-positioned at the promoters of Runx3-CBF-activated genes in naïve CD8+ T cells and then further stabilize binding, or can acquire access to new promoters or active enhancers during CD8+ TEFF cell differentiation.
Figure 6. The Runx-CBF complex acts on promoters and enhancers to activate the cytotoxic program during CD8+ TEFF cell differentiation.
ChIP-Seq tracks of CBFβ binding peaks and histone modifications at the Prf1 (a), Prdm1 (b) and Gzmb (c) gene loci (top panels), with vertical bars on top of CBFβ ChIP-Seq tracks (raw data) denoting the MACS-called high-confidence CBFβ peaks and green rectangles marking the gene TSS. In (c), a cyan filled rectangle highlights active enhancer upstream of Gzmb, and blue and red bars above the H3K4me1 ChIP-Seq tracks (island-filtered) denote active enhancers in WT or Runx3−/− P14 CD8+ TEFF cells, respectively. Shown in the bottom panels are relative enrichment of CBFβ binding to the Prf1 TSS (a), Prdm1 TSS (b), and Gzmb TSS and a –22 kb upstream enhancer (c) determined by ChIP-qPCR in Cbfb−/− or WT naïve CD8+ T (TN) cells, or WT P14 TEFF cells sort-purified from recipient spleens on day 5 after adoptive transfer and LCMV-Arm infection. Data are means ± s.d. (n = 4 to 6) from 3 independent experiments. ns, not statistically significant; **, p<0.01 (Student’s t-test).
Runx3 suppresses Tcf1 to repress the TFH program in TEFF cells
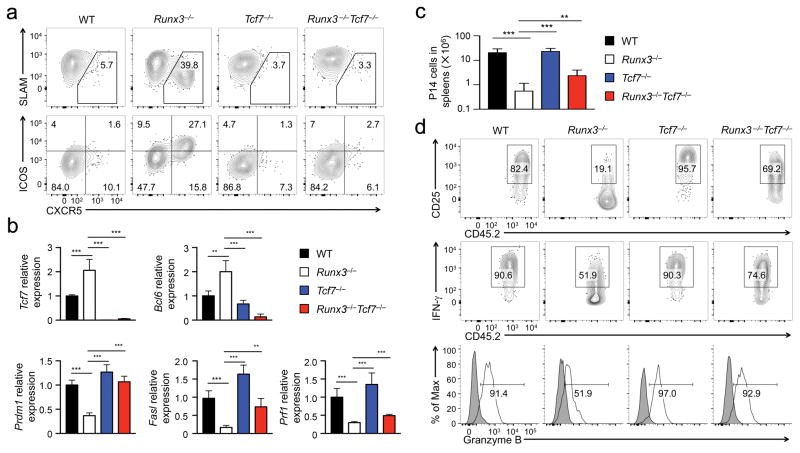
To further define the mechanism by which Runx3 represses the TFH program in CD8+ TEFF cells, we focused in Tcf1, which is critical for inducing Bcl-6 and repressing Blimp-1 in CD4+ TFH cells23,34,35. We adoptively transferred P14 CD8+ T cells from Runx3−/−, hCD2-Cre+Rosa26GFPTcf7fl/fl (hereafter Tcf7−/−), hCD2-Cre+Rosa26GFPRunx3fl/flTcf7fl/fl (Runx3−/−Tcf7−/−) and control (with genotype of Runx3+/+Tcf7+/+, Runx3+/−Tcf7+/+, Runx3+/+Tcf7+/−, or Runx3+/−Tcf7+/−, referred to as wild-type because CD8+ T cells in these genotypes behaved similarly in all assays) P14 TCR transgenic mice into congenic recipient mice, followed by infection with LCMV-Arm. On 6 dpi, unlike control or Tcf7−/− P14 CD8+ TEFF cells, only Runx3−/− P14 CD8+ TEFF cells contained a distinct CXCR5+SLAMloICOShi subset, which was completely abrogated in Runx3−/−Tcf7−/− P14 CD8+ TEFF cells (Fig. 7a). In addition, the increased Bcl6 and Il21 transcripts in Runx3−/− P14 CD8+ TEFF cells were abolished in Runx3−/−Tcf7−/− cells (Fig. 7b), suggesting that Tcf1 is responsible for inducing the TFH program in CD8+ TEFF cells. Furthermore, the induction of CXCR5+SLAMloICOShi CD8+ TEFF cells was abrogated when Runx3−/−Tcf7−/− P14 CD8+ T cells were activated by LM-GP33, LCMV Cl13 and GP33-KLH immunization (Supplementary Fig. 4a,c and Supplementary Fig. 7a), indicating that Runx3-mediated repression of Tcf1 is a conserved molecular circuit that prevents activation of the TFH program in CD8+ TEFF cells activated by diverse stimuli.
Figure 7. Runx3 represses Tcf1 expression to prevent activation of the TFH program and to assist activation of the cytotoxic program in CD8+ TEFF cells.
(a) Expression of CXCR5, SLAM and ICOS detected by surface staining on WT, Runx3−/−, Tcf7−/−, or Runx3−/−Tcf7−/− P14 CD8+ TEFF cells in recipient spleens on day 6 after adoptive transfer and LCMV-Arm infection. (b) Expression of Tcf7, Bcl6, Prdm1, Fasl and Prf1 genes determined by qRT-PCR in WT, Runx3−/−, Tcf7−/−, or Runx3−/−Tcf7−/− P14 TEFF cells sort-purified from recipient spleens on day 4 after adoptive transfer and LCMV-Arm infection. (c) Numbers of WT, Runx3−/−, Tcf7−/−, or Runx3−/−Tcf7−/− P14 TEFF cells determined in recipient spleens on day 6 after LCMV-Arm infection. (d) Expression of CD25 and granzyme B, and GP33 peptide-stimulated IFN-γ production determined by surface or intracellular staining in WT, Runx3−/−, Tcf7−/−, or Runx3−/−Tcf7−/− P14 TEFF cells in recipient spleens on day 6 after LCMV-Arm infection. Data are from 2 experiments (n = 4–5), and in b and d, data are means ± s.d., **, p<0.01; ***, p<0.001 (one-way ANOVA followed by Bonferroni’s test).
Compared with Runx3−/− P14 CD8+ TEFF cells, Runx3−/−Tcf7−/− P14 CD8+ TEFF cells showed about 4-fold increase in clonal expansion on day 6 after LCMV-Arm infection, albeit still lower than wild-type P14 CD8+ TEFF cells (Fig. 7c). In addition, the decreased CD25 expression, IFN-γ and granzyme B production observed in Runx3−/− P14 CD8+ TEFF cells was restored in Runx3−/−Tcf7−/− cells to a level close to wild-type P14 CD8+ TEFF cells (Fig. 7d). Further, expression of Prdm1, Fasl and Prf1 was higher in Tcf7−/−Runx3−/− P14 CD8+ TEFF cells than Runx3−/− cells (Fig. 7b), indicating that repression of Tcf1 by Runx3 is intrinsically necessary for Runx3 to fully activate the cytotoxic program and promote clonal expansion of CD8+ TEFF cells. Collectively, our data suggest that Runx3 constitutes a key module of the transcriptional network that allows activated CD8+ T cells to become cytotoxic effector cells and prevents deviation to helper lineages in response to acute infections (Supplementary Fig. 7b).
Discussion
Using in vivo infection models, here we show that Runx3 has a dual function, i.e., activation of the cytotoxic program and repression of the TFH program, during CD8+ T cell responses. Differentiation of CD8+ TEFF cells is orchestrated by multiple transcriptional and epigenetic regulators14. In our recent study mapping transcription factor motifs in active enhancers during CD8+ T cell responses, Runx motif is among the most enriched during the transition of naïve to CD8+ TEFF cells36. Unlike T-bet, Eomes or Blimp-1, which are strongly upregulated during CD8+ TEFF cell differentiation, Runx3 is only minimally induced in CD8+ TEFF cells compared with naïve CD8+ T cells36,37. As such, Runx3 appears as a ‘housekeeping’ factor that acquires regulatory roles in CD8+ TEFF cells by gaining access to new regulatory elements (compared with naïve CD8+ T cells) and/or cooperate with newly induced factors to achieve stabilized binding to key targets in the cytotoxic program. While being critical for optimal upregulation of Blimp-1 in CD8+ TEFF cells, Runx3-CBFβ does not appear to be required for induction of T-bet or Eomes. Whether Runx3 and the T-bet-Eomes pathways act in parallel and/or cooperatively to program CD8+ TEFF cell differentiation merits further investigation.
Maintaining the identity of activated CD8+ T cells is critical for their dedicated cytotoxic functions4, as indicated by the combined deficiency of T-bet together with Eomes or Blimp-1, a situation in which CD8+ TEFF cells aberrantly produce IL-17 and cause wasting inflammatory diseases15,16. Our observations indicate that Runx3 is required for preventing activation of the TFH program in CD8+ TEFF cells in response to acute infections. By deploying H3K27me3 repressive mark to TFH cell-associated gene loci, Runx3 thus provides constant supervision of CD8+ TEFF cell identity. Because epigenetic spreading of repressive histone marks may differ in individual cells within a population, only a portion of the Runx3-deficient CD8+ TEFF cells showed strong upregulation of CXCR5 and Bcl-6. Of note, key gene loci in the TFH lineage, such as Tcf7 and Bcl6 were in a bivalent state, decorated with both active and repressive histone marks in wild-type CD8+ TEFF cells. This observation suggests that these genes are in a poised status for potential activation, and hence some T cell lineage plasticity is embedded in CD8+ TEFF cells. In fact, CD8+ T cells have the capacity to produce IL-4 or IL-9 under in vitro polarization conditions, at specific anatomical locations or under specific allergic or inflammatory conditions in vivo38. Thus, beyond activation of the cytotoxic program, Runx3 might cooperate with other key factors, such as T-bet, Eomes and Blimp-1, to prevent the activation of alternative helper programs in the context of acute infections.
Our results indicate that loss of Tcf1 repression mediates activation of the TFH program in Runx3-deficient CD8+ TEFF cells, and this is consistent with the known role of Tcf1 in promoting CD4+ TFH cell differentiation23,34,35. On the other hand, deletion of Tcf1 in Runx3−/− CD8+ TEFF cells also improved activation of the cytotoxic program, in particular IFN-γ production and granzyme B expression, and enhanced expression of CD25 and Blimp-1, which may in turn help clonal expansion in response to acute infections. In fact, forced expression of CD25 in Runx3−/− CD8+ TEFF cells did improve clonal expansion, albeit showing little effect on increasing IFN-γ and granzyme B production (Q.S. and H.-H. X., unpublished data). These observations indicate that activation of the cytotoxic program and repression of the TFH program are not independent events, but are at least partly interconnected and coordinated by Runx3 and Tcf1. In naïve CD8+ T cells, loss of Tcf1 alone causes upregulation of perforin, granzyme B and Blimp-1 without overt activation signals8. Thus, Tcf1 may function as a ‘brake’ to restrain induction of the cytotoxic program in CD8+ T cells, and this may explain why Tcf1 is strongly downregulated in fully differentiated CD8+ effector T cells39. Thus, Runx3-mediated Tcf1 repression is critical for CD8+ TEFF cells to fully acquire cytotoxicity and prevent deviation to the TFH lineage.
During chronic infection, antigen-specific CD8+ T cells initially acquire effector functions but gradually become less functional due to antigen persistence in the hosts40. A portion of the exhausted CD8+ T cells have been found to gain TFH cell-like features, including expression of CXCR5, Bcl-6 and Tcf1, with CXCR5+CD8+ T cells maintaining the pool of exhausted CD8+ T cells and contributing to viral curtailment more than their CXCR5–CD8+ counterparts26–28. Therefore, depending on the context of CD8+ T cell activation, e.g., duration of antigen exposure and cytokine milieu, activation of the TFH program in CD8+ T cells may have beneficial effect. When Runx3−/− CD8+ T cells were activated in response to protein immunization, the CXCR5+CD8+ TEFF cells migrated into the B cell follicles and helped antibody production, and this B cell help function was not accompanied by germinal center formation, consistent with a current view of extrafollicular help to B cells29–31. Considering that the expression levels of key TFH factors such as Bcl6 remained lower in Runx3−/− CD8+ TEFF cells than wild-type CD4+ TFH T cells, it is thus not unexpected that the B cell help from the Runx3−/− CXCR5+CD8+ TEFF cells was not as potent as CD4+ TFH cells. Of note, exhausted CXCR5+ or Tcf1hi CD8+ T cells induced by chronic infection express about 60–70% of Runx3 transcripts compared with CXCR5– or Tcf1lo CD8+ T cells27,41. Runx3 may thus represent a novel target, and the resulting TFH plasticity in CD8+ effector T cells may benefit the control of chronic viral infections and/or tumor immunotherapy. Collectively, our study showed that CD8+ T cell identity is under constant supervision both during development and after activation. The underlying regulatory circuits should provide useful tools to prevent unnecessary identity diversion and at the same time confer the plasticity desired.
METHODS
Mice
C57BL/6J (B6), B6.SJL, Runx3fl/fl, Cbfbfl/fl, Bcl6fl/fl, and Rosa26GFP mice were from the Jackson Laboratory. Tcf7fl/fl mice were previously described and hCD2-Cre mice were provided by Paul E. Love (NICHD, NIH)7,23. All compound mouse strains used in this work were from in-house breeding at the University of Iowa animal care facility. All mice analyzed were 6–12 weeks of age, and both genders are used without randomization or blinding. All mouse experiments were performed under protocols approved by the Institutional Animal Use and Care Committees of the University of Iowa.
Peptide stimulation, active caspase detection, and flow cytometry
Single-cell suspensions were prepared from the spleen, LNs, or peripheral blood, and surface or intracellularly stained as described39,42. For analysis at 48–60 hrs and day 4 post-infection, the spleen was first treated with 100 U/ml Collagenase II (Life Technologies) at 37oC for 30 min to maximize cell recovery. The fluorochrome-conjugated antibodies were as follows: anti-CD8 (53-6.7), anti-TCRβ (H57-597), anti-DX5 (DX5), anti-NK1.1 (PK136), anti-Thy1.2 (53-2.1), anti-CD4 (RM4-5), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD69 (H1.2F3), anti-CD40L (MR1), anti-CD45.2 (104), anti-ICOS (C398.4A), anti-CD25 (M1/69), anti-IFN-γ (XMG1.2), anti-TNFα (MP6-XT22), anti-Eomes (Dan11mag), anti-T-bet (eBio4B10), and anti-IL-2 (JES6-5H4) from eBiosciences; anti-Bcl6 (K112-91) and anti-Runx3 (R3-5G4) from BD Biosciences; anti-human granzyme B (FGB12) and corresponding isotype control from Invitrogen/Life Technologies, anti-Tcf1 (C63D9) from Cell Signaling Technology; anti-SLAM (TC15-12F12.2) from BioLegend. For detection of CXCR5, three-step staining protocol was used with unconjugated anti-CXCR5 (2G8; BD Biosciences)23. For detection of Bcl6 or Tcf1, surface-stained cells were fixed and permeabilized with the Foxp3/Transcription Factor Staining Buffer Set (eBiosciences), followed by incubation with corresponding fluorochrome-conjugated antibodies. GP33- and OVA-specific MHC-I tetramers were generated in-house at the Badovinac lab. Peptide-stimulated cytokine production and detection by intracellular staining were as described39. Active Caspsase-3/7 was detected using the Vybrant FAM caspase-3/7 assay kit (Invitrogen/Life Technologies) as described39. Data were collected on an LSRII with Violet and a FACSVerse (BD Biosciences) and were analyzed with FlowJo software (TreeStar).
Adoptive transfer and viral or bacterial infection
Naïve P14 CD8+ T cells were isolated from the LNs from WT, Runx3−/−, Tcf7−/−, Runx3−/−Tcf7−/− P14 TCR transgenic mice. For characterization of CD8+ effector T cell responses on 4, 6, or 8 dpi, 2 × 104 Vα2+ P14 CD8+ T cells were intravenously (i.v.) injected into CD45.1+ B6.SJL recipient mice and infected intraperitoneally (i.p) with 2 × 105 PFU of LCMV-Arm, or 2 × 106 PFU of LCMV-Cl13. To obtain enough cells for RNA-Seq, ChIP-Seq for histone marks, 2 × 105 cells were transferred and P14 CD8+ TEFF cells were sort-purified on 4 dpi. For characterization of early CD8+ T cell activation and division within 60 hrs post-infection, P14 CD8+ T cells were labeled with 10 μM Cell Trace Violet (Invitrogen/Life Sciences), and 1×106 of labeled cells were transferred followed by i.v. infection with 2 × 106 PFU of LCMV-Arm. In some experiments, Runx3−/− or WT mice were directly infected with 2 × 105 PFU of LCMV-Armstrong (i.p.) or 5 × 106 CFU of Listeria monocytogenes expressing both GP33 and Ovalbumin (OVA) 257 epitopes (LM-GP33-OVA, i.v.). Viral titers in the spleen, lung and liver were quantified with standard plaque assay on VERO cells as previously described43.
Immunization and Enzyme-Linked Immunosorbent Assay (ELISA)
The LCMV GP33-41 peptide (KAVYNFATC) was synthesized and conjugated with KLH to the cysteine by the GenScript. The GP33-KLH conjugates (50 μg/mouse; 25 μg/rear footpad) were mixed with Addavax (Invivogen) at 1:1 volume ratio, then with polyinosine-polycytidylic acid (10 μg/mouse, Sigma-Aldrich) and used as the immunogen. Runx3−/− or WT P14 CD8+ T cells were sort-purified to eliminate contamination by CD4+ T cells and adoptively transferred into CD45.1+ B6.SJL or CD45.1+CD4-Cre+Bcl6fl/fl (Bcl6−/−) mice at 1 × 105 cells per recipient. Twenty-four hours later, the recipients were immunized with the immunogen by subcutaneous injection to the rear footpads. Seven days later, the inguinal LNs and spleens were harvested for characterization of activated CD8+ T cells. On days 7 and 21 post-immunization, sera were collected for ELISA, and inguinal LNs harvested for confocal microscopy analysis.
KLH-specific IgG in the sera was measured by ELISA as previously described44. In brief, Nunc MaxiSorp flat-bottom 96 well plate (eBiosciences) was coated with 1 μg/ml Imject mcKLH (Thermo Fisher Scientific) overnight, and then incubated with serial diluted serum samples. The KLH-specific IgG was detected by Horseradish peroxidase (HRP)-conjugated goat-anti-mouse IgG (H+L) secondary antibody (Thermo Fisher Scientific) coupled with TMB substrate (BD Biosciences). The absorbance at 450 nm was read on a Synergy H1M microplate reader (BioTek Instruments).
RNA-Seq and data analysis
Total RNA was extracted from Runx3−/− or WT CD45.2+GFP+ P14 CD8+ TEFF cells sorted on 4 dpi in the adoptive transfer and LCMV-Arm infection experiments, and two biological replicates were obtained for each genotype. RNA-Seq was performed as previously described23. The sequencing quality of RNA-seq libraries was assessed by FastQC v0.10.1 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). RNA-Seq libraries were mapped to mouse genome using Tophat (v2.1.0)45, and the mapped reads were then processed by Cuffdiff (v2.2.1)46 to estimate expression levels of all genes and identify differentially expressed genes. The expression level of a gene is expressed as a gene-level Fragments Per Kilobase of transcripts per Million mapped reads (FPKM) value. The reproducibility of RNA-Seq data was evaluated by computing Pearson’s correlation of FPKM values for all genes between biological replicates. The Pearson’s correlation coefficient between the two biological replicates was 0.955 for the WT samples, and 0.962 for the Runx3−/− samples, indicating strong reproducibility. Upregulated or downregulated genes in Runx3−/− CD8+ T cells were identified by requiring ≥ 2-fold expression changes and false discovery rate (FDR) < 0.05, as well as FPKM ≥ 1 in Runx3−/− CD8+ TEFF cells for upregulated genes, or FPKM ≥ 1 in control CD8+ TEFF cells for downregulated genes. UCSC genes from the iGenome mouse mm9 assembly (http://support.illumina.com/sequencing/sequencing_software/igenome.html) were used for gene annotation. The RNAseq data are deposited at the GEO (accession number GSE81888).
Gene set enrichment analysis (GSEA)
GSEA was performed with GSEA software from the Broad Institute47, and used to determine the enrichment of gene sets in Runx3−/− or WT CD8+ TEFF cells. TFH-associated gene set was generated in-house based on published results (GEO accession code GSE21380)23.
ChIP-Seq of histone marks and enhancer analysis
WT or Runx3−/− P14 CD8+ TEFF cells were sorted from CD45.1+ recipient mice on 4 dpi in adoptive transfer and LCMV-Arm infection experiments, and used for ChIP-Seq of H3K4me1, H3K4me3, H3K27ac, and H3K27me3 as described8. The ChIP-Seq data were processed with SICER (v1.1)48 to identify islands significantly enriched with each histone mark with the setting of FDR < 10–4. Active enhancers were defined as H3K4me1hi H3K4me3neg/lo H3K27aclo H3K27me3neg/lo regions that located 1 kb away from transcriptional start sites (TSS). Specifically, the H3K4me1 islands were first identified, and then any portion(s) that overlap with H3K4me3 islands were removed. The resulting H3K4me1hi H3K4me3neg/lo regions were then compared with gene promoters (defined as –1 kb to + 1 kb regions flanking the TSS), and further modified as follows: 1) if more than half of an H3K4me1hi H3K4me3neg/lo – region overlapped with a gene promoter, this region was removed in its entirety, and 2) if the overlap was less than half of the length of the H3K4me1hi H3K4me3neg/lo region, the overlapping portion was removed from the region. Further overlay with H3K27ac and H3K27me3 islands thus identified gene promoter-excluded H3K4me1hi H3K4me3neg/lo H3K27aclo H3K27me3neg/lo regions, which were considered as active enhancers. During the data processing outlined above, internal truncation and/or trimming of disqualified genomic regions may have generated short fragments that retained the required histone mark features, and any fragments < 500 bp were excluded from the active enhancer repertoire. The histone mark ChIP-Seq data are also under GEO accession number GSE81888.
ChIP-Seq of CBFβ in CD8+ TEFF cells and data processing
hCD2-Cre+Rosa26GFPCbfbFL/FL (Cbfb−/−) naïve CD8+ T cells were isolated by negative selection from uninfected mice. CD45.2+KLRG1hiIL-7Rαlo CD8+ TEFF cells were sorted from CD45.1+ recipient mice on 8 dpi in adoptive transfer and LCMV-Arm infection experiments. The cells were incubated with 2 mM disuccinimidyl glutarate (DSG, Sigma Aldrich) at room temperature for 45 min, and then cross-linked for 10 min with 1% formaldehyde in medium. The fixed cells were processed with a truChIP Chromatin Shearing Reagent Kit (Covaris) and sonicated for 5 min on Covaris S2 ultrasonicator. The sheared chromatin from 3–5 × 106 CD8+ T cells was immunoprecipitated with 5 μg of purified anti-CBFβ antibody32 and was washed as described23. DNA segments from ChIP were end-repaired and ligated to indexed Illumina adaptors followed by low-cycle PCR. The resulting libraries were sequenced with the Illumina HiSeq-2000 platform.
The sequencing quality of ChIP-Seq libraries was assessed by FastQC. Bowtie2 v2.2.649 was used to align the sequencing reads to the mm9 mouse genome. UCSC genes from the iGenome mouse mm9 assembly were used for gene annotation. MACS v1.4.250 was used for peak calling with CBFβ ChIP-Seq in Cbfb−/− CD8+ T cells as a negative control. We used a stringent setting, i.e., ≥ 4 fold enrichment, p-value < 10−5, and FDR < 5%, and identified 12,981 high-confidence CBFβ binding peaks in CD8+ TEFF cells.
To determine the overlap of CBFβ binding peaks with active enhancers, CBFβ binding peaks were compared with active enhancers defined above, and at least one base pair overlap was required to consider potential direct association of CBFβ with an active enhancer. To determine the overlap of CBFβ binding peaks with Runx3 binding peaks, the Runx3 ChIP-Seq data were retrieved from GSE5013122 and processed using the same protocol and stringent setting as above. At least one base pair overlap was required to consider a CBFβ peak and a Runx3 peak overlap with each other.
The profile of a histone mark flanking the CBFβ binding peaks in CD8+ TEFF cells was generated as follows. The CBFβ peaks were aligned by their summits, and the island-filtered reads of the histone mark were counted in a resolution of 100 bps and with smoothing window of 400 bps within ±5 kb region flanking the summits. The read counts in each window were then normalized by the number of CBFβ peaks and by the window size, and expressed as reads per kb. The profile was further normalized by the total number of island-filtered reads in the histone mark library as reads per kilobase per million reads (RPKM). The CBFβ ChIP-Seq data are also under GEO accession number GSE81888.
De novo motif analysis
Among the 12,981 high-confidence CBFβ peaks in CD8+ TEFF cells, 5,186 were at the promoter region, and 1,758 overlapped with the putative enhancers (defined as H3K4Me1hi H3K4Me3neg/lo regions that are located with 5 kbs of TSS). The top 3,000 most significant CBFβ peaks at the promoters as ordered by p-value and all CBFβ peaks overlapping with the putative enhancers were used for motif analysis. The sequences of ± 200 bps flanking the peak summits, as identified by MACS, were used in MEME-ChIP for de novo motif discovery51.
Immunohistochemistry
Fresh spleens or LNs were snap-frozen in Tissue-Tek optimum cutting temperature (O.C.T.) compound (Sakura Finetek). Cryosections of 10 μm were cut, fixed in 4% of paraformaldehyde for 10 min followed by another 10 min incubation in pre-chilled acetone at −20 °C, and then washed three times with PBS for immunostaining. Following incubation in blocking buffer (CAS-Block, Invitrogen), the samples were incubated with fluorescence-labeled antibodies overnight at 4 °C. The primary antibodies used are anti-mouse CD8-Alexa Fluor 594 (53-6.7), anti-mouse B220-BV510 or B220-Alexa Fluor 647 (RA3-6B2 for both fluorochromes), and anti-mouse CD45.2-Alexa Fluor 488 (clone 104, all from BioLegend). Confocal images of cryosections were acquired using a Zeiss LSM710 confocal fluorescence microscopy and were processed with Imaris software (Bitplane).
Chromatin immunoprecipitation (ChIP)
hCD2-Cre+Rosa26GFPCbfbFL/FL (Cbfb−/−) or WT naïve CD8+ T cells were isolated by negative selection from uninfected mice. P14 CD8+ TEFF cells were sorted from CD45.1+ recipient mice on 5 dpi in adoptive transfer and LCMV-Arm infection experiments. The purified cells were cross-linked for 10 min with 1% formaldehyde in medium, were processed with a truChIP Chromatin Shearing Reagent Kit (Covaris) and sonicated for 5 min on Covaris S2 ultrasonicator. The sheared chromatin from 3–5 × 106 CD8+ cells was immunoprecipitated with 5 μg of purified anti-CBF-β antibody32 or control rabbit IgG and was washed as described23. The immunoprecipitated DNA segments were used for quantification by PCR. For calculation of enriched binding by CBF-β, the signal at the genomic region of interest in each ChIP sample were first normalized to that at the Hprt promoter, and the enrichment by anti-CBF-β was then normalized to that by IgG in corresponding ChIP sample.
The primers used for ChIP-PCR are:
Prf1 TSS, 5′-agcactgcaccatgtcttca and 5′-atgcgctgtcaggaagagtt;
Gzmb TSS, 5′-taaccacagcagaacccaca and 5′-tccaaaacactgatgctcca;
Gzmb –22 kb enhancer, 5′-ccacctctagcagcacttcc and 5′-tgagcctctgtcatctgtgg;
Prdm1 TSS, 5′-ctgccgcagacttctttacc and 5′-tttgcaaacagaggaagctg;
Bcl6 TSS, 5′-ggcagcaacagcaataatca and 5′-ctgcggagcaatggtaaag;
Tcf7 –37 kb region, 5′-tttctgctccccactcaaac and 5′-ttcctgaggtgacccatttc;
Hprt TSS, 5′-tgagcgcaagttgaatctg and 5′-ggacgcagcaactgacatt.
Quantitative RT-PCR
P14 CD8+ TEFF cells were sorted from recipient mice in adoptive transfer and LCMV-Arm infection experiments. Total RNA was extracted, reverse-transcribed, and target gene transcripts were measured with quantitative PCR as described7. The primers used are as follows:
Prdm1, 5′-cctgccaaccaggaacttct and 5′-gttgctttccgtttgtgtgaga;
Prf1, 5′-gatgtgaaccctaggccaga and 5′-ggtttttgtaccaggcgaaa;
Fasl, 5′-gcagaaggaactggcagaac and 5′-ttaaatgggccacactcctc;
Tcf7, 5′-caatctgctcatgccctacc and 5′-cttgcttctggctgatgtcc;
Bcl6, 5′-cctgagggaaggcaatatca and 5′-cggctgttcaggaactcttc;
Actb, 5′-cggttccgatgccctgaggctctt and 5′-cgtcacacttcatgatggaattga.
Statistical analysis
For comparison between two experimental groups, Student’s t-test was used. For multiple group comparison, one way ANOVA was used to first determine whether any of the differences between the means are statistically significant, followed by 1) unpaired Student’s t-test to determine the statistical significance for a specific pair, 2) post hoc tests using Tukey’s test and Bonferroni’s test to more stringently determine the statistical significance of differences between all possible pairs52. In Fig. 4d, the SMARTA CD4+ T cell recipient group was included as a positive control for the experimental system and was not included in the multi-group comparisons so as not to artificially reduce the statistical power of comparison among CD8+ T cell recipient groups. For comparison of numbers wild-type, Runx3−/−, Tcf7−/−, Runx3−/−Tcf7−/− P14 CD8+ TEFF cells in response to various challenges, the values were log10 transformed for post hoc tests because the numbers of Runx3−/− CD8+ TEFF cells were 1–2 logs lower than those of wild-type cells. Both unpaired t-test and post hoc tests yielded consistent outcomes, although statistical power differed in some cases. In figures, the level of statistical significance was marked based on the most stringent Bonferroni’s test, and that by unpaired t-test and Tukey’s test was provided in the “Source data-ANOVA/post-host test” file.
Data Availability
High throughput sequencing data are deposited at the GEO with accession number GSE81888. Experimental protocols are described above or in cited references, and more details can be provided upon requests.
Supplementary Material
Acknowledgments
We thank J. Fishbaugh, H. Vignes and M. Shey (University of Iowa Flow Cytometry Core Facility) for cell sorting, J. Bair and E. Snir (Genomics Division, Iowa Institute of Human Genetics) for ChIP-Seq, J. Shao (University of Iowa Central Microscopy Research Facility) for assistance with immunofluorescence staining, I. Antoshechkin (California Institute of Technology) for RNA-Seq, S. Crotty (La Jolla Institute) for sharing the protein immunization protocol, W. Chen (Beijing Institute of Genomics) for help with RNA-Seq data analysis and Y. Groner (Weizmann Institute of Science, Israel) for sharing high-throughput data on Runx3. This study is supported by grants from the NIH (AI112579 and AI115149 to H.-H.X., AI119160 to H.-H.X. and V.P.B., AI042767 to J.T.H., AI114543 and GM113961 to V.P.B., AI121080 to H.-H.X. and W.P., AI113806 to W.P.) and the US Department of Veteran Affairs (I01 BX002903 to H.-H.X.). The flow cytometry core facility at the University of Iowa is supported by the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center, and also by grants from the NCI (P30CA086862) and the National Center for Research Resources of the NIH (S10 OD016199). M.D.M. is supported by a T32 Post-doctoral Training Grant (4T32AI007260-30). JAG is a recipient of the Ballard and Seashore Dissertation Fellowship. The authors declare no conflict of interests.
Footnotes
Author contribution
Q.S. performed most of the experiments with help of S.X., F.L., S.M.H., J.A.G., S.P.K., N.V.B.B., Y.S., and M.D.M; Z.Z. analyzed the high-throughput data under the supervision of W.P.; S.M.V., I.T., J.T.H., and V.P.B contributed critical reagents and provided scientific insights; H.-H.X. conceived the project and supervised the overall study.
Accession codes
Gene Expression Omnibus: RNA-Seq and ChIP-Seq data have been deposited under accession number GSE81888.
References
- 1.Natoli G. Maintaining cell identity through global control of genomic organization. Immunity. 2010;33:12–24. doi: 10.1016/j.immuni.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Steinke FC, Xue HH. From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunol Res. 2014;59:45–55. doi: 10.1007/s12026-014-8545-9. [DOI] [PubMed] [Google Scholar]
- 3.Taniuchi I, Ellmeier W. Transcriptional and epigenetic regulation of CD4/CD8 lineage choice. Adv Immunol. 2011;110:71–110. doi: 10.1016/B978-0-12-387663-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 4.Gullicksrud JA, Shan Q, Xue HH. Front Biol. 2017 doi: 10.1007/s11515-017-1445-3. [DOI] [Google Scholar]
- 5.He X, Park K, Kappes DJ. The role of ThPOK in control of CD4/CD8 lineage commitment. Annu Rev Immunol. 2010;28:295–320. doi: 10.1146/annurev.immunol.25.022106.141715. [DOI] [PubMed] [Google Scholar]
- 6.Vacchio MS, et al. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nature immunology. 2014;15:947–956. doi: 10.1038/ni.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinke FC, et al. TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4(+) T cell fate and interact with Runx3 to silence Cd4 in CD8(+) T cells. Nature immunology. 2014;15:646–656. doi: 10.1038/ni.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing S, et al. Tcf1 and Lef1 transcription factors establish CD8(+) T cell identity through intrinsic HDAC activity. Nature immunology. 2016;17:695–703. doi: 10.1038/ni.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 10.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nature immunology. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Intlekofer AM, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin A, et al. A molecular threshold for effector CD8(+) T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nature immunology. 2016;17:422–432. doi: 10.1038/ni.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins A, Littman DR, Taniuchi I. RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nat Rev Immunol. 2009;9:106–115. doi: 10.1038/nri2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djuretic IM, Cruz-Guilloty F, Rao A. Regulation of gene expression in peripheral T cells by Runx transcription factors. Adv Immunol. 2009;104:1–23. doi: 10.1016/S0065-2776(08)04001-7. [DOI] [PubMed] [Google Scholar]
- 19.Setoguchi R, et al. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- 20.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. The Journal of experimental medicine. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Guilloty F, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. The Journal of experimental medicine. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotem J, et al. Runx3-mediated transcriptional program in cytotoxic lymphocytes. PLoS One. 2013;8:e80467. doi: 10.1371/journal.pone.0080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YS, et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nature immunology. 2015;16:980–990. doi: 10.1038/ni.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallies A, et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nature immunology. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 25.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He R, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 27.Im SJ, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong YA, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nature immunology. 2016;17:1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- 29.Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends Immunol. 2012;33:590–597. doi: 10.1016/j.it.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarlinton D, Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science. 2013;341:1205–1211. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- 31.Miyauchi K, et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nature immunology. 2016;17:1447–1458. doi: 10.1038/ni.3563. [DOI] [PubMed] [Google Scholar]
- 32.Naoe Y, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. The Journal of experimental medicine. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heintzman ND, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu T, et al. TCF1 Is Required for the T Follicular Helper Cell Response to Viral Infection. Cell Rep. 2015;12:2099–2110. doi: 10.1016/j.celrep.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L, et al. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nature immunology. 2015;16:991–999. doi: 10.1038/ni.3229. [DOI] [PubMed] [Google Scholar]
- 36.He B, et al. CD8+ T Cells Utilize Highly Dynamic Enhancer Repertoires and Regulatory Circuitry in Response to Infections. Immunity. 2016;45:1341–1354. doi: 10.1016/j.immuni.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Best JA, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nature immunology. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittrucker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8(+) T cells. Arch Immunol Ther Exp (Warsz) 2014;62:449–458. doi: 10.1007/s00005-014-0293-y. [DOI] [PubMed] [Google Scholar]
- 39.Zhao DM, et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utzschneider DT, et al. T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Zhou X, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen H, et al. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 44.Jing X, Zhao DM, Waldschmidt TJ, Xue HH. GABPbeta2 is dispensible for normal lymphocyte development but moderately affects B cell responses. J Biol Chem. 2008;283:24326–24333. doi: 10.1074/jbc.M804487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zang C, et al. A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics. 2009;25:1952–1958. doi: 10.1093/bioinformatics/btp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langmead B. Aligning short sequencing reads with Bowtie. Curr Protoc Bioinformatics. 2010;Chapter 11(Unit 11):17. doi: 10.1002/0471250953.bi1107s32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. gb-2008-9-9-r137 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey TL, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamb TJ, Graham AL, Petrie A. T testing the immune system. Immunity. 2008;28:288–292. doi: 10.1016/j.immuni.2008.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
High throughput sequencing data are deposited at the GEO with accession number GSE81888. Experimental protocols are described above or in cited references, and more details can be provided upon requests.