Abstract
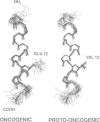
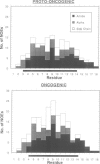
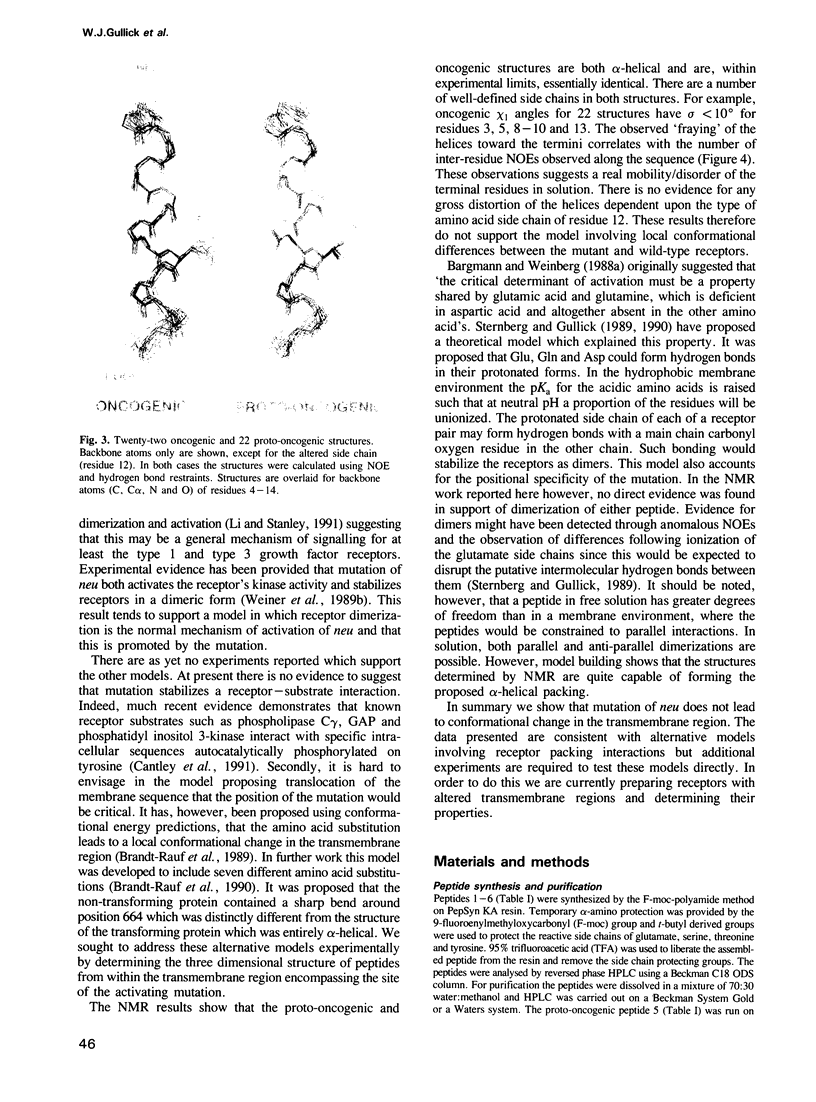
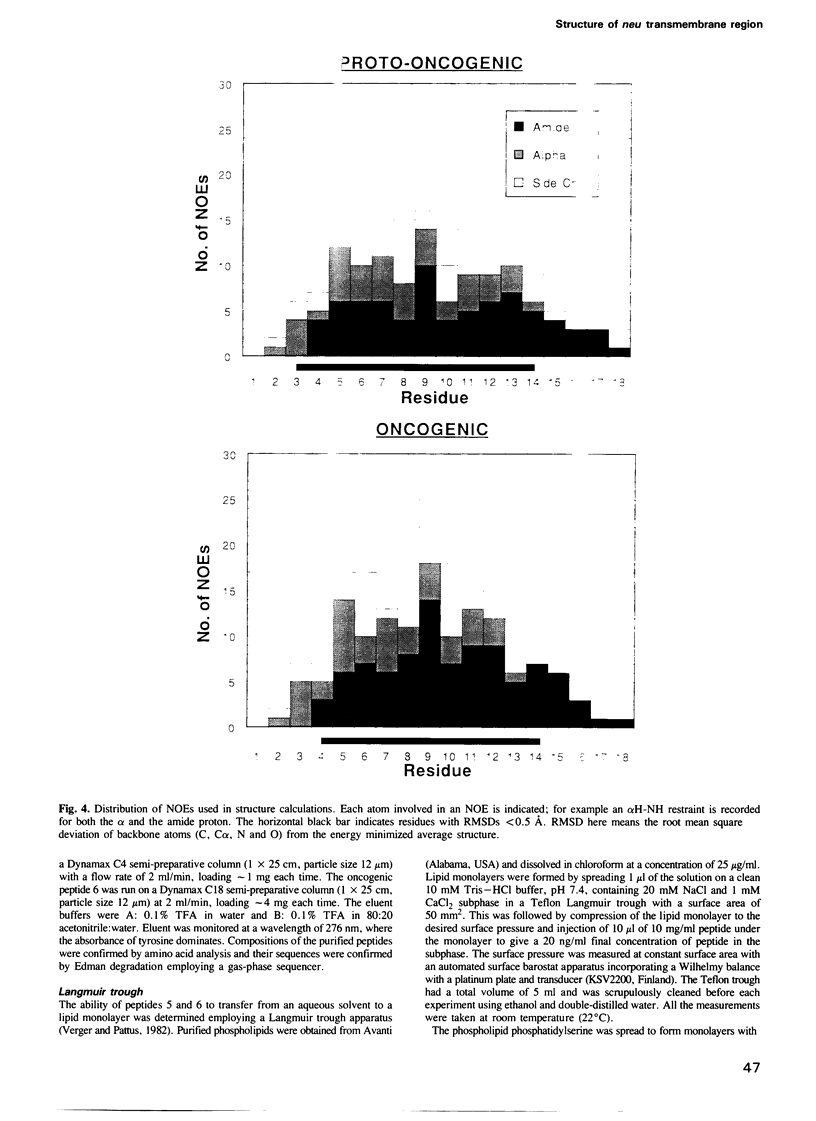
The neu proto-oncogene may be converted into a dominantly transforming oncogene by a single point mutation. Substitution of a valine residue at position 664 in the transmembrane region with glutamic acid activates the tyrosine kinase of the molecule and is associated with increased receptor dimerization. Previously we have proposed a model in which the glutamic acid side chain stabilizes receptor dimerization by hydrogen bonding. Other models have been proposed in which the mutation leads to a conformational change in the transmembrane region mimicking that assumed to occur following binding of a natural ligand. Synthetic peptides representing part of the transmembrane region were prepared. Some residues were replaced with serine in order to improve peptide solubility to allow purification and analysis. Both the peptides containing valine and glutamic acid dissolved in water and in an artificial lipid monolayer. The structures of the peptides were determined by NMR spectroscopy to be alpha-helical. No significant difference in conformation was observed between the two peptides. This result does not support the model proposing a conformational change. The receptor structures determined experimentally do allow alternative models involving receptor transmembrane region packing.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986 Jan 16;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988 Jul;7(7):2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt-Rauf P. W., Pincus M. R., Chen J. M. Conformational changes induced by the transforming amino acid substitution in the transmembrane domain of the neu oncogene-encoded p185 protein. J Protein Chem. 1989 Dec;8(6):749–756. doi: 10.1007/BF01024899. [DOI] [PubMed] [Google Scholar]
- Brandt-Rauf P. W., Rackovsky S., Pincus M. R. Correlation of the structure of the transmembrane domain of the neu oncogene-encoded p185 protein with its function. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8660–8664. doi: 10.1073/pnas.87.21.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze A. L., Harvey T. S., Bazzo R., Campbell I. D. Solution structure of human calcitonin gene-related peptide by 1H NMR and distance geometry with restrained molecular dynamics. Biochemistry. 1991 Jan 15;30(2):575–582. doi: 10.1021/bi00216a036. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Ingraham H. A., Cochet C., Walton G. M., Lazar C. S., Sowadski J. M., Rosenfeld M. G., Gill G. N. Structural analysis of the transmembrane domain of the epidermal growth factor receptor. J Biol Chem. 1991 Mar 25;266(9):5750–5755. [PubMed] [Google Scholar]
- Hall P. A., Hughes C. M., Staddon S. L., Richman P. I., Gullick W. J., Lemoine N. R. The c-erb B-2 proto-oncogene in human pancreatic cancer. J Pathol. 1990 Jul;161(3):195–200. doi: 10.1002/path.1711610305. [DOI] [PubMed] [Google Scholar]
- Lemoine N. R., Jain S., Silvestre F., Lopes C., Hughes C. M., McLelland E., Gullick W. J., Filipe M. I. Amplification and overexpression of the EGF receptor and c-erbB-2 proto-oncogenes in human stomach cancer. Br J Cancer. 1991 Jul;64(1):79–83. doi: 10.1038/bjc.1991.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine N. R., Staddon S., Dickson C., Barnes D. M., Gullick W. J. Absence of activating transmembrane mutations in the c-erbB-2 proto-oncogene in human breast cancer. Oncogene. 1990 Feb;5(2):237–239. [PubMed] [Google Scholar]
- Lemoine N. R., Wyllie F. S., Lillehaug J. R., Staddon S. L., Hughes C. M., Aasland R., Shaw J., Varhaug J. E., Brown C. L., Gullick W. J. Absence of abnormalities of the c-erbB-1 and c-erbB-2 proto-oncogenes in human thyroid neoplasia. Eur J Cancer. 1990;26(7):777–779. doi: 10.1016/0277-5379(90)90149-n. [DOI] [PubMed] [Google Scholar]
- Li W., Schlessinger J. Platelet-derived growth factor (PDGF)-induced disulfide-linked dimerization of PDGF receptor in living cells. Mol Cell Biol. 1991 Jul;11(7):3756–3761. doi: 10.1128/mcb.11.7.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Stanley E. R. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991 Feb;10(2):277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey D., King G. F., Cooke R. M., Doak D. G., Harvey T. S., Campbell I. D. High resolution 1H NMR study of the solution structure of the S4 segment of the sodium channel protein. FEBS Lett. 1989 Oct 23;257(1):113–117. doi: 10.1016/0014-5793(89)81799-5. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Segatto O., King C. R., Pierce J. H., Di Fiore P. P., Aaronson S. A. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988 Dec;8(12):5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Padhy L. C., Murray M., Weinberg R. A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981 Mar 19;290(5803):261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Gullick W. J. A sequence motif in the transmembrane region of growth factor receptors with tyrosine kinase activity mediates dimerization. Protein Eng. 1990 Mar;3(4):245–248. doi: 10.1093/protein/3.4.245. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Gullick W. J. Neu receptor dimerization. Nature. 1989 Jun 22;339(6226):587–587. doi: 10.1038/339587a0. [DOI] [PubMed] [Google Scholar]
- Tuzi N. L., Venter D. J., Kumar S., Staddon S. L., Lemoine N. R., Gullick W. J. Expression of growth factor receptors in human brain tumours. Br J Cancer. 1991 Feb;63(2):227–233. doi: 10.1038/bjc.1991.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Weiner D. B., Kokai Y., Wada T., Cohen J. A., Williams W. V., Greene M. I. Linkage of tyrosine kinase activity with transforming ability of the p185neu oncoprotein. Oncogene. 1989 Oct;4(10):1175–1183. [PubMed] [Google Scholar]
- Weiner D. B., Liu J., Cohen J. A., Williams W. V., Greene M. I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989 May 18;339(6221):230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- Wides R. J., Zak N. B., Shilo B. Z. Enhancement of tyrosine kinase activity of the Drosophila epidermal growth factor receptor homolog by alterations of the transmembrane domain. Eur J Biochem. 1990 May 20;189(3):637–645. doi: 10.1111/j.1432-1033.1990.tb15532.x. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Wider G., Wagner G., Braun W. Sequential resonance assignments as a basis for determination of spatial protein structures by high resolution proton nuclear magnetic resonance. J Mol Biol. 1982 Mar 5;155(3):311–319. doi: 10.1016/0022-2836(82)90007-9. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987 Mar 10;26(5):1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]