SYNOPSIS
The monogenic autoinflammatory diseases are a group of illnesses with prominent rheumatic manifestations that are characterized by genetically-determined recurrent sterile inflammation, and are thus inborn errors of innate immunity. Molecular targeted therapies against inflammatory cytokines such as IL-1 and TNF and intracellular cytokine signaling pathways have proven effective in many cases. Emerging next generation sequencing technologies have accelerated the identification of previously unreported genes causing autoinflammatory diseases. In this review we will cover several of the prominent recent advances in the field of autoinflammatory diseases, including gene discoveries, the elucidation of new pathogenic mechanisms, and the development of effective targeted therapies.
Keywords: haploinsufficiency of A20 (HA20), otulipenia, deficiency of ADA2 (DADA2), interferonopathy, CANDLE/PRAAS, SAVI, NLRC4 inflammasome, pyrin inflammasome
INTRODUCTION
Autoinflammatory diseases (AID) are a group of disorders characterized by seemingly unprovoked inflammation that may be recurrent or sometimes nearly continuous. The term "autoinflammatory" first appeared in the literature in 1999 to describe two monogenic disorders with recurrent fevers and episodes of systemic inflammation without high-titer autoantibodies or antigen-specific T cells: familial Mediterranean fever (FMF) and the then newly-described TNF receptor associated periodic syndrome (TRAPS)1. At present more than 20 monogenic AID have been reported. The clinical manifestations of AID are typically driven by genetically-determined dysregulation of innate immunity, which results in overproduction of inflammatory cytokines, such as IL-1β, IL-6, IL-18, TNF, and type I interferon (IFN). Specific treatments targeting these cytokine signaling pathways have been proven to be effective in many AID patients, highlighting the importance of accurate genetic diagnosis and detailed molecular pathophysiology.
In this article, we review some of the recent advances in the field of AID over the last three years, including the discovery of several newly identified monogenic disorders (Table 1). We also focus on recent insights into the pathogenesis of FMF to demonstrate how genetics and basic biology have synergized to demystify one important mechanism of host-pathogen interaction.
Table 1.
Newly identified monogenic autoinflammatory disorders.
| Disease | Gene | Protein | Phenotypes | Disease Mechanism |
|---|---|---|---|---|
|
| ||||
| HA20 | TNFAIP3 | A20 |
|
Haploinsufficiency of A20 leads to exacerbated NF-κB signaling and NLRP3 inflammasome activation |
|
| ||||
| Otulipenia/ORAS | OTULIN | OTULIN |
|
Loss of OTULIN leads to impaired removal of linear-Ub from proinflammatory signaling complexes |
|
| ||||
| DADA2 | CECR1 | ADA2 |
|
Reduced serum level of ADA2 resulting in:
|
|
| ||||
| SAVI | TMEM173 | STING |
|
Gain-of-function in STING leads to the constitutive activation of IFNβ signaling |
|
| ||||
| CANDLE | PSMB8 | β5i |
|
Defects in proteasome formation, also associated with upregulation of type I IFN |
| PSMB4 | β7 | |||
| PSMA3 | α7 | |||
| PSMB9 | β1i | |||
| POMP | POMP | |||
|
| ||||
| NLRC4-related autoinflammatory syndromes | NLRC4 | NLRC4 |
|
Gain-of-function in NLRC4 leads to abnormal activation of NLRC4 inflammasome, resulting in aberrant production of IL-1β and IL-18, and dysregulation of pyroptotic cell death |
THE DEUBIQUITINASE DEFICIENCIES
NF-κB denotes a group of transcription factors that regulate the expression of genes involved in the cell cycle, immune response, differentiation, and DNA repair. This signaling pathway is in part regulated by ubiquitination, a protein post-transcriptional modification process2,3. The deubiquitinases (DUBs) are a group of enzymes that specifically remove ubiquitin (Ub) moieties from target proteins, and their dysregulation has been reported to result in various human diseases4. Several DUBs, including A20, CYLD, OTULIN, and OTUD7B (Cezanne), act as negative regulators of NF-κB signaling2. Prior to 2016, CYLD was the only DUB for which germline mutations had been implicated in a Mendelian human disease5.
Haploinsufficiency of A20 (HA20)
A20 is a DUB that plays a key inhibitory role in the NF-κB proinflammatory pathway. The inhibitory function of A20 is coordinately effected by its N-terminal ovarian tumor (OTU) domain-mediated DUB activity and by its C-terminal zinc finger-mediated E3 ubiquitin ligase activity. Thus, A20 removes lysine 63 (K63)-linked Ub chains from proinflammatory signaling complexes, leading to their disassembly, and then conjugates the constituent proteins with lysine 48 (K48)-linked Ub chains, marking them for proteasomal degradation. Hence, the net effect of A20 is anti-inflammatory, and a deficiency of A20 would be predicted to cause unchecked inflammation.
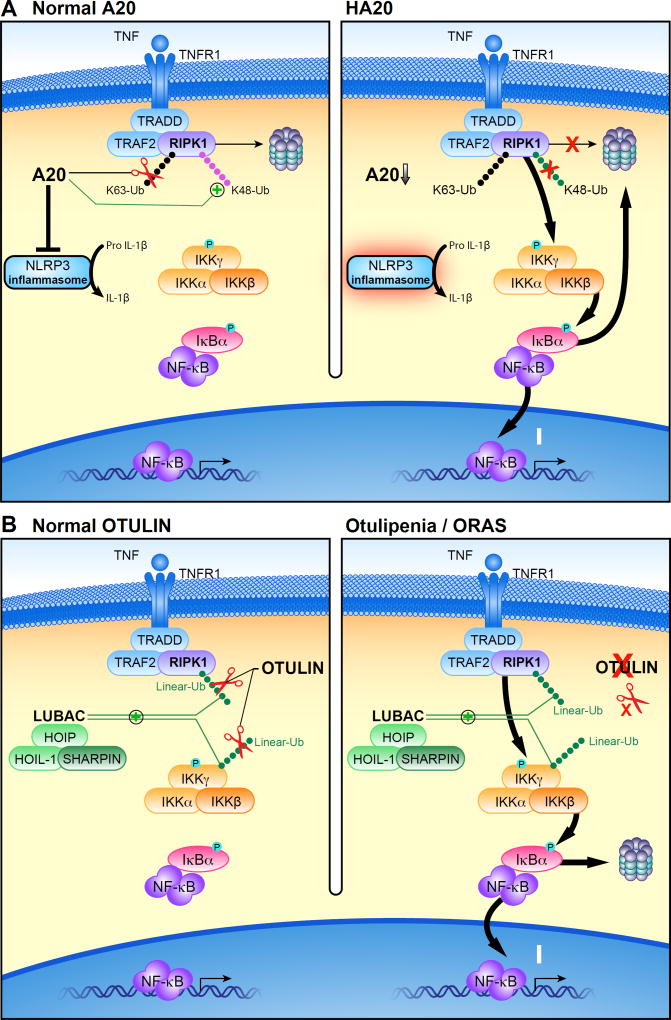
In 2016, Zhou et al reported six families with dominantly inherited truncating mutations in the TNFAIP3 gene, which encodes A206. Clinical manifestations included early-onset fevers, arthralgia, oral and genital ulcers, and ocular inflammation, in some cases resembling Behçet's disease. Five of the mutations were in the OTU domain, while one was in the zinc finger domain. Mutant A20 demonstrated no inhibitory effect on the NF-κB pathway, while a mixture of wild type and mutant A20 had substantial inhibitory activity, suggesting that the mutant proteins are likely to act through haploinsufficiency rather than a dominant-negative effect. In vitro reconstitution experiments showed accumulation of K63-Ub on RIPK1, one of the A20 substrates, an effect that was also confirmed in patients' cells. Patients' peripheral blood mononuclear cells (PBMCs) and fibroblasts also demonstrated strong phosphorylation of IκBα, IKKα/β, and p38 with and without TNF stimulation, consistent with constitutive NF-κB activity. Spontaneous NLRP3 inflammasome activation leading to IL-1β release was observed in PBMCs, and one of the patients showed a good clinical response to IL-1β inhibition, consistent with previous reports suggesting the role of A20 as a negative regulator of the NLRP3 inflammasome in mice7,8. Zhou et al dubbed this novel Mendelian disease haploinsufficiency of A20 (HA20) (Figure 1A).
Figure 1. Proposed mechanisms of pathogenesis in deubiquitinase deficiencies.
(A) After binding TNF, a signaling complex is recruited to the TNF receptor. The addition of lysine 63 (K63)-linked ubiquitin (Ub) chains to RIPK1 stabilizes its signaling complex and leads to phosphorylation of the IKK complex, degradation of IκBα, and translocation of the NF-κB heterodimer into the nucleus. Under normal conditions (left), A20 removes K63-linked Ub chains from the RIPK1 complex and instead conjugates its constituent proteins with lysine 48 (K48)-linked Ub chains to mark them for proteasomal degradation. In haploinsufficiency of A20 (HA20), heterozygous loss of function of A20 results in the impaired suppressive function of A20, leading to excessive activation of NFκB signaling (right).
(B) The linear ubiquitin assembly complex (LUBAC) conjugates methionine 1-linked ubiquitin (linear-Ub) to targets including NEMO and RIPK1, which potentiates NF-κB signaling. OTULIN restricts this signaling by hydrolyzing linear-Ub on LUBAC and its target proteins (left). In otulipenia/OTULIN-related autoinflammatory syndrome (ORAS), biallelic loss of function of OTULIN results in loss of this suppressive function, leading to accentuated activation of NF-κB signaling (right). For the sake of simplicity, ubiquitination on TRAF2 and TNFR1 are not shown in these figures.
Recent studies suggest an essential role of A20 in the development and appropriate regulation of immune cells, and its dysregulation has been linked to various human diseases. Common nucleotide variants in TNFAIP3 have been associated with multiple autoimmune diseases, including systemic lupus erythematosus (SLE), type I diabetes, inflammatory bowel disease, ankylosing arthritis, Sjögren's syndrome, and rheumatoid arthritis9. Furthermore, somatic loss-of-function mutations in A20 have been described in B cell lymphoma, which suggests its role as a tumor-suppressor gene10. Complete loss of A20 in mice (Tnfaip3−/−) resulted in early lethality due to persistent NF-κB activation and severe multiorgan inflammation11, whereas immune-cell specific ablation resulted in autoimmunity, such as SLE12. One of the reported HA20 cases initially carried the diagnosis of SLE6, and recently a new case of HA20 from Japan was reported to have autoimmune lymphoproliferative syndrome (ALPS)13. Taken together, these reports underscore the importance of A20 in immune regulation, and suggest that further study is needed to define the full clinical spectrum of HA20.
Otulipenia/Otulin-Related Autoinflammatory Syndrome (ORAS)
Linear (or methionine 1-linked) ubiquitination is catalyzed by the linear ubiquitin assembly complex (LUBAC)14,15, consisting of HOIL-1, HOIP, and SHARPIN. LUBAC plays a critical role in the activation of NF-κB signaling by ligating linear-Ub to its target proteins, which include NEMO and RIPK1. OTULIN (FAM105B) is a DUB that, as a cysteine protease, exclusively hydrolyzes linear-Ub, prevents baseline accumulation of linear-Ub on LUBAC components, and restricts ubiquitination of LUBAC target proteins (Figure 1B)16,17,18. OTULIN has an N-terminal PUB-interacting site through which it interacts with HOIP19, and a C-terminal OTU domain that mediates its DUB activity.
Zhou et al and Damgaard et al independently reported that homozygous mutations of OTULIN cause a novel systemic autoinflammatory disorder20,21. These patients were characterized by neonatal-onset fever, neutrophilic dermatosis, panniculitis, lipodystrophy, joint swelling, diarrhea, and failure to thrive. Due to their dermatologic findings, two patients were initially diagnosed as chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE). Two of the mutations were missense substitutions that were predicted to be damaging and the other was a frameshift mutation. Indeed, protein expression of OTULIN was severely reduced in all the patients, suggesting the instability of the mutant proteins. Zhou and colleagues further demonstrated that patients’ cells showed enhanced phosphorylation of IκBα and linear-Ub accumulation on NEMO after cytokine stimulation. Overproduction of inflammatory cytokines including TNF, IL-1β, IL-6, IL-17, IL-18 and IFNγ was detected in patients’ serum samples. Anti-TNF therapy was effective in controlling disease activity as well as suppressing inflammatory markers in the blood.
OTULIN-null mice (gumby/gumby) are embryonic lethal due to defects in angiogenesis18. Damgaard et al generated mice with an inducible system of immune cell-specific OTULIN ablation. Strikingly, the immune cell-specific OTULIN ablation resulted in rapid weight loss and systemic inflammation, which was completely reversed by anti-TNF treatment. These authors further tested the effects of lineage-specific OTULIN deletion in multiple cell types. Only OTULIN disruption in the myeloid cell lineage led to inflammatory phenotypes, which included enlargement of lymphoid organs, immune cell infiltration in the liver, and the elevation of serum inflammatory cytokines. T- or B- cell specific OTULIN ablation did not result in an overt phenotype. However, HOIP and SHARPIN expression levels were strongly reduced, presumably due to protein destabilization, which suggests a possible unexpected mechanism of lineage-specific LUBAC regulation.
While loss of OTULIN leads to excessive inflammation through accumulation of linear-Ub, germline deficiencies of LUBAC also result in autoinflammation. Boisson et al reported three RBCK1 (HOIL-1) deficient and one RNF31 (HOIP) deficient patients, characterized by immunodeficiency, rash, gastrointestinal manifestations, myopathy, and systemic autoinflammation22,23. The patients' fibroblasts showed less linear-Ub accumulation and reduced NF-κB signaling, relative to controls, while patients' monocytes were hyperactive upon IL-1β stimulation. Furthermore, loss of SHARPIN in mice is responsible for the paradoxical phenotypes of the chronic proliferative dermatitis mouse (cpdm), in which systemic multiorgan inflammation coexists with immunodeficiency24,25,26. These reports underscore the importance of linear ubiquitination in both pro- and anti-inflammatory pathways.
DEFICIENCY OF ADA2 (DADA2)
In 2014 Zhou et al27 and Navon-Elkan et al28 identified biallelic loss-of-function CECR1 mutations in patients presenting with fevers and early-onset strokes and/or with vasculitis resembling polyarteritis nodosa (PAN), a systemic necrotizing vasculitis typically affecting medium-sized muscular arteries29. Zhou and her colleagues reported nine patients with fevers, early-onset (< 5 years old) lacunar strokes, livedoid rash, hepatosplenomegaly, cytopenia, and systemic vasculopathy, including two patients with PAN and one with small-vessel vasculitis. Eight of the 9 patients had histories of lacunar strokes mainly affecting the deep-brain nuclei and the brain stem. Several strokes were hemorrhagic, or underwent hemorrhagic transformation, leading to long-term disability. Most of the strokes occurred during episodes of systemic inflammation. These patients also presented with various sequelae of systemic vascular disease, including livedo racemosa, myositis, portal hypertension, and ophthalmologic complications. Indeed, biopsy samples from skin, liver, and brain exhibited vasculopathic changes, including impaired endothelial integrity, endothelial cellular activation, and inflammation. Four patients had hypogammaglobulinemia, and two of them had multiple episodes of bacterial and viral infections.
Simultaneously and independently, Navon Elkan and her colleagues reported 24 patients with PAN with biallelic CECR1 mutations, 19 of whom were of Georgian Jewish ancestry. Among them, 18 presented with childhood-onset PAN (<10 years old), including 6 who received the diagnosis during infancy (< 1 year old). Most of the patients had cutaneous involvement, most commonly livedo racemosa. Five patients had episodes of either strokes or intracranial hemorrhage, whereas 10 had signs of peripheral neuropathy. Aneurysm formation in visceral arteries was observed in 6 patients, with associated renal hypertension and gastrointestinal manifestations.
CECR1 encodes adenosine deaminase 2 (ADA2), which can convert adenosine to inosine and 2'-deoxyadenosine to 2'-deoxyinosine30. Although ADA2 has partial structural homology with ADA (ADA1), the deficiency of which causes human severe combined immunodeficiency (SCID) through the intracellular accumulation of toxic nucleotides, these two enzymes differ in many aspects30. Whereas ADA1 acts as a monomer and is primarily localized intracellularly, ADA2 acts as a dimer and is secreted into the extracellular space. The patients described in both reports had a marked reduction of ADA2 protein concentrations and ADA2-specific enzymatic activity in the blood, suggesting that the detected mutations were loss-of-function. In the structural analysis, the missense mutations were predicted to affect the catalytic and dimerization domains or protein stability. Knock-down of cecr1b in zebrafish embryos resulted in intracranial hemorrhages and neutropenia, which were rescued by co-injection with human wild-type CECR1 (but not mutant CECR1), establishing the pathogenicity of the mutations. Zhou and her colleagues therefore proposed the term deficiency of adenosine deaminase 2 (DADA2) to denote the human disease.
ADA2 is expressed in the myeloid lineage and, once secreted, it induces differentiation of monocytes into macrophages, possibly by binding proteoglycan-like structures on the cellular surface31. Indeed, DADA2 patient monocytes showed impaired differentiation toward the anti-inflammatory (M2) macrophage population under standard culture conditions, thus leading to polarized differentiation toward the pro-inflammatory (M1) macrophage subset27. A recent transcriptome-wide analysis utilizing DADA2 patients' blood samples displayed a strong upregulation of neutrophil-related genes as well as a moderate interferon signature, and the authors also reported the accumulation of myeloperoxidase in patients' polymorphonuclear cells32. Zhou et al further reported that the DADA2 patients' brain and skin samples showed substantial endothelial activation and damage, and upregulation of inflammatory cytokines27. Co-culture of patients' monocytes with a human primary endothelial cell layer led to considerable disruption of its integrity. These studies suggest that the deficiency of ADA2 results in vascular damage at least in part mediated by skewed monocytic differentiation and neutrophil activation.
It is noteworthy that all of the Georgian Jewish patients in the report from Navon Elkan were homozygous for a mutation encoding a p.Gly47Arg substitution. The carrier frequency of this mutation in the endogamous Georgian Jewish population was 0.102, which is consistent with the apparently high prevalence of this disease in this population. Conserved haplotypes were detected around several missense mutations including p.Gly47Arg, suggesting the existence of a possible founder effect27. Interestingly, a heterozygous p.Tyr453Cys CECR1 mutation was identified in two siblings with late-onset lacunar strokes in the Siblings with Ischemic Stroke Study (SWISS)33,27. Although no GWAS loci for stroke have been identified in the CECR1 gene region, the effect of this gene on non-Mendelian cases of stroke and other vascular diseases should be further pursued.
Recent reports have broadened the clinical spectrum of DADA2 beyond the typical clinical picture of systemic inflammation presented in the first papers. CECR1 mutations have been demonstrated in patients with autoimmunity, lymphoproliferation, and a combined immunodeficiency (CID)34, as well as patients presenting primarily with common variable immunodeficiency (CVID)35. The lymphoproliferative picture is shared by a mutation-positive patient who was diagnosed with Castleman's disease and responded to anti IL-6 treatment36. Biallelic CECR1 mutations have also been found in patients presenting with anemia, thrombocytopenia, and splenomegaly, leading to the initial clinical diagnosis of Diamond-Blackfan anemia or storage disease37. Recently a biallelic 770-kb deletion of chromosome 22q11.1 encompassing both CECR1 and IL17RA (encoding the IL-17 receptor A) was reported in two siblings with a history of both mucocutaneous infection and early-onset systemic vasculitis38. This finding, taken together with the 28-kb deletion in the CECR1 locus reported by Zhou et al27, indicates the importance of structural genomic analysis in the genetic diagnosis of DADA2.
Given the specter of stroke, vasculitis, and the other possible manifestations of DADA2, treatment strategies have been the subject of intense investigation. In many cases, corticosteroids, methotrexate, cyclophosphamide, azathioprine, and IL-1 inhibitors have been ineffective. Navon Elkan et al reported the use of anti-TNF agents in ten DADA2 vasculitis patients, among which eight demonstrated a complete response28. This strategy has been further supported by a recent report from Ombrello and colleagues, demonstrating that anti-TNF treatment has completely prevented the recurrence of strokes in 15 DADA2 patients with a previous history of stroke39. Hematopoietic stem cell transplantation (HSCT) is another possible therapeutic strategy, because the pathogenesis of DADA2 appears to derive mainly from the myeloid cell lineage. Indeed, the effectiveness of HSCT was initially reported by Van Eyck et al40, followed by a number of papers41,37, in which HSCT normalized the plasma level of ADA2 and suppressed disease manifestations. It will be important to establish the risk-benefit ratios for anti-TNF, HSCT, and other potential therapies in various clinical settings across the widening spectrum of DADA2.
Lastly, although the zebrafish data of Zhou et al clearly establish the importance of CECR1 in vascular and myeloid development, the field has been hampered by the lack of an obvious murine CECR1 orthologue that would permit the development of a mouse model. Whether through the development of alternative animal models or through the more detailed study of leukocyte and endothelial biology in DADA2 patients, there is still much to be learned about the basic biology of this disease.
TYPE I INTERFERONOPATHIES
Type I interferons (mainly IFN-α and -β) are a group of cytokines that play an important role in host defense. Binding of type I IFN to the IFN-α/ β receptor (IFNAR) induces the transcription of hundreds of genes called IFN-stimulated genes (ISGs) by activating the JAK-STAT signaling pathway. The overproduction of type I IFN leads to a group of Mendelian inherited diseases called type I interferonopathies. For example, Aicardi-Goutières disease derives from either loss-of-function mutations in cytoplasmic nucleic acid metabolism or gain-of-function mutations in pattern recognition receptors such as MDA5 (encoded by the IFIH1 gene), leading to severe encephalopathy and systemic autoimmunity resembling SLE (see article by Hiraki and Silverman in this issue for further discussion of Aicardi- Goutières disease). Upregulation of ISGs in the peripheral blood ("IFN-signature") was proposed as a screening method for interferonopathy, analagous to the upregulation of type I IFN signaling in SLE patients42,43,44.
STING-Associated Vasculopathy with Onset in Infancy (SAVI)
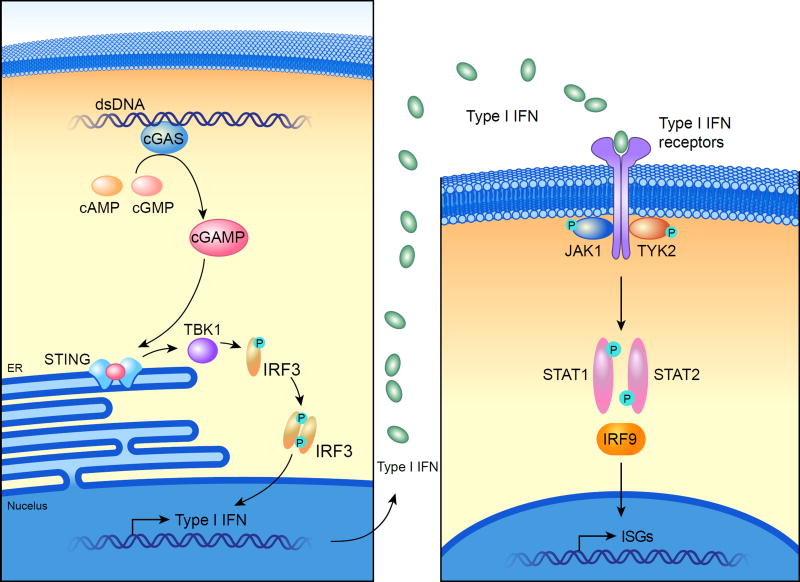
Recently the cGAS-cGAMP-STING pathway was identified as the mechanism for the detection of cytoplasmic double-stranded DNA (dsDNA)45. Upon recognition and binding of cytoplasmic dsDNA, cyclic GMP-AMP synthase (cGAS) synthesizes cyclic GMP-AMP (cGAMP) as a second messenger, which then activates the stimulator of interferon genes (STING), an endoplasmic reticulum (ER)-resident adapter molecule. cGAMP induces the translocation of STING from the ER to the ER-Golgi intermediate compartment (ERGIC) to recruit TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3), resulting in upregulation of type I IFN transcription (Figure 2).
Figure 2. The STING-type I interferon (IFN) pathway.
Cytosolic sensing of double stranded DNA (dsDNA) by cyclic GMP-AMP synthase (cGAS) leads to the production of cyclic GMP-AMP (cGAMP) as a second messenger, which then activates the stimulator of interferon genes (STING) in the endoplasmic reticulum (ER) and further upregulates type I interferon (IFN) transcription via the recruitment of TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3). The binding of type I IFN molecules to their receptors initiates the transcription of hundreds of IFN-stimulated genes (ISGs) via the activation of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway.
In 2014 Liu et al identified de novo germline or somatic mosaic gain-of-function mutations in TMEM173, encoding STING, in six patients with early-onset autoinflammatory disorders manifesting dermatologic and pulmonary involvement46. Skin lesions were seen in all six patients, with telangiectatic, pustular, or blistering rashes and gangrenous lesions, the latter of which were exacerbated by cold exposure and even required surgical amputations. Microscopically these skin lesions were characterized by marked vascular inflammation around capillaries with fibrin and immune complex deposition. Concomitantly various degrees of interstitial lung disease were observed in five of six patients. Lung biopsies showed a scattered lymphocytic inflammatory infiltrate, interstitial fibrosis, and emphysematous changes. Strong IFN signatures as well as constitutive phosphorylation of STAT1 in patients' lymphocytes were observed, consistent with the constitutive activity of mutant STING in an in vitro assay. Stimulation of patients' fibroblasts with cGAMP induced further IFNB1 upregulation. Patients' vascular endothelial cell layers from lesional skin samples expressed markers of endothelial inflammation and activation. These authors proposed the term STING-associated vasculopathy with onset in infancy (SAVI) to denote this condition.
To date, more than 20 patients from multiple pedigrees have been reported with gain-of-function mutations in TMEM17346,47,48,49,50,51,52,53,54. Most of the mutations encode amino acid substitutions located close to the dimerization site of STING, and in fact two recombinant mutants (p.N154S and p.V155M) were reported to form a stable homodimer46. Dobbs et al recently demonstrated that disease-associated STING mutants trafficked to the ERGIC without cGAMP stimulation, and also that these active mutants were less susceptible to degradation, both of which could explain the constitutively active characteristics of STING mutants in SAVI55. Melki et al identified three novel missense mutations in STING (p.C206Y, p.R281Q, and p.R284G), all of which lie outside the linker region important for homo-dimerization54. The precise molecular mechanism of STING mutants in SAVI remains to be defined.
While therapies such as corticosteroids and DMARDs, or various inhibitors of TNF, IL-1, and IL-6, have been reported to elicit minimal clinical responses, accumulating evidence suggests the effectiveness of JAK inhibition in SAVI. Ex vivo treatment with any of three JAK inhibitors blocked the constitutive phosphorylation of STAT1 in a patient's B and CD4+ T cells46. Recently two papers reported the clinical efficacy of JAK inhibitors in SAVI52,56, with reduction in febrile episodes and almost complete resolution of dermatologic lesions. Early genetic diagnosis and treatment may prevent the eventual need for surgical amputations and the irreversible pulmonary damage of SAVI.
Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated Temperature (CANDLE)/Proteasome-Associated Autoinflammatory Syndrome (PRAAS)
Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE), characterized by fever, panniculitis, and brain calcification, is another form of type I interferonopathy with biallelic loss-of-function mutations in PSMB8, which encodes the inducible proteasome component β5i57,58,59,60. It is one of several clinical conditions that collectively are denoted the proteasome-associated autoinflammatory syndrome (PRAAS). Although a strong IFN signature in CANDLE patients suggested the association between proteasome dysfunction and IFN signaling, the direct molecular mechanism had not been well defined. Brehm et al reported eight patients with mutations in four proteasome genes, PSMA3 (encoding the α7 subunit), PSMB4 (encoding β7), PSMB9 (encoding β1i), and POMP (encoding the proteasome maturation protein), and also one novel mutation in PSMB861. Of note, six of the reported patients were compound heterozygous for two different genes described above, which suggested a "digenic inheritance" model in CANDLE. In addition, one patient was heterozygous for the POMP frameshift mutation, which likely causes haploinsufficiency, suggesting a novel autosomal-dominant mode of inheritance. The authors also demonstrated that proteasome inhibition in fibroblasts from healthy individuals, either by knockdown or pharmacological inhibition, resulted in strong upregulation of IFN related genes, which recapitulated the patients' IFN signature. A clinical trial of a JAK inhibitor for CANDLE is ongoing (NCT01724580), with promising preliminary results62.
NLRC4-RELATED AUTOINFLAMMATORY SYNDROMES
NLRC4, a member of the nucleotide-binding and oligomerization domain (NOD)-like receptor (NLR) family, assembles with NAIP and ASC to form an inflammasome complex upon the intracellular entry of ligands such as bacterial flagellin or components of the bacterial type 3 secretion system. As a result, the NLRC4 inflammasome mediates the autocatalysis of procaspase-1 to the enzymatically active caspase-1, which subsequently catalyzes the cleavage of pro-IL-1β and pro-IL-18 to their biologically active mature forms, and also induces a rapid lytic cell death called pyroptosis.
In 2014 Canna el al and Romberg et al independently reported that heterozygous gain-of-function mutations of NLRC4 (p.T337S and p.V341A, respectively) caused an autoinflammatory disease. The phenotypes of these patients were characterized by early-onset multiple fever episodes, enterocolitis, splenomegaly, macrophage activation syndrome (MAS)-like flares, and persistent serum IL-18 elevation63,64. In both reports, in vitro overexpression of mutant NLRC4 protein led to increased cleavage of caspase-1, and patients' macrophages exhibited increased production of IL-1β and IL-18, more frequent formation of ASC specks, and increased susceptibility to cell death, suggesting that these mutations result in constitutive activation of the NLRC4 inflammasome.
Concurrently, Kitamura et al reported that another mutation in NLRC4 (p.H443P) caused a somewhat milder phenotype with cold-induced fever and urticaria, which resembled familial cold autoinflammatory syndrome (FCAS)65. Transgenic mice expressing this mutant NLRC4 allele developed severe inflammatory infiltrates in the skin and joints, erosion of bones, splenomegaly, and elevated levels of IL-1β, IL-17A, and G-CSF in the blood. Hypercytokinemia could be induced by adoptive transfer of bone marrow from mutant mice into wild type recipients. Cold exposure of mutant mice augmented the cutaneous inflammation, which recapitulated the patients' phenotype.
Recently, a fourth mutation of NLRC4 (p.T177A) was reported in a patient with periodic fever, rash, arthralgia, sensorineural hearing loss, chronic meningitis, and cerebral atrophy, all of which are consistent with neonatal onset multisystem inflammatory disease (NOMID)66. In this paper, multiple iPSC-derived monocytic lineage cells from the patient showed a differential pattern of inflammasome activation, and whole exome sequencing between these normal and inflammatory groups of iPSCs identified the NLRC4 p.T177A mutation, suggesting that the patient had somatic mosaicism of this NLRC4 mutation.
A structural study showed that ADP-bound NLRC4 is in an autoinhibited state that is achieved by an ADP-mediated intramolecular interaction67. Disruption of this interaction by ligand binding may facilitate conformational changes and attenuate ADP binding, both of which could result in NLRC4 inflammasome activation. It is noteworthy that all of the mutations identified in human NLRC4-related autoinflammatory syndromes are in close proximity to the ADP-binding site and that p.Thr177 and p.His443 individually form a hydrogen bond to the ADP molecule. This clustering of disease-causing mutations in the nucleotide-binding domain (NBD), as well as the phenotypic variability and the presence of somatic mosaicism discussed above, all suggest the similarities between NLRC4-related autoinflammatory syndromes and cryopyrin-associated periodic syndrome (CAPS) caused by NLRP3 mutations.
Hemophagocytic disorders such as MAS and hemophagocytic lymphohistiocytosis (HLH) are life-threatening sepsis-like conditions notable for acute cytopenias, hyperferritinemia, coagulopathy, and multiple organ damage, and if not promptly treated, they are associated with high mortality rates. In addition to NLRC4-MAS, several conditions such as familial HLH and systemic onset juvenile idiopathic arthritis (sJIA) are predisposed to MAS. During the flares, serum total IL-18 as well as free IL-18 are extraordinary high, although its role in the pathogenesis of MAS/HLH remains controversial68. Recently administration of recombinant human IL-18 binding protein successfully rescued a patient with NLRC4-MAS, who did not respond to a combination of immunosuppressive drugs, anti-IL-1, anti-TNF, and α4β7 integrin inhibitory therapies69. This not only substantiates a proinflammatory role for IL-18 in MAS, but also raises a possible new treatment option for various MAS/HLH-inducing clinical conditions.
RECENT ADVANCES IN FMF AND THE PYRIN INFLAMMASOME
FMF is the longest-recognized and one of the most extensively studied hereditary autoinflammatory disorders. It is characterized by recurrent 1–3 day febrile attacks accompanied by serositis, synovitis, and/or cutaneous inflammation. Two independent consortia identified MEFV, the gene mutated in FMF, by positional cloning in 199770,71. Since that time, the mechanism by which mutations in pyrin, the encoded protein, cause FMF has been the topic of intense investigation. Although at first FMF was hypothesized to be an autosomal recessive disease deriving from biallelic loss-of-function mutations mainly in exon 10 of MEFV (encoding the B30.2 domain of pyrin), subsequent reports of 30–40% of FMF patients carrying only one mutated allele of MEFV, coupled with the conspicuous absence of null mutations, raised the possibility that FMF is caused by gain-of-function mutations in MEFV.
Human pyrin consists of an N-terminal PYRIN (PYD) domain, a B-box zinc-finger domain, a coiled-coil domain, and a C-terminal ~200 amino acid B30.2 domain (also known as the rfp/PRY/SPRY domain). As noted above, the majority of FMF-causing mutations are located in the B30.2 domain72. The PYD domain interacts with an adapter protein, apoptosis-associated speck-like protein with a caspase recruitment domain (ASC), through a homotypic PYD-PYD interaction73. Chae et al reported in 2011 that homozygous knockin mice harboring any one of three human B30.2 domains with an FMF mutation showed constitutive activation of caspase-1 in macrophages and IL-1β secretion after lipopolysaccharide (LPS) stimulation (without a second signal), which was completely dependent on ASC and caspase-1 but not on NLRP374. This paper also demonstrated that this prominent inflammatory phenotype was not induced by knockout of the murine Mefv gene, and crosses between knockin and knockout mice supported the hypothesis that FMF-associated mutations are gain-of-function with a gene-dosage effect.
In 2014 Xu et al published a breakthrough paper that shed considerable light on the mechanism by which pyrin contributes to host defense75. These authors demonstrated that pyrin inflammasome activation is induced by modifications of host RhoA by certain bacterial toxins. RhoA inactivation is a shared mechanism of bacterial virulence that prevents the reorganization of the host actin cytoskeleton and thus inhibits leukocyte migration, phagocytosis, and degranulation. These pyrin inflammasome-activating modifications of RhoA include monoglucosylation, adenylation, ADP-ribosylation, and deamidation, occurring on different residues. Thus, it was hypothesized that pyrin would recognize these RhoA modifications by sensing a downstream effect, although the molecular details were at the time unknown.
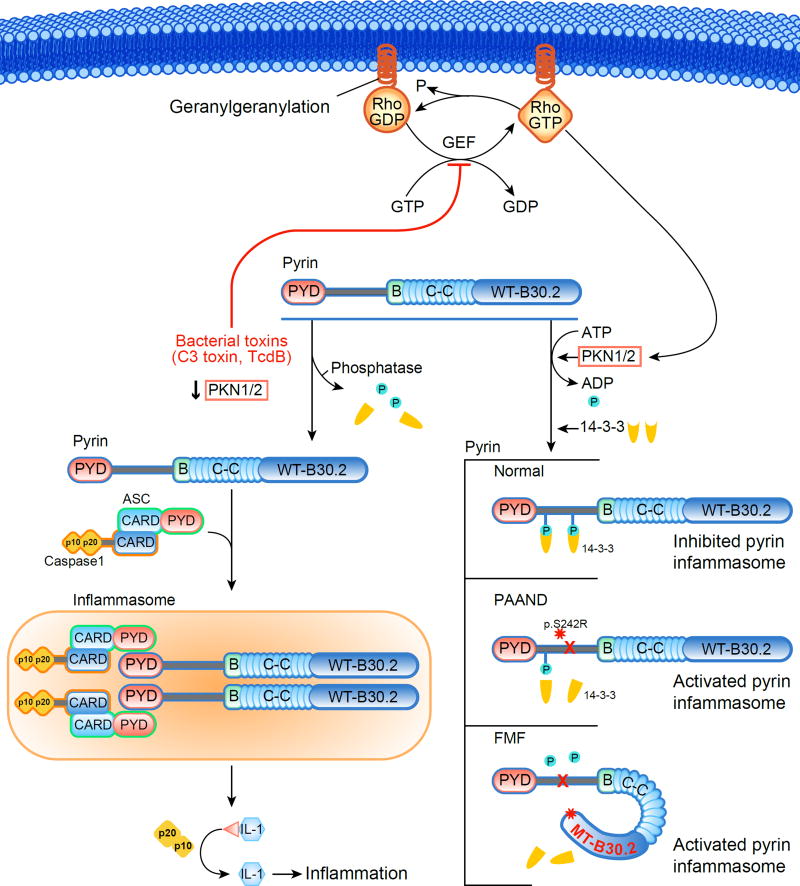
In 2016 Park and colleagues elucidated the molecular mechanism by which RhoA inactivation leads to pyrin inflammasome activation (Figure 3)76. Under physiological conditions RhoA induces the phosphorylation of pyrin on its residues p.Ser208 and p.Ser242 through two effector kinases, PKN1 and PKN2. Pyrin phosphorylation on these residues permits its interaction with 14-3-3, a regulatory protein, that in turn inhibits pyrin inflammasome formation. Of note, the binding of 14-3-3 and PKN1/2 to FMF-associated mutant pyrin is substantially reduced, and the constitutive activation of the pyrin inflammasome in FMF knockin mice as well as FMF patient cells are inhibited by pharmacological PKN activation. Bacterial inactivation of RhoA diminishes PKN activity and thus favors the dephosphorylated form of pyrin that can assemble an inflammasome. This paradigm, sometimes termed the ‘guard mechanism’76, by which microbial products activate innate immunity through their indirect biologic effects on the cell, is common among plants, but the pyrin inflammasome represents the first documented example of the guard mechanism in the animal kingdom.
Figure 3. Mechanism of pyrin inflammasome regulation and the diseases caused by pyrin dysregulation.
In the resting state Rho family guanosine triphosphate hydrolases (GTPases) are activated by guanine nucleotide exchange factor (GEF). This in turn activates protein kinase N (PKN) 1/2-mediated inactivation of pyrin through its phosphorylation and the recruitment of 14-3-3, a regulatory protein. During certain bacterial infections, RhoA inactivation by bacterial toxins leads to the downstream activation of the pyrin inflammasome by preventing the activation of PKN 1/2, with the resultant dephosphorylation of pyrin and dissociation of 14-3-3. In pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND) and familial Mediterranean fever (FMF), mutations of pyrin diminish the binding of 14-3-3 to pyrin and lead to spontaneous or more easily triggered activation of the pyrin inflammasome.
The importance of pyrin p.Ser242 phosphorylation was further highlighted by the recent identification of a novel pyrin-related disorder distinct from FMF, called pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND). In 2016 Masters et al reported the heterozygous p.Ser242Arg mutation in a group of patients with neutrophilic dermatosis (severe acne, sterile skin abscess, and pyoderma gangrenosum), recurrent long-lasting fever episodes, arthralgia, and myalgia/myositis, associated with the upregulation of systemic inflammatory markers77. The prominent dermatologic manifestations of these patients resemble the cutaneous features of pyogenic arthritis with pyoderma gangrenosum and acne (PAPA) syndrome, which is caused by mutations in PSTPIP1, an interactor of pyrin. The p.Ser242Arg mutation of pyrin dramatically reduced its affinity to 14-3-3, and resulted in constitutive activation of the pyrin inflammasome.
Quite unexpectedly, Park's report above also demonstrated a role for the pyrin inflammasome in the pathophysiology of another AID, mevalonate kinase deficiency (also known as hyper-IgD syndrome, or HIDS). HIDS is caused by recessive loss-of-function mutations in MVK, encoding mevalonate kinase, an enzyme that is important both in cholesterol biosynthesis and in the synthesis of nonsterol isoprenes, such as geranylgeranyl pyrophosphate. Pharmacological inhibition of cholesterol synthesis by statins, which mimics the molecular pathogenesis of HIDS, had been shown to induce IL-1β release, but the molecular mechanism was unknown78,79. Reasoning that geranylgeranylation of RhoA is important for its trafficking to the plasma membrane80, and that the impairment of RhoA geranylgeranylation would inhibit its activity and thus result in pyrin inflammasome activation, Park et al showed that statin treatment dissociates RhoA from the plasma membrane to the cytosol, resulting in IL-1β production. This IL-1β production is blocked by supplementation of geranylgeranyl pyrophosphate or pharmacological activation of PKNs, is dependent only on the pyrin inflammasome (but not on NLRP3, NLRC4, or AIM2), and is associated with decreased binding of pyrin to 14-3-3 proteins.
Finally, these recent genetic and functional data further clarify the pharmacological mechanism of colchicine, a medication used for rheumatic diseases (such as Behçet's disease) and is also one of the most commonly used and effective treatments for FMF81,82. Colchicine is known to enhance the activity of RhoA by depolymerizing microtubules and thus releasing guanine nucleotide exchange factor (GEF)-H1, an RhoA activator83. Park et al demonstrated76 that colchicine reverses the effect of RhoA inhibition by C3 toxin from Clostridium botulinum, and furthermore, suppresses the spontaneous activation of the pyrin inflammasome in FMF knockin mice and in FMF patients’ cells, but not in the cells from CAPS patients. In addition, Feng Shao and colleagues84 demonstrated that multiple microtubule-targeting drugs including colchicine, vinblastine, and paclitaxel inhibit pyrin inflammasome-mediated ASC aggregation. These data are consistent with the clinical response of FMF patients to colchicine treatment and the exquisite specificity of colchicine for FMF among the hereditary periodic fever syndromes.
SUMMARY
Recent genomic technologies including next generation sequencing (NGS) have substantially contributed to the advancement of the field of AID by catalyzing the discovery of new causative genes and pathways in AID patients. Some of these patients did not present with classical symptoms of AID, underscoring the heterogeneity of the clinical manifestations of AID. Also, the wide spectrum of clinical symptoms deriving from mutations in a single gene as exemplified by DADA2 highlights the importance of further clinical studies and the elucidation of pathogenic mechanisms. Conversely, the broad range of effects of A20 mutations from low to high penetrance and even somatic changes exemplifies the importance of comprehensive genetic approaches. The success of novel targeted therapies for previously uncontrollable AID patients emphasizes the importance of promoting translational research in this field. Exciting challenges remain to deliver rapid and accurate genetic diagnosis and effective treatments to currently undiagnosed AID patients.
KEY POINTS.
Two deubiquitinase deficiencies, haploinsufficiency of A20 (HA20) and otulipenia, derive from the impairment of the negative regulation in NF-κB signaling.
Deficiency of ADA2 (DADA2) results in clinical manifestations including recurrent lacunar strokes, polyarteritis nodosa-like vasculitis, hypogammaglobulinemia, Diamond-Blackfan anemia, and bone marrow failure.
STING-associated vasculopathy with onset in infancy (SAVI) is characterized by severe dermatologic and pulmonary lesions.
Clinical features of NLRC4-related autoinflammatory syndromes vary from cold-induced fever to chronic central nervous system inflammation or macrophage activation syndrome.
RhoA GTPase suppresses the pyrin inflammasome by stimulating pyrin phosphorylation, which in turn favors the binding of inhibitory 14-3-3 proteins to pyrin. Certain bacterial toxins inactivate RhoA and thereby derepress the pyrin inflammasome. Mutations in MEFV and MVK predispose to autoinflammatory disease by decreasing 14-3-3 interaction with pyrin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Disclose any relationship with a commercial company that has a direct financial interest in subject matter or materials discussed in article or with a company making a competing product. If nothing to disclose, please state “The Authors have nothing to disclose.”
References
- 1.McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97(1):133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 2.Zinngrebe J, Montinaro A, Peltzer N, Walczak H. Ubiquitin in the immune system. EMBO Rep. 2014;15(1):28–45. doi: 10.1002/embr.201338025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panday A, Inda ME, Bagam P, Sahoo MK, Osorio D, Batra S. Transcription Factor NF-kappaB: An Update on Intervention Strategies. Arch Immunol Ther Exp (Warsz) 2016;64(6):463–483. doi: 10.1007/s00005-016-0405-y. [DOI] [PubMed] [Google Scholar]
- 4.Hanpude P, Bhattacharya S, Dey AK, Maiti TK. Deubiquitinating enzymes in cellular signaling and disease regulation. IUBMB Life. 2015;67(7):544–555. doi: 10.1002/iub.1402. [DOI] [PubMed] [Google Scholar]
- 5.Rajan N, Ashworth A. Inherited cylindromas: lessons from a rare tumour. Lancet Oncol. 2015;16(9):e460–469. doi: 10.1016/S1470-2045(15)00245-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Wang H, Schwartz DM, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 2016;48(1):67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vande Walle L, Van Opdenbosch N, Jacques P, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512(7512):69–73. doi: 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong BH, Onizawa M, Oses-Prieto JA, et al. A20 restricts ubiquitination of pro-interleukin-1beta protein complexes and suppresses NLRP3 inflammasome activity. Immunity. 2015;42(1):55–67. doi: 10.1016/j.immuni.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Peng LL, Wang Y, et al. Roles of A20 in autoimmune diseases. Immunol Res. 2016;64(2):337–344. doi: 10.1007/s12026-015-8677-6. [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Sanada M, Kato I, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459(7247):712–716. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- 11.Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12(11):774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takagi M, Ogata S, Ueno H, et al. Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki K, Iwai K. Roles of linear ubiquitinylation, a crucial regulator of NF-kappaB and cell death, in the immune system. Immunol Rev. 2015;266(1):175–189. doi: 10.1111/imr.12308. [DOI] [PubMed] [Google Scholar]
- 15.Kirisako T, Kamei K, Murata S, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25(20):4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keusekotten K, Elliott PR, Glockner L, et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell. 2013;153(6):1312–1326. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fill BK, Damgaard RB, Wagner SA, et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol Cell. 2013;50(6):818–830. doi: 10.1016/j.molcel.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivkin E, Almeida SM, Ceccarelli DF, et al. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature. 2013;498(7454):318–324. doi: 10.1038/nature12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaeffer V, Akutsu M, Olma MH, Gomes LC, Kawasaki M, Dikic I. Binding of OTULIN to the PUB domain of HOIP controls NF-kappaB signaling. Mol Cell. 2014;54(3):349–361. doi: 10.1016/j.molcel.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Yu X, Demirkaya E, et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc Natl Acad Sci U S A. 2016;113(36):10127–10132. doi: 10.1073/pnas.1612594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damgaard RB, Walker JA, Marco-Casanova P, et al. The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity. Cell. 2016;166(5):1215–1230. e1220. doi: 10.1016/j.cell.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisson B, Laplantine E, Prando C, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol. 2012;13(12):1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boisson B, Laplantine E, Dobbs K, et al. Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J Exp Med. 2015;212(6):939–951. doi: 10.1084/jem.20141130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokunaga F, Nakagawa T, Nakahara M, et al. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471(7340):633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda F, Deribe YL, Skanland SS, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471(7340):637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerlach B, Cordier SM, Schmukle AC, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, Yang D, Ombrello AK, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–920. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navon Elkan P, Pierce SB, Segel R, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370(10):921–931. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- 29.Forbess L, Bannykh S. Polyarteritis nodosa. Rheum Dis Clin North Am. 2015;41(1):33–46. doi: 10.1016/j.rdc.2014.09.005. vii. [DOI] [PubMed] [Google Scholar]
- 30.Zavialov AV, Engstrom A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J. 2005;391(Pt 1):51–57. doi: 10.1042/BJ20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zavialov AV, Yu X, Spillmann D, Lauvau G, Zavialov AV. Structural basis for the growth factor activity of human adenosine deaminase ADA2. J Biol Chem. 2010;285(16):12367–12377. doi: 10.1074/jbc.M109.083527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belot A, Wassmer E, Twilt M, et al. Mutations in CECR1 associated with a neutrophil signature in peripheral blood. Pediatr Rheumatol Online J. 2014;12:44. doi: 10.1186/1546-0096-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meschia JF, Brown RD, Jr, Brott TG, Chukwudelunzu FE, Hardy J, Rich SS. The Siblings With Ischemic Stroke Study (SWISS) protocol. BMC Med Genet. 2002;3:1. doi: 10.1186/1471-2350-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Eyck L, Jr, Hershfield MS, Pombal D, et al. Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase 2 deficiency. J Allergy Clin Immunol. 2015;135(1):283–287. e285. doi: 10.1016/j.jaci.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schepp J, Bulashevska A, Mannhardt-Laakmann W, et al. Deficiency of Adenosine Deaminase 2 Causes Antibody Deficiency. J Clin Immunol. 2016;36(3):179–186. doi: 10.1007/s10875-016-0245-x. [DOI] [PubMed] [Google Scholar]
- 36.Van Eyck L, Liston A, Wouters C. Mutant ADA2 in vasculopathies. N Engl J Med. 2014;371(5):480. doi: 10.1056/NEJMc1405506. [DOI] [PubMed] [Google Scholar]
- 37.Van Montfrans JM, Hartman EA, Braun KP, et al. Phenotypic variability in patients with ADA2 deficiency due to identical homozygous R169Q mutations. Rheumatology (Oxford) 2016;55(5):902–910. doi: 10.1093/rheumatology/kev439. [DOI] [PubMed] [Google Scholar]
- 38.Fellmann F, Angelini F, Wassenberg J, et al. IL-17 receptor A and adenosine deaminase 2 deficiency in siblings with recurrent infections and chronic inflammation. J Allergy Clin Immunol. 2016;137(4):1189–1196. e1181–1182. doi: 10.1016/j.jaci.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 39.Amanda K, Ombrello KB, Hoffmann Patrycja, Toro Camilo, Stone Deborah L, Pinto-Patarroyo Gineth, Jones Anne, Romeo Tina, Soldatos Ariane, Zhou Qing, Deuitch Natalie, Qin Jing, Aksentijevich Ivona, Kastner Daniel L. The Deficiency of Adenosine Deaminase Type 2 (DADA2)-Results of Anti-TNF Treatment in a Cohort of Patients with a History of Stroke. Paper presented at: 2016 ACR/ARHP Annual Meeting; September 28, 2016, 2016; Washington DC. [Google Scholar]
- 40.Van Eyck L, Liston A, Meyts I. Mutant ADA2 in vasculopathies. N Engl J Med. 2014;371(5):478–479. doi: 10.1056/NEJMc1405506. [DOI] [PubMed] [Google Scholar]
- 41.van Montfrans J, Zavialov A, Zhou Q. Mutant ADA2 in vasculopathies. N Engl J Med. 2014;371(5):478. doi: 10.1056/NEJMc1405506. [DOI] [PubMed] [Google Scholar]
- 42.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25(4):401–406. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- 43.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36(8):511–518. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- 44.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao J, Zhou X, Jiang Z. cGAS-cGAMP-STING: The three musketeers of cytosolic DNA sensing and signaling. IUBMB Life. 2016;68(11):858–870. doi: 10.1002/iub.1566. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Jesus AA, Marrero B, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeremiah N, Neven B, Gentili M, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124(12):5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munoz J, Rodiere M, Jeremiah N, et al. Stimulator of Interferon Genes-Associated Vasculopathy With Onset in Infancy: A Mimic of Childhood Granulomatosis With Polyangiitis. JAMA Dermatol. 2015;151(8):872–877. doi: 10.1001/jamadermatol.2015.0251. [DOI] [PubMed] [Google Scholar]
- 49.Omoyinmi E, Melo Gomes S, Nanthapisal S, et al. Stimulator of interferon genes-associated vasculitis of infancy. Arthritis Rheumatol. 2015;67(3):808. doi: 10.1002/art.38998. [DOI] [PubMed] [Google Scholar]
- 50.Picard C, Thouvenin G, Kannengiesser C, et al. Severe Pulmonary Fibrosis as the First Manifestation of Interferonopathy (TMEM173 Mutation) Chest. 2016;150(3):e65–71. doi: 10.1016/j.chest.2016.02.682. [DOI] [PubMed] [Google Scholar]
- 51.Konig N, Fiehn C, Wolf C, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis. 2017;76(2):468–472. doi: 10.1136/annrheumdis-2016-209841. [DOI] [PubMed] [Google Scholar]
- 52.Fremond ML, Rodero MP, Jeremiah N, et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol. 2016;138(6):1752–1755. doi: 10.1016/j.jaci.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Clarke SL, Pellowe EJ, de Jesus AA, Goldbach-Mansky R, Hilliard TN, Ramanan AV. Interstitial Lung Disease Caused by STING-associated Vasculopathy with Onset in Infancy. Am J Respir Crit Care Med. 2016;194(5):639–642. doi: 10.1164/rccm.201510-2102LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melki I, Rose Y, Uggenti C, et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 55.Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING Activation by Translocation from the ER Is Associated with Infection and Autoinflammatory Disease. Cell Host Microbe. 2015;18(2):157–168. doi: 10.1016/j.chom.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seo J, Kang JA, Suh DI, et al. Tofacitinib relieves symptoms of stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy caused by 2 de novo variants in TMEM173. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Ramot Y, Torrelo A, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012;64(3):895–907. doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitamura A, Maekawa Y, Uehara H, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest. 2011;121(10):4150–4160. doi: 10.1172/JCI58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arima K, Kinoshita A, Mishima H, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A. 2011;108(36):14914–14919. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agarwal AK, Xing C, DeMartino GN, et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87(6):866–872. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brehm A, Liu Y, Sheikh A, et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest. 2015;125(11):4196–4211. doi: 10.1172/JCI81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez GAM, Reinhardt AL, Brogan P, et al. Chronic Atypical Neutrophilic Dermatosis With Lipodystrophy and Elevated Temperatures (CANDLE): Clinical Characterization and Initial Response To Janus Kinase Inhibition With Baricitinib. Arthritis Rheum-Us. 2013;65:S758–S759. [Google Scholar]
- 63.Canna SW, de Jesus AA, Gouni S, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46(10):1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romberg N, Al Moussawi K, Nelson-Williams C, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46(10):1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med. 2014;211(12):2385–2396. doi: 10.1084/jem.20141091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawasaki Y, Oda H, Ito J, et al. Identification of a High-Frequency Somatic NLRC4 Mutation as a Cause of Autoinflammation by Pluripotent Cell-Based Phenotype Dissection. Arthritis Rheumatol. 2017;69(2):447–459. doi: 10.1002/art.39960. [DOI] [PubMed] [Google Scholar]
- 67.Hu Z, Zhou Q, Zhang C, et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science. 2015;350(6259):399–404. doi: 10.1126/science.aac5489. [DOI] [PubMed] [Google Scholar]
- 68.Bracaglia C, Prencipe G, De Benedetti F. Macrophage Activation Syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J. 2017;15(1):5. doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canna SW, Girard C, Malle L, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell. 1997;90(4):797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 71.French FMFC. A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17(1):25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 72.Manukyan G, Aminov R. Update on Pyrin Functions and Mechanisms of Familial Mediterranean Fever. Front Microbiol. 2016;7:456. doi: 10.3389/fmicb.2016.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards N, Schaner P, Diaz A, et al. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J Biol Chem. 2001;276(42):39320–39329. doi: 10.1074/jbc.M104730200. [DOI] [PubMed] [Google Scholar]
- 74.Chae JJ, Cho YH, Lee GS, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34(5):755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu H, Yang J, Gao W, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513(7517):237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 76.Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17(8):914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masters SL, Lagou V, Jeru I, et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med. 2016;8(332):332ra345. doi: 10.1126/scitranslmed.aaf1471. [DOI] [PubMed] [Google Scholar]
- 78.Kuijk LM, Beekman JM, Koster J, Waterham HR, Frenkel J, Coffer PJ. HMG-CoA reductase inhibition induces IL-1beta release through Rac1/PI3K/PKB-dependent caspase-1 activation. Blood. 2008;112(9):3563–3573. doi: 10.1182/blood-2008-03-144667. [DOI] [PubMed] [Google Scholar]
- 79.Mandey SH, Kuijk LM, Frenkel J, Waterham HR. A role for geranylgeranylation in interleukin-1beta secretion. Arthritis Rheum. 2006;54(11):3690–3695. doi: 10.1002/art.22194. [DOI] [PubMed] [Google Scholar]
- 80.Garcia-Mata R, Boulter E, Burridge K. The ’invisible hand’ : regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12(8):493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldfinger SE. Colchicine for familial Mediterranean fever. N Engl J Med. 1972;287(25):1302. doi: 10.1056/NEJM197212212872514. [DOI] [PubMed] [Google Scholar]
- 82.Mandhare A, Banerjee P. Therapeutic use of colchicine and its derivatives: a patent review. Expert Opin Ther Pat. 2016:1–18. doi: 10.1080/13543776.2016.1214268. [DOI] [PubMed] [Google Scholar]
- 83.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4(4):294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 84.Gao W, Yang J, Liu W, Wang Y, Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A. 2016;113(33):E4857–4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]