Abstract
Breast and cervical cancers are dangerous threats with regard to the health of women. The two malignancies have reached the highest record in terms of cancer-related deaths among women worldwide. Despite the use of novel strategies with the aim to treat and cure advanced stages of cancer, post-therapeutic relapse believed to be caused by cancer stem cells is one of the challenges encountered during tumor therapy. Therefore, further attention should be paid to cancer stem cells when developing novel anti-tumor therapeutic approaches. Low-intensity laser irradiation is a form of phototherapy making use of visible light in the wavelength range of 630–905 nm. Low-intensity laser irradiation has shown remarkable results in a wide range of medical applications due to its biphasic dose and wavelength effect at a cellular level. Overall, this article focuses on the cellular responses of healthy and cancer cells after treatment with low-intensity laser irradiation alone or in combination with a photosensitizer as photodynamic therapy and the influence that various wavelengths and fluencies could have on the therapeutic outcome. Attention will be paid to the biomodulative effect of low-intensity laser irradiation on cancer stem cells.
Keywords: Stem cells, cancer stem cells, breast cancer, cervical cancer, low-intensity laser irradiation, photodynamic therapy
Introduction
Breast cancer is presently the second most commonly diagnosed invasive cancer, after lung cancer, predominantly affecting woman and the leading cause of cancer-related deaths in women worldwide.1 With approximately 1.7 million new cases diagnosed in 2012, breast cancer accounted for 12% of all cancer and 25% of cancer affecting women worldwide. Cervical cancer occupies the second and third position on the list of the most commonly diagnosed cancers in women and the leading cause of cancer-related death worldwide, respectively.2 With nearly 527,600 new cases diagnosed in 2012, cervical cancer accounted for 7.9% of all cancer affecting women (Table 1).2,3
Table 1.
Comparison of breast and cervical cancer statistical analysis.
| Breast cancer | Cervical cancer | |
|---|---|---|
| Incidence | 1,700,000 | 527,600 |
| Incidence rate (%) | 12 | 7.9 |
| Incidence rate in female (%) | 25 | 7.9 |
| Rank | 2 | 7 |
| Mortality | 521,900 | 265,700 |
| Mortality rate (%) | 6.4 | 3.2 |
| Mortality rate in female (%) | 14.7 | 7.5 |
| 5-year prevalence rate (%) | 19.2 | 4.8 |
| 5-year prevalence rate in female (%) | 36.3 | 9 |
| Estimated incidence in 2016 in the US | 246,660 | 12,990 |
| Estimated incidence rate in 2016 in the US (%) | 14.6 | 0.8 |
| Estimated mortality in 2016 in the US | 40,450 | 4120 |
| Estimated mortality rate in 2016 in the US (%) | 6.8 | 0.7 |
Post-therapeutic cancer recurrence is believed to be caused by cancer stem cells (CSCs).4 Stem cells are embryonic or adult (somatic), undifferentiated cells that have the remarkable potential to differentiate into any cell type of the living organism.5 CSCs and normal stem cells share phenotypic similarities, including the self-renewal, the differentiation, and the proliferation abilities.
The capacity of low-intensity laser irradiation (LILI) to enhance natural functions of the mitochondria such as the metabolic energy synthesis of adenosine triphosphate (ATP) and programmed cell death activation has been observed in both normal and cancer cells and has made LILI a novel approach in disorders whose treatment effectiveness relies on cellular biostimulation or bioinhibition. Light absorption is made possible by the chromophores (photoacceptors) located in the mitochondrial inner membrane.6 The proliferative cellular response to LILI is believed to be the result of a change in the redox state of mitochondrial redox couples, which in turn regulates a number of signaling pathways and transcription factors that are involved in cell proliferation, growth, and motility.7,8
Breast cancer
Breast cancer is a life-threatening heterogeneous disease caused by multiple alterations of epithelial cells found in the milk-producing lobules and the milk ducts within breast tissues.9 Based on their immunohistochemical (IHC) characteristics and their expression of protein receptors, breast cancers are classified clinically into four subtypes, namely, lumina A, lumina B, human epidermal growth factor receptor 2 (HER2), and triple-negative breast cancers (TNBC). They all require different therapeutic approaches and have different prognosis.10
The estrogen and progesterone receptor protein overexpression is observed in both the lumina A and B breast cancer subtypes, which represent 40% and 20% of all breast cancers, respectively.11 Given their estrogen positive (ER+) and progesterone positive (PR+) status, both lumina A and B show favorable responses to the endocrine therapy using drugs such as tamoxifen, toremifene, and fulvestran, which decrease or stop the estrogen production in cancer cells, thus disrupting their growth.12 The knowledge of the gene expression profile and protein synthesis turns out to be helpful in determining the behavior of a given cancer in order to decide on the suitable treatment. The deregulation and overexpression of the enhancer of zeste homolog 2 (EZH2) protein have been associated with CSC formation, angiogenesis, progression, metastasis, epithelial–mesenchymal transition (EMT), drug resistance, and poor prognosis in breast and cervical cancer.13
Notch signaling pathway appears to be involved in the tumorigenesis, angiogenesis, cancer cell growth, and resistance to the anti-estrogen therapy through its interaction with the estrogen pathway.14
Signaling pathway crosstalk plays a crucial role in the cellular responses to different changes that occur in their environment. Cancer cells misuse of this intercommunication causes harmful effects in the entire organism.11 One of the signaling networks misuse is the crosstalk between Notch and the HER2 signaling pathway which has been associated with the development of breast cancer.11,14 HER2 protein is a receptor found in healthy breast cells and is involved in the cellular proliferation, division, and repair. HER2 gene amplification could lead to the malignancy of healthy breast cells. This happens in nearly 10%–20% of cases and the breast cancer is said to be HER2-positive (Figure 1).12
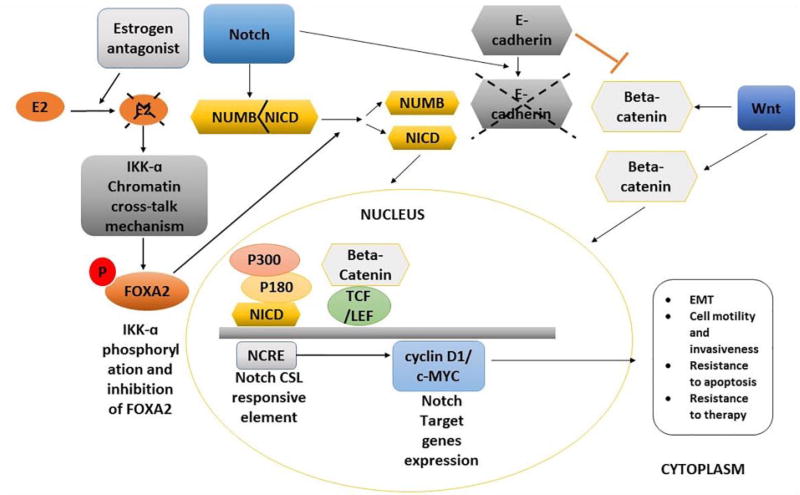
Figure 1.
Proposed mechanism of the influence of Notch and Wnt pathways in the oncogenesis and stemness maintenance of breast cancer cells in the absence of estrogen. Following the estrogen inhibition by estrogen antagonist drugs, Notch pathway activation induces the expression of estrogen-responsive genes via the IKK-α cooperative chromatin recruitment of Notch-CSL-MAML1 transcriptional complex (NTC). NTC promotes the recruitment of transcriptional co-activator proteins such as p180 and p300 on the estrogen-responsive gene promoters resulting in the expression of the downstream Notch target genes including cyclin D1 and c-MYC, both involved in tumor formation, rapid progression, aggressiveness, and poor prognosis. E-cadherin inhibition as a consequence of Notch activation induces the accumulation of free β-catenin (β-catenin) proteins in the cytoplasm followed by their entry in the nucleus facilitated by Wnt pathway. Inside the nucleus, β-catenin binds to the TCF/LEF protein and acts as a transcriptional co-activator of the Wnt target gene c-MYC whose upregulation has been associated with the acquisition of mesenchymal characteristics by epithelial cells also known as the EMT.
Cervical cancer
Cervical cancer develops in the lower part of the female uterus called cervix. Malignant lesions of the cervix are caused by a synergy between infection by the human papilloma virus (HPV) and the genetic and epigenetic alterations of healthy stem cells.15
The mechanism behind cervical cancer development due to HPV infection is the disturbance of vital cellular pathways such as notch caused by the E6 and E7 viral oncoproteins, whose overexpression has been associated with cancer malignancy.16 The gene expression profiling of cervical cancer cells has shown aberrant methylations of the CpG Island within the promoters of several tumor-suppressor genes including p53, which is normally involved in the positive regulation of apoptosis and negative regulation of cell growth and migration.17 Currently, hysterectomy and radiation therapy are used to treat and cure cervical cancer at early stage.18 However, due to the lack of regular screening, the cancer is already at a malignant stage at the time of diagnosis and the post-therapeutic results are far from optimal. At this stage, the recurrence rate usually stands at 50% within a year after therapy.19
Stem cells
Stem cells are embryonic or adult undifferentiated cells that differ from other cells by their capacity to renew themselves and to transit from the undifferentiated to the differentiated state under specific physiological or experimental conditions.20 Regardless of their origins, all stem cells divide through mitosis and can either renew themselves and give rise to undifferentiated daughter cells or become organ specific and give rise to differentiated daughter cells.21 Based on their potency, stem cells are classified as totipotent, pluripotent, and multipotent. Totipotent stem cells are either from the zygote, spore, or morula embryonic tissues and have the potential to give rise to an entire functional organism given their ability to differentiate into any type of adult and embryonic cells.22 Pluripotent stem cells are also from the embryonic tissues but can only give rise to adult cells. Multipotent stem cells are from the adult tissues and can only differentiate into a limited range of cells.22 Adult differentiated cells can be genetically reprogrammed into “induced pluripotent stem cells (IPSCs)” by forcing the expression of certain genes under specific conditions.23
CSCs
CSCs are malignant cells believed to originate from genetically or epigenetically altered healthy stem cells. They represent a minority of undifferentiated side-population (SP) cells possessing stem-like properties among cancerous cells of a heterogenic malignant tumor.20,24 In addition, CSCs also possess tumorigenic phenotypes including the multidrug resistance, expression of anti-apoptotic proteins, drug efflux pumps, clonal long-term repopulation capacity, uncontrolled proliferation, metastasis, and epithelial–mesenchymal plasticity.25,26
Intratumoral heterogeneity, which consists of the simultaneous presence of several types of cells within a single neoplasm, is one of the main features observed in cancer cell populations of the majority of solid and hematopoietic malignancies.27 The different cell subpopulations are distinguished from each other by characteristics such as their morphology and surface antigen expression. Due to these variations, different responses to treatments are expected and consequently the choice of appropriate treatment is more challenging.27
CSCs are biomarker-defined cell populations that can be characterized and isolated from the tumor mass based on their specific cell surface biomarkers.28 Among an entire breast tumor cell population, breast CSCs are the ones possessing the CD44+/CD24−/low phenotype, while in cervical cancer, CSCs are CD133+ cells.28,29 These biomarkers have been associated with tumor growth and cancer cell aggregation given their involvement in cancer cell migration and matrix adhesion. The stemness properties in CSCs as well as in their healthy counterparts are believed to be maintained by the same signaling pathways, namely, the Notch, Hedgehog, and Wnt signaling pathways.30 These pathways are involved in the regulation of vital stem cell properties such as the self-renewal, differentiation, and fate determination in embryonic and adult stem cells.31 Considering their role at the cellular level, it is understood that their slightest perturbation could be at the origin of drastic changes in the organism.30,32 Several signaling pathways involved in the regulation of normal functions in healthy stem cells appear to be altered in CSCs.33
Biphasic dose and wavelength-related cellular response to LILI treatment
LILI, also known as photobiomodulation, is a non-thermal and non-toxic phototherapy that uses coherent monochromatic low-intensity light, usually corresponding to the visible red (400–720 nm) and the near-infrared (NIR; 700–1000 nm) range of the light spectrum to induce photobiological processes at the cellular level.34 The clinical use of LILI has so far shown no side effects in patients, making it a promising therapeutic approach in a wide range of clinical applications.8
In the past decades, the medical application of LILI has been focused on its non-invasive biostimulatory effect. LILI has been used in wound healing, stimulation of the immune system, swelling reduction, and acute/chronic pain relief.35,36 Since the introduction of laser in cancer therapy, the use of LILI has mostly been focused on the reduction of acute and chronic symptoms caused by the cancer condition itself or the cancer treatment.37 Further attention should be turned to the possible bioinhibitory effect of LILI used alone at specific wavelengths and fluencies or in the form of photodynamic therapy (PDT) as a potential therapeutic tool for CSC eradication.8
The biomodulative effects of LILI vary from cellular proliferation to the programmed cell death. LILI is said to have a biphasic dose and wavelength-dependent effect.7 Either effect can be beneficial for therapeutic purposes. The biostimulatory effect is believed to be linked to the increased ATP production, and the bioinhibitory effect has been linked to the oxidative stress due to the reactive oxygen species (ROS) overload.38 The following are some concrete examples of research reports supporting the biphasic dose effect hypothesis of LILI in non-cancerous and cancer cells. Research results supporting the biostimulatory effect of LILI were observed in human adipose–derived stem cells (hADSCs) where a statistically significant increase in cell proliferation could be seen 48 h post-irradiation when using 5 J/cm2 at a wavelength of 636 nm.39 In a study on diabetic induced human skin fibroblast cells (WS1), an increase in the cellular proliferation and viability was observed when cells were treated with a wavelength of 830 nm and fluencies of 5, 10, and 15 J/cm2, whereas no significant change could be seen when using wavelength of 680 nm and the same fluencies.40 This confirms the wavelength dependency of cellular bio-activation due to LILI. The dose dependency was observed in neoplastic cells (EMT-6 and RIF-1) when wavelength of 632.8 nm and fluence of 180 mJ/cm2 promoted cell growth, while fluencies ranging from 400 to 600 mJ/cm2 induced growth inhibition.35
Effect of LILI on CSCs and mechanism of action
As normal cells and CSCs share similarities in their mitochondrial content, the chromophores located in the inner membrane of CSCs are expected to play a photoacceptor role as the ones found in normal cell. As previously mentioned, the photobiological effect of LILI highly depends on the cell properties and light parameters. This could be seen in a study on lung cancer that aimed to evaluate the photo stimulatory effect of low and high fluences of LILI on lung CSCs isolated from the A549 cell line which has been demonstrated to have a chemo and multidrug resistance phenotype.3,41 One of the outcomes of this study confirmed that unlike non-cancer cells that undergo apoptosis through ROS overproduction after exposure to high dose of light, CSCs on the contrary have the capacity to self-renew after being exposed to the same laboratory condition.41 When using low fluencies ranging from 5 to 10 J/cm2 at 600 and 800 nm, a statistically significant increase in both viability and proliferation was observed in CSCs. Unexpectedly, the same biostimulatory effect could be observed after exposure to higher fluence of 20 J/cm2.41 However, a statistically significant decrease in viability and proliferation going hand to hand with an increase in apoptosis could be seen upon exposure to 40 J/cm2.3 The outcome of this study supports that the biostimulatory and bioinhibitory effect of LILI relies on the fluence and wavelength of light. This is in agreement with the “The Arndt-Schultz Law” which basically states that weak stimuli (referring to the irradiation time or dose of light) increase physiologic activity, medium stimuli inhibit activity, and very strong stimuli stop activity.42
Although the exact mechanism behind the bioinhibitory effect of high-fluence LILI is unclear, the possibility of the excessive production of harmful ROS such as singlet oxygen and hydroxyl radicals should be considered. It is proposed that prolonged exposure to laser light at a certain wavelength can prompt the mitochondria to produce excess ROS that escape the control of the antioxidant defence mechanism of CSCs.
As light-induced apoptosis requires the involvement of the mitochondrial respiratory chain, the shift in cell redox potential (increased oxidation) induced by high-fluence LILI treatment on CSCs could be a trigger for apoptosis through an oxidative stress.7 The presence of free radical outside the mitochondria as a consequence of the oxidative stress causes the formation of BH123 aggregate facilitating the release of cytochrome c from the intermembrane to the cytoplasm, which acts as a signal for the activation of the initiator caspase (caspase 9) which in turn activates the executioner caspase (caspase 3) and eventually leads to programmed cell death (Figure 2).43
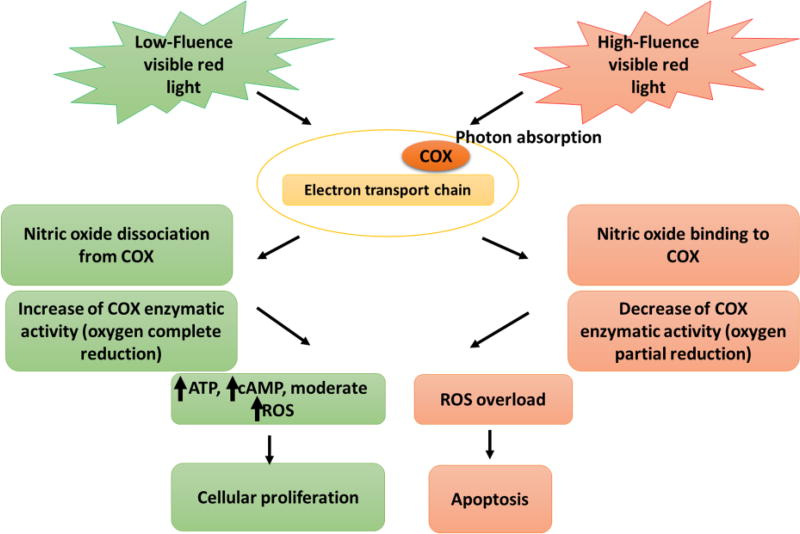
Figure 2.
Proposed mechanism of the biphasic dose effect of LILI treatment on CSCs. Light photon from laser is absorbed by the cytochrome c oxidase (COX) of the respiratory chain. The energy of photons donated by low-fluence visible red light is sufficient to dissociate nitric oxide (NO) from COX and enhance the COX reduction capacity which eventually leads to CSC proliferation through ATP, cAMP, and moderate ROS production. By contrast, the energy of photons donated by high-fluence visible red light is sufficient to decrease the COX reduction capacity leading to the massive conversion of dioxygen into ROS which prompts the programmed cell death. High level of ROS within the inner membrane of the mitochondria serves as signal for the opening of the mitochondrial permeability which triggers caspase-3 activation leading to the release of COX in the outer membrane which in turn serves as signal for the activation of pro-apoptotic enzymes.
Effect of high-fluence LILI on the immune system
It is well known that cancer cells in general have a weakening effect on the innate and adaptive immune system of the host organism. Unlike the majority of conventional cancer therapies like chemotherapy and radiation therapy which are toxic to the bone narrow and tend to further weaken the immune system, phototherapy on the contrary has been proven to strengthen immunity through the activation of tumor-specific cytotoxic T-cells.44,45
While the immune stimulatory effects of PDT have been extensively studied, we cannot say the same for high-fluence LILI. As PDT and high-fluence LILI share similarities in their outcome which is the activation of apoptosis through excessive ROS production, it is suggested that LILI could have immune stimulatory effects as a consequence of the traumatic insult to the tumor mass and the microenvironment in which they live. Just like in PDT, an inflammatory reaction could raise from the above traumatic insult following high-fluence LILI treatment.44 As a reminder, an excessive ROS production results in an irreversible damage of the disorganized blood vessel networks that make up the tumor vasculature causing a lethal drop in the nutrient and oxygen supply.46 This shut down of the tumor angiogenic switch which is usually continuously on may constitute a great advancement in challenges encounter during tumor therapy.47,48
PDT
The development of drugs that would only be toxic toward a targeted biomarker-defined cancer cell population is one of the objectives pursued by scientists. PDT, which associates low-intensity light with a photosensitizer (PS), has proved its effectiveness in numerous cancer therapies and approaches in vitro and in vivo.49 The mechanism of action behind the effectiveness of PDT is the oxidative stress overload caused by the photo-active PS which is first excited into an unstable singlet state quickly followed by its relaxation into a more stable triplet state.8
For the photochemical process to occur, the wavelength of the light must correspond to the absorption spectra of the PS.8 Light absorption by tissue is directly proportional to the increase in the wavelength from the red to the deep red region of the electromagnetic spectrum.50 PSs with absorption peaks between 600 and 800 nm are the most efficient and provide sufficient energy to convert oxygen molecules into their excited singlet state. Shortly after being injected to the cancer location, the PS is absorbed by cancer cells and some healthy surrounding cells. Afterward, only cancer cells have retained the PS and the visible light can be applied to the cancer cells site.37 The application of PDT in cancer treatment has only been approved for a limited number of cancer types (mostly localized cancers).37 Currently, Porfimer is one of the most commonly used PSs in the treatment of cancers such as the non-small-cell lung cancer, cancer of the esophagus, and Barrett’s esophagus with dysplasia (pre-cancer).37 Further researches aiming the application of PDT in a wider range of cancer types including breast and cervical cancers and the production of appropriate PSs are being conducted.
PDT treatment of CSCs
Studies are being carried out to evaluate the effect of PDT on CSCs and to find appropriate photosensitizer that could be used for various types of cancer. A 5-aminolevulinic acid (5-ALA)–mediated PDT study (ALA-PDT) done on head and neck cancer–derived cancer stem cells (HNC-CSCs) using wavelengths of 635 ± 5 nm revealed a significant decrease in the aldehyde dehydrogenase 1 (ALDH1) activity, mammosphere formation, and CD44 biomarker expression in HNC-CSCs.51 This set of results show that ALA-PDT affects the stemness of HNC-CSCs by disturbing their self-renewal capacity. In a study done on HT29 cell line from colorectal cancer, after CSC isolation based on their expression of the CD133 biomarker, the effect of PDT on both CD133+ and CD133− cells using protoporphyrin IX (PpIX) as PS and a light-emitting diode (LED) laser of 632 nm using fluencies of 2 and 5 J/cm2 was evaluated.49 Measurement of cell viability showed 80% of cell death in CD133− cells when using 2 J/cm2. The same outcome was obtained in CD133+ when using 5 J/cm2. One could conclude that although PpIX-mediated PDT has shown effectiveness in CD133+ CSCs eradication, higher fluence is required compared to CD133− cells.49 This confirms that CSCs show more resistance than the differentiated cancer cells.49
Targeted photodynamic nanotherapy
The selectivity in the systemic drug delivery and the delivery of hydrophobic PSs such as porphyrin and phthalocyanine derivatives are challenging.52 The use of nanoparticles (NPs) in the systemic delivery of PS is in constant evolution. Mostly, biodegradable polymers such as polylactide (PLA) and polyglycolide (PGA) and carbon nanotubes with strong absorption in the NIR such as the single-walled nanotubes (SWNTs) are used as nanocarriers.52,53 The PSs are encapsulated in NPs attached to a ligand or antibody that have affinity with specific CSC surface markers.54 The PS interaction with the NP can either be hydrophobic or electrostatic.52 NPs are also involved in drug loading and drug release into CSCs, charge transfer, and free radical formation. This novel technique has been proven to enhance the cytotoxic effect of PDT in CSCs. A study on MCF-7 breast CSCs using methylene blue (MB) as PS investigated and compared the cytotoxic effect of MB when used alone and when encapsulated in dioctyl sulfosuccinate sodium salt NP (MB NP). CSCs self-renewal was investigated by assessing the mammosphere formation ability of CSCs after both MB and MB NP mediated PDT. When compared to the untreated control, colony formation on soft agar gel decreased to 8% with MB used alone and 1% with MB NPs. This indicated that nanoencapsulation of the PS enhances its cytotoxic effect.55
Conclusion
Considering the life-threatening status of breast and cervical cancers, there is an urgent need to develop appropriate therapeutic approaches to cure these malignancies. Over the last years, overwhelming evidence has confirmed the involvement of CSCs in driving cancer in most human affecting malignancies including breast and cervical cancers. The post-therapeutic recurrence observed in malignant cancers has pushed scientists to find alternative therapies to eradicate CSCs. In the quest for effective treatments, it has been discovered that treatment with LILI alone or as PDT could be a potential therapeutic tool, given its ability to trigger apoptotic cell death. Unlike chemotherapy, PDT offers a better post-therapeutic life quality without any known side effects. LILI and PDT could be used as adjuvant therapy to chemotherapy, radiotherapy, and surgery.32 It is worth to consider that LILI treatment might have a bioinhibitory effect on breast and cervical CSCs. Presently, the number of studies in breast and cervical cancer treatment using LILI alone or as PDT is limited; hence, there is a need to increase the number of studies on that subject.
Acknowledgments
The University of Johannesburg, Laser Research Centre (LRC), National Research Funding (NRF), Council for Scientific and Industrial Research (CSIR), and the National Laser Centre (NLC) are acknowledged for their support. All authors agreed to the submission of this article to Stem Cell Research and Therapy. N.E.K. wrote the manuscript and participated in conception and design. H.A. edited the manuscript and provided final approval. M.R.H. edited the manuscript. All authors read and approved the final manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NRF Masters Innovation Scholarship, Supervisor Linked Bursary (University of Johannesburg).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.International Agency for Research on Cancer and Word Health Organization. Latest world cancer statistics. Global cancer burden rises to 14.1 million new cases in 2012: Marked increase in breast cancers must be addressed. [accessed 12 December 2015];2013 https://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf.
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamse H, Crous A. Proceedings of SPIE. Vol. 9596. San Francisco, CA: Feb 13, 2016. Biochemical responses of isolated lung CSCs after application of low intensity laser irradiation. [DOI] [Google Scholar]
- 4.Hong IS, Lee HY, Nam JS. Cancer stem cells: the “Achilles heel” of chemo-resistant tumors. Recent Pat Anticancer Drug Discov. 2015;10(1):2–22. doi: 10.2174/1574892809666141129172658. [DOI] [PubMed] [Google Scholar]
- 5.Enderling H, Hlatky L, Hahnfeldt P. Cancer stem cells: a minor cancer subpopulation that redefines global cancer features. Front Oncol. 2013;3:76. doi: 10.3389/fonc.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mroz P, Yaroslavsky A, Kharkwal GB, et al. Cell death pathways in photodynamic therapy of cancer. Cancers. 2011;3:2516–2539. doi: 10.3390/cancers3022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamblin MR. Mechanisms of low level light therapy. [accessed 31 July 2016];2008 http://photobiology.info/Hamblin.html.
- 8.Crous AM, Abrahamse H. Lung cancer stem cells and low-intensity laser irradiation: a potential future therapy? Stem Cell Res Ther. 2013;4:129. doi: 10.1186/scrt340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Breast cancer facts & figures 2013–2014. [accessed 7 December 2016];2013 http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf.
- 10.Tian F, Wang Y, Seiler M, et al. Functional characterization of breast cancer using pathway profiles. BMC Med Genomics. 2014;7:45. doi: 10.1186/1755-8794-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker AT, Zlobin A, Osipo C. Notch-EGFR/HER2 bidirectional crosstalk in breast cancer. Front Oncol. 2014;4:360. doi: 10.3389/fonc.2014.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23:60–64. doi: 10.1038/modpathol.2010.33. [DOI] [PubMed] [Google Scholar]
- 13.Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer. 2012;106:243–247. doi: 10.1038/bjc.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hussaini H, Subramanyam D, Reedijk M, et al. Notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. 2011;10(1):9–15. doi: 10.1158/1535-7163.MCT-10-0677. [DOI] [PubMed] [Google Scholar]
- 15.López J, Ruíz G, Organista JN, Gariglio P, et al. Human papillomavirus infections and cancer stem cells of tumors from the uterine cervix. Open Virol J. 2012;6:232–240. doi: 10.2174/1874357901206010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. 2011;6(9):1083–1098. doi: 10.2217/fmb.11.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pol BV, Klingelhutz AJ. The papillomavirus episteme papillomavirus E6 oncoproteins. Virology. 2013;445(0):115–137. doi: 10.1016/j.virol.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrivastavaa SK, Mahantshettya U, Narayanb K. Principles of radiation therapy in low-resource and well-developed settings, with particular reference to cervical cancer. Int J Gynecol Obstet. 2015;131(2):153–158. doi: 10.1016/j.ijgo.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhang SL, Wang Y, Zhou T, et al. Isolation and characterization of cancer stem cells from cervical cancer HeLa cells. Cytotechnology. 2012;64(4):477–484. doi: 10.1007/s10616-012-9436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reya T, Morrison T, Michael F, et al. Stem cells, cancer, and cancer stem cells. Nature. 2011;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 21.Tomasetti C, Levy D. Role of symmetric and asymmetric division of stem cells in developing drug resistance. Proc Natl Acad Sci USA. 2011;107(39):16766–16771. doi: 10.1073/pnas.1007726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hongxiang H, Yongming T, Min H, et al. Stem cells: general features and characteristics. In: Gholamrezanezhad A, editor. Stem cells in clinic and research. Baltimore, MD: InTech; 2011. pp. 2–10. [DOI] [Google Scholar]
- 23.Lemey C, Milhavet O, Lemaitre JM. iPSCs as a major opportunity to understand and cure age-related diseases. Biogerontology. 2015;16(4):399–410. doi: 10.1007/s10522-015-9579-7. [DOI] [PubMed] [Google Scholar]
- 24.Jang M, Kim SS, Lee J. Cancer cell metabolism: implications for therapeutic targets. Exp Mol Med. 2013;45:e45. doi: 10.1038/emm.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohr M, Zänker KS, Dittmar T. Cancer (stem) cell differentiation: an inherent or acquired property? Med Hypotheses. 2015;85(6):1012–1018. doi: 10.1016/j.mehy.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Plaks V, Kong N, Zena W. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell Perspective. 2015;16(3):225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannuti A, Foreman K, Rizzo P, et al. Targeting cancer stem cells through Notch signaling. Clin Cancer Res. 2010;16(12):3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romano M, De Francesco F, Pirozzi G, et al. Expression of cancer stem cell biomarkers as a tool for a correct therapeutic approach to hepatocellular carcinoma. Oncoscience. 2015;2(5):443–456. doi: 10.18632/oncoscience.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Zeng J, Luo L, et al. Identification of a cancer stem cell-like side population in the HeLa human cervical carcinoma cell line. Oncol Lett. 2013;6(6):1673–1680. doi: 10.3892/ol.2013.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H, Jia J, Wang X, et al. CD44+/CD24− breast cancer cells isolated from MCF-7 cultures exhibit enhanced angiogenic properties. Clin Transl Oncol. 2013;15(1):46–54. doi: 10.1007/s12094-012-0891-2. [DOI] [PubMed] [Google Scholar]
- 31.Vishnoi K, Tyagi A, Singh SM, et al. Cervical cancer stem cells and their association with human papillomavirus: are they ready as anticancer targets? In: Gandhi V, Mehta K, Grover R, et al., editors. Multi-targeted approach to treatment of cancer. New York: Springer; 2015. pp. 377–399. [Google Scholar]
- 32.Malik F, Korkaya H, Clouthier SG, et al. Principles of stem cell biology and cancer: future applications and therapeutics. 1. Chichester: Wiley-Blackwell; 2015. [Google Scholar]
- 33.Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta. 2012;1830(2):2481–2495. doi: 10.1016/j.bbagen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Abrahamse H. Inducing stem cell differentiation using low intensity laser irradiation: a possible novel therapeutic intervention. Cent Eur J Biol. 2011;6:695. [Google Scholar]
- 35.Al-Watban FA, Andres BL. Laser biomodulation of normal and neoplastic cells. Lasers Med Sci. 2012;27(5):1039–1043. doi: 10.1007/s10103-011-1040-9. [DOI] [PubMed] [Google Scholar]
- 36.Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Cancer Society. Photodynamic therapy. [accessed 31 July 2016];2015 http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/photodynamic-therapy.
- 38.Kim HP. Lightening up light therapy: activation of retrograde signaling pathway by photobiomodulation. Biomol Ther. 2014;22(6):491–496. doi: 10.4062/biomolther.2014.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Villiers JA, Houreld NN, Abrahamse H. Influence of low intensity laser irradiation on isolated human adipose derived stem cells over 72 hours and their differentiation potential into smooth muscle cells using retinoic acid. Stem Cell Rev. 2011;7:869–882. doi: 10.1007/s12015-011-9244-8. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamse H, Houreld NN, Muller S, et al. Fluence and wavelength of low intensity laser irradiation affect activity and proliferation of human adipose derived stem cells. Med Technol SA. 2010;24(2):15–20. [Google Scholar]
- 41.Abrahamse H, Crous A. Biochemical responses of isolated lung CSCs after application of low intensity laser irradiation. Photomed Laser Surg. 2016;34:525–532. doi: 10.1089/pho.2015.3979. [DOI] [PubMed] [Google Scholar]
- 42.Huang YY, Chen AC, Carroll JD, et al. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochim Biophy Acta. 2008;1777:8877–8881. doi: 10.1016/j.bbabio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Reginato E, Wolf P, Hamblin MR. Immune response after photodynamic therapy increases anti-cancer and antibacterial effects. World J Immunol. 2014;4(1):1–11. doi: 10.5411/wji.v4.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St Denis TG, Aziz K, Waheed AA, et al. Combination approaches to potentiate immune response after photodynamic therapy for cancer. Photochem Photobiol Sci. 2011;10(5):792–801. doi: 10.1039/c0pp00326c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 49.Feng MW, You SH, Jyuan SY, et al. Cell death of colorectal cancer stem-like cell was induced by photodynamic therapy with protoporphyrin IX. [accessed 7 December 2016];2009 http://www.senkyo.co.jp/apcc20th_abstract/pdf/0080_P-44)00173.pdf.
- 50.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hang CY, Chia CY. Photodynamic therapy with 5-aminolevulinic acid (ALA) impairs tumor initiating and chemo-resistance property in head and neck cancer-derived cancer stem cells. PLoS One. 2014;9:e87129. doi: 10.1371/journal.pone.0087129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Moo KH. Polymeric nanocarrier systems for photodynamic therapy. Biomater Res. 2014;18:19. doi: 10.1186/2055-7124-18-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon JU, Jadeja P, Tambe P, et al. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics. 2013;3(3):152–166. doi: 10.7150/thno.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usacheva M, Swaminathan SK, Kirtane AR, et al. Enhanced photodynamic therapy and effective elimination of cancer stem cells using surfactant–polymer nanoparticles. Mol Pharm. 2014;11:3186–3195. doi: 10.1021/mp5003619. [DOI] [PubMed] [Google Scholar]