Abstract
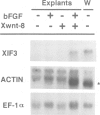
In Xenopus, growth factors of the TGF-beta, FGF and Wnt oncogene families have been proposed to play a role in generating embryonic pattern. In this paper we examine potential interactions between the bFGF and Xwnt-8 signaling pathways in the induction and dorsal-ventral patterning of mesoderm. Injection of Xwnt-8 mRNA into 2-cell Xenopus embryos does not induce mesoderm formation in animal cap ectoderm isolated from these embryos at the blastula stage, but alters the response of this tissue to mesoderm induction by bFGF. While animal cap explants isolated from non-injected embryos differentiate to form ventral types of mesoderm and muscle in response to bFGF, explants from Xwnt-8 injected embryos form dorsal mesodermal and neural tissues in response to the same concentration of bFGF, even if the ectoderm is isolated from the prospective ventral sides of embryos or from UV-ventralized animals. Our results support a model whereby dorso-ventral mesodermal patterning can be attained by a single mesoderm inducing agent, possibly bFGF, which is uniformly distributed across the prospective dorsal-ventral axis, and which acts in concert with a dorsally localized signal, possibly a Wnt protein, which either alters the response of ectoderm to induction or modifies the character of mesoderm after its induction.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Bradley R. S., Brown A. M. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990 May;9(5):1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B., Gimlich R. L. Lithium-induced teratogenesis in frog embryos prevented by a polyphosphoinositide cycle intermediate or a diacylglycerol analog. Dev Biol. 1989 Apr;132(2):315–324. doi: 10.1016/0012-1606(89)90228-5. [DOI] [PubMed] [Google Scholar]
- Cardellini P. Reversal of dorsoventral polarity in Xenopus laevis embryos by 180 degrees rotation of the animal micromeres at the eight-cell stage. Dev Biol. 1988 Aug;128(2):428–434. doi: 10.1016/0012-1606(88)90304-1. [DOI] [PubMed] [Google Scholar]
- Cho K. W., De Robertis E. M. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990 Nov;4(11):1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Christian J. L., McMahon J. A., McMahon A. P., Moon R. T. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991 Apr;111(4):1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987 Apr;99(4):527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987 Jun;100(2):279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Dale L., Smith J. C., Slack J. M. Mesoderm induction in Xenopus laevis: a quantitative study using a cell lineage label and tissue-specific antibodies. J Embryol Exp Morphol. 1985 Oct;89:289–312. [PubMed] [Google Scholar]
- De Robertis E. M., Morita E. A., Cho K. W. Gradient fields and homeobox genes. Development. 1991 Jul;112(3):669–678. doi: 10.1242/dev.112.3.669. [DOI] [PubMed] [Google Scholar]
- Dent J. A., Polson A. G., Klymkowsky M. W. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989 Jan;105(1):61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Danilchik M., Doniach T., Roberts S., Rowning B., Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107 (Suppl):37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- Gillespie L. L., Paterno G. D., Slack J. M. Analysis of competence: receptors for fibroblast growth factor in early Xenopus embryos. Development. 1989 May;106(1):203–208. doi: 10.1242/dev.106.1.203. [DOI] [PubMed] [Google Scholar]
- Gimlich R. L. Acquisition of developmental autonomy in the equatorial region of the Xenopus embryo. Dev Biol. 1986 Jun;115(2):340–352. doi: 10.1016/0012-1606(86)90254-x. [DOI] [PubMed] [Google Scholar]
- Gimlich R. L., Gerhart J. C. Early cellular interactions promote embryonic axis formation in Xenopus laevis. Dev Biol. 1984 Jul;104(1):117–130. doi: 10.1016/0012-1606(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Green J. B., Howes G., Symes K., Cooke J., Smith J. C. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990 Jan;108(1):173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Jones E. A., Woodland H. R. Spatial aspects of neural induction in Xenopus laevis. Development. 1989 Dec;107(4):785–791. doi: 10.1242/dev.107.4.785. [DOI] [PubMed] [Google Scholar]
- Kageura H. Spatial distribution of the capacity to initiate a secondary embryo in the 32-cell embryo of Xenopus laevis. Dev Biol. 1990 Dec;142(2):432–438. doi: 10.1016/0012-1606(90)90365-p. [DOI] [PubMed] [Google Scholar]
- Kageura H., Yamana K. Pattern regulation in defect embryos of Xenopus laevis. Dev Biol. 1984 Feb;101(2):410–415. doi: 10.1016/0012-1606(84)90155-6. [DOI] [PubMed] [Google Scholar]
- Kao K. R., Elinson R. P. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988 May;127(1):64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Abraham J. A., Haaparanta T., Palisi T. M., Kirschner M. W. The presence of fibroblast growth factor in the frog egg: its role as a natural mesoderm inducer. Science. 1988 Nov 18;242(4881):1053–1056. doi: 10.1126/science.3194757. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Brockes J. P. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984 Mar 1;308(5954):67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989 Sep 22;58(6):1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Brennan S., Dathan N., Fairman S., Gurdon J. B. Cell type-specific activation of actin genes in the early amphibian embryo. Nature. 1984 Oct 25;311(5988):716–721. doi: 10.1038/311716a0. [DOI] [PubMed] [Google Scholar]
- Musci T. J., Amaya E., Kirschner M. W. Regulation of the fibroblast growth factor receptor in early Xenopus embryos. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8365–8369. doi: 10.1073/pnas.87.21.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990 Jun;10(6):2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. Retinoic acid modifies mesodermal patterning in early Xenopus embryos. Genes Dev. 1991 Feb;5(2):175–187. doi: 10.1101/gad.5.2.175. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Interaction between peptide growth factors and homoeobox genes in the establishment of antero-posterior polarity in frog embryos. Nature. 1989 Sep 7;341(6237):33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- Sharpe C. R., Pluck A., Gurdon J. B. XIF3, a Xenopus peripherin gene, requires an inductive signal for enhanced expression in anterior neural tissue. Development. 1989 Dec;107(4):701–714. doi: 10.1242/dev.107.4.701. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Gillespie L. L., Godsave S. F., Isaacs H. V., Paterno G. D. The role of fibroblast growth factor in early Xenopus development. Development. 1989;107 (Suppl):141–148. doi: 10.1242/dev.107.Supplement.141. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Isaacs H. V., Darlington B. G. Inductive effects of fibroblast growth factor and lithium ion on Xenopus blastula ectoderm. Development. 1988 Jul;103(3):581–590. doi: 10.1242/dev.103.3.581. [DOI] [PubMed] [Google Scholar]
- Smith J. C. Mesoderm induction and mesoderm-inducing factors in early amphibian development. Development. 1989 Apr;105(4):665–677. doi: 10.1242/dev.105.4.665. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Slack J. M. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J Embryol Exp Morphol. 1983 Dec;78:299–317. [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991 Nov 15;67(4):741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Sokol S., Melton D. A. Pre-existent pattern in Xenopus animal pole cells revealed by induction with activin. Nature. 1991 May 30;351(6325):409–411. doi: 10.1038/351409a0. [DOI] [PubMed] [Google Scholar]
- Sokol S., Wong G. G., Melton D. A. A mouse macrophage factor induces head structures and organizes a body axis in Xenopus. Science. 1990 Aug 3;249(4968):561–564. doi: 10.1126/science.2382134. [DOI] [PubMed] [Google Scholar]
- Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990 Nov 2;63(3):485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- van den Eijnden-Van Raaij A. J., van Zoelent E. J., van Nimmen K., Koster C. H., Snoek G. T., Durston A. J., Huylebroeck D. Activin-like factor from a Xenopus laevis cell line responsible for mesoderm induction. Nature. 1990 Jun 21;345(6277):732–734. doi: 10.1038/345732a0. [DOI] [PubMed] [Google Scholar]