Abstract
BACKGROUND
Cardiac fibroblasts are a critical cell population responsible for myocardial extracellular matrix homeostasis. Upon injury or pathological stimulation, these cells transform to an activated myofibroblast state and play a fundamental role in myocardial fibrosis and remodeling. Chronic sympathetic overstimulation, a hallmark of heart failure (HF), induces pathological signaling through G protein βγ subunits and their interaction with G protein-coupled receptor kinase 2 (GRK2).
OBJECTIVES
We hypothesized that Gβγ-GRK2 inhibition and/or ablation after myocardial injury would attenuate pathological myofibroblast activation and cardiac remodeling.
METHODS
The therapeutic potential of small molecule Gβγ-GRK2 inhibition, alone or in combination with activated fibroblast- or myocyte-specific GRK2 ablation – each initiated after myocardial ischemia-reperfusion (I/R) injury – was investigated to evaluate possible salutary effects on post-I/R fibroblast activation, pathological remodeling, and cardiac dysfunction.
RESULTS
Small molecule Gβγ-GRK2 inhibition initiated 1 week post-injury was cardioprotective in the I/R model of chronic HF, including preservation of cardiac contractility and reduction in cardiac fibrotic remodeling. Systemic small molecule Gβγ-GRK2 inhibition initiated 1 week post-I/R in cardiomyocyte-restricted GRK2 ablated mice (also post-I/R) still demonstrated significant cardioprotection, suggesting a potential protective role beyond the cardiomyocyte. Inducible ablation of GRK2 in activated fibroblasts (i.e. myofibroblasts) post-I/R injury demonstrated significant functional cardioprotection with reduced myofibroblast transformation and fibrosis. Systemic small molecule Gβγ-GRK2 inhibition initiated 1 week post-I/R provided little to no further protection in mice with ablation of GRK2 in activated fibroblasts alone. Finally, Gβγ-GRK2 inhibition significantly attenuated activation characteristics of failing human cardiac fibroblasts isolated from end-stage HF patients.
CONCLUSIONS
These findings suggested consideration of a paradigm shift in our understanding of the therapeutic role of Gβγ-GRK2 inhibition in treating HF and the potential therapeutic role for Gβγ-GRK2 inhibition in limiting pathological myofibroblast activation, interstitial fibrosis, and HF progression.
Keywords: cardiac fibroblast, cardioprotection, fibrosis, remodeling
Introduction
Heart failure (HF) is a devastating disease characterized by interstitial fibrosis, chamber remodeling, and reduced ventricular compliance. Cardiovascular disease remains the predominant cause of mortality in the United States, presenting a considerable economic burden with estimated annual direct and indirect costs totaling about $320 billion (1). Regardless of etiology, HF generally involves adverse myocardial remodeling, characterized by excessive deposition of extracellular matrix proteins by pathologically activated cardiac fibroblasts; this reduces tissue compliance, promotes arrhythmogenesis, and accelerates HF progression (2). Despite the critical importance of fibrosis in HF, there are essentially no clinical interventions that effectively target the cardiac fibroblast nor its pathological contributions to disease progression.
The adrenergic system plays a fundamental role in the physiological regulation of the myocardium; however, chronic stimulation can induce cardiac hypertrophy and fibrosis, important components of HF pathophysiology (3). Stimulation of the β-adrenergic receptor (β-AR) induces conformational changes in G protein βγ subunits, ultimately resulting in activation and membrane recruitment of G protein-coupled receptor kinase 2 (GRK2) (4). Expression of GRK2 is known to be elevated in patients with HF (4) and recent studies have suggested that this is associated with β-AR uncoupling and downregulation in fibroblasts, which can promote a pro-fibrotic phenotype (5).
We and others have explored several approaches to specifically target GRK2 and its interaction with Gβγ subunits. These efforts have demonstrated the therapeutic potential of Gβγ-GRK2 inhibitory peptides (6) or compounds (7–9). The beneficial effects of the small molecule gallein, which selectively inhibits the interaction between Gβγ and GRK2 (7,10), were recently demonstrated in several animal models of HF (7,8). While the Gβγ-GRK2 interface represents an important target of therapeutic interventions for HF, the mechanisms and therapeutic potential of Gβγ-GRK2 inhibition specifically within cardiac fibroblasts and the progression of fibrosis have yet to be elucidated.
In the present study, small molecule inhibition of Gβγ-GRK2 initiated 1 week following myocardial ischemia-reperfusion (I/R) ameliorated the progression of cardiac dysfunction as well as pathological cardiac remodeling, particularly regarding infarct expansion. Furthermore, inducible ablation of GRK2 in the pathologically activated cardiac fibroblast 1 week post-I/R was found to be equally cardioprotective, whereas gallein provided significant cardioprotection in animals with post-I/R ablation of GRK2 in cardiomyocytes. This cardioprotection in vivo correlated with a reduction in the activation state of primary mouse and human HF-derived cardiac fibroblasts when treated with gallein. These data support a paradigm shift in proposed mechanisms behind the protective effects of Gβγ-GRK2 inhibition in the treatment of HF.
Methods
We recently reported a possible therapeutic role for interdicting pathological Gβγ-GRK2 binding interactions with the small molecule gallein (8). In the current study, gallein was evaluated for its therapeutic efficacy in a more clinically relevant I/R model of HF; mice were subjected to I/R through coronary artery occlusion, followed by 4 weeks of reperfusion. Gallein administration was initiated 1 week post-I/R at 2.5 mg/kg/day and titrated to a maximum dose of 10 mg/kg/day over 3 weeks, followed by assessment of cardiac function by echocardiography and histological analysis of fibrotic remodeling 4 weeks after injury. To biochemically assess injury severity, transcript expression of fibrotic and HF markers was assessed by quantitative polymerase chain reaction (qPCR).
Conditional cardiomyocyte-targeted GRK2 knockout mice were achieved by crossing GRK2fl/fl animals with mice possessing tamoxifen-inducible Cre recombinase under the control of the endogenous promotor for α-myosin heavy chain (α-MHCMCM) (11). GRK2fl/fl animals were also crossed with mice expressing inducible Cre recombinase under the control of the endogenous promoter for periostin (PostnMCM) (12). Tamoxifen administration via the chow was initiated after surgery and continued for 2 weeks to achieve inducible GRK2 ablation in a cell-specific manner.
Detailed materials and methods are included in the Online Appendix.
Results
Optimum dosing and administration, along with initial therapeutic efficacy of gallein, were first evaluated and established in wild-type C57Bl/6 mice subjected to I/R injury. Gallein treatment conferred substantial protection against myocardial dysfunction and dilation (Online Figures 1A through C). Furthermore, examination of collagen deposition by Masson’s trichrome staining showed a reduction in fibrotic expansion in animals treated with gallein (Online Figure 1D).
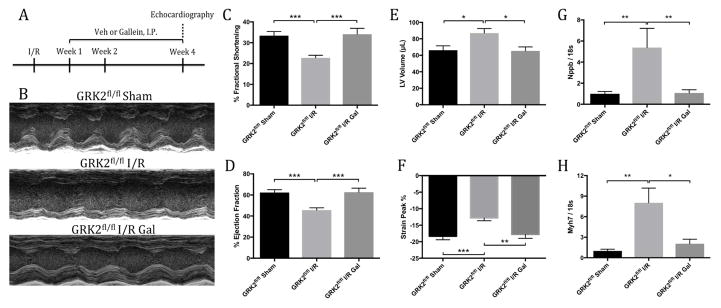
Corroboration of the cardioprotective attributes of small molecule Gβγ-GRK2 inhibition was assessed in animals possessing the GRK2fl/fl allele, as these mice would serve as controls for subsequent genetic ablation studies when crossed with various Cre lines. Gallein treatment was initiated 1 week post-injury as described to investigate its therapeutic efficacy in treating extant HF (Figure 1A). Animals receiving vehicle demonstrated significant deterioration in overall cardiac function 4 weeks post-I/R. Remarkably, mice treated with gallein initiated 1 week post-I/R exhibited significant preservation of contractile performance and ventricular volumes (Figure 1B) as measured by percent fractional shortening (Figure 1C), ejection fraction (Figure 1D), and left ventricular (LV) volume (Figure 1E). Echocardiographic strain analysis supported this finding, revealing significant restoration in peak wall contraction in animals receiving gallein, indicating attenuation of progressive LV dysfunction (Figure 1F). Subsequent qPCR analysis of injured mice receiving gallein post-I/R demonstrated a significant reduction in the messenger ribonucleic acid (mRNA) levels of natriuretic peptide B (Nppb) (Figure 1G), β-myosin heavy chain (Myh7) (Figure 1H) and natriuretic peptide A (Nppa) (Online Figure 2A) in comparison to vehicle-treated control mice.
Figure 1. Cardiac Function and HF Marker Expression.
(A) Timeline of ischemia-reperfusion (I/R) injury surgery and gallein (Gal) or vehicle (Veh) administration in mice. Effect of G protein-coupled receptor kinase 2 (GRK2) inhibition seen in (B) representative m-mode echocardiographic images and cardiac functional parameters including (C) percent fractional shortening, (D) ejection fraction, (E) left ventricular (LV) end-diastolic volume and (F) peak percentage by strain analysis. Gene expression levels of heart failure (HF) markers (G) Nppb (statistical analysis following log transformation) and (H) Myh7 normalized to 18s ribosomal sub-unit as determined by quantitative polymerase chain reaction (qPCR). *p < 0.05; **p < 0.01; ***p < 0.001.
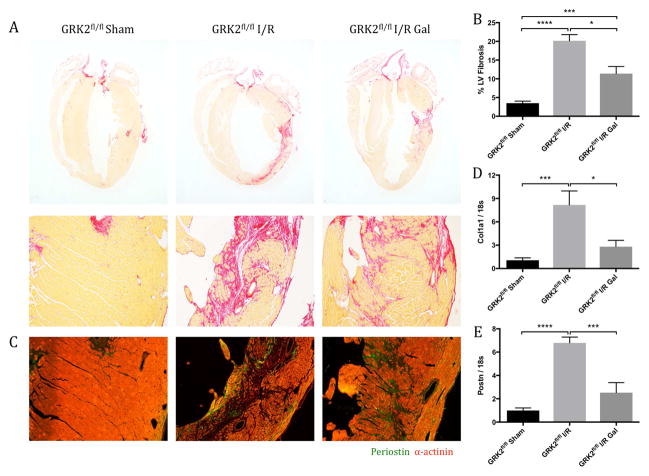
The observed preservation of cardiac function by post-I/R gallein treatment occurred commensurate with an overall reduction in post-I/R fibrosis, particularly with regard to infarct expansion. Serial cardiac longitudinal sections were stained by picrosirius red to determine the extent of collagen deposition following injury (Figure 2A). Fibrosis was quantified in relation to total LV area, revealing a significant reduction in pathological fibrotic expansion from the initial infarct region in gallein-treated mice (Figure 2B). Immunofluorescence revealed a parallel reduction in the presence of periostin, which is produced specifically by activated fibroblasts following tissue injury (12), in gallein-treated mice compared with vehicle controls (Figure 2C). Furthermore, gene expression analysis revealed a concomitant reduction in the transcript expression of collagen type I α1 (col1α1) (Figure 2D), periostin (Postn) (Figure 2E), and fibronectin 1 (Fn1) (Online Figure 2C), along with trends toward a decrease in the expression of collagen type III α1 (Col3α1) (Online Figure 2B) in mice treated with gallein. Overall, pharmacological Gβγ-GRK2 inhibition initiated post-I/R provided functional protection and reduced post-I/R infarct expansion following ischemic myocardial injury.
Figure 2. Fibrotic Scar Formation and Marker Expression.
The effect of Gβγ-GRK2 inhibition on fibrotic scar formation (A) was assessed by picrosirius red staining, and (B) quantification was expressed as percentage of collagen deposition over total LV area. (C) Representative images show periostin expression within the ventricular free wall. Messenger ribonucleic acid levels of fibrosis markers (D) Col1a1 and (E) Postn were measured by qPCR and normalized to 18s ribosomal sub-unit. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Abbreviations as in Figure 1.
Effect of Cardiomyocyte-Specific GRK2 Inhibition
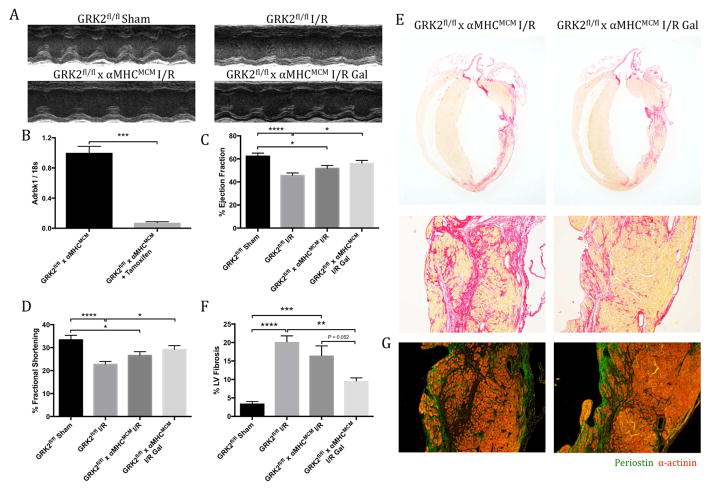
The therapeutic efficacy of cardiomyocyte-specific GRK2 inhibition and ablation has been investigated extensively for its role in slowing HF progression in numerous animal models (13,14). Cardiomyocyte-restricted GRK2 ablation was achieved by placing GRK2fl/fl x α-MHCMCM mice on tamoxifen chow following I/R, and functional assessment was performed by echocardiography 4 weeks post-injury (Figure 3A and Online Figure 3A). Successful knockdown was confirmed in isolated cardiomyocytes by a reduction in both transcript expression of GRK2 (Adrbk1) (Figure 3B) and protein expression (Online Figure 3B). GRK2fl/fl control mice subjected to I/R injury showed significant signs of impaired cardiac function compared with sham controls, as measured by echocardiography 4 weeks post-injury (Figures 3C and 3D). Cardiac functional assessment of αMHCMCM mice by investigator-blinded echocardiography revealed a nearly identical level of cardiac dysfunction following I/R as was observed in the GRK2fl/fl mice, consistent with previous reports (Online Table 1 and Online Figure 4) (13). Cardiomyocyte-specific GRK2 ablation offered modest protection from myocardial dysfunction compared with GRK2fl/fl control mice as measured by percent ejection fraction (Figure 3C) (p = 0.277) and fractional shortening (Figure 3D) (p = 0.276). Histologically, cardiomyocyte-specific GRK2 ablation only modestly reduced LV fibrosis, as observed by picrosirius red staining (Figures 3E and 3F) or periostin immunofluorescence (Figure 3G). However, there did appear to be some preservation in cardiomyocyte viability, as previously described (Figure 3E) (13).
Figure 3. Cardiac Function and Fibrosis in Cardiomyocyte-specific GRK2 Knockout Mice Post-I/R.
(A) Representative m-mode echocardiographic images showing ventricular contractility. (B) GRK2 transcript expression in cardiomyocytes isolated from GRK2fl/fl x α-MHCMCM mice by qPCR analysis. Function quantified by (C) percent ejection fraction and (D) fractional shortening. Collagen deposition assessed by (E) picrosirius red staining, with the fibrotic area quantified as percentage of collagen deposition over total left ventricular area (F). (G) Representative images of periostin (Postn) expression within the ventricular free wall 4 weeks post-I/R. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. α-MHC = α-myosin heavy chain; other abbreviations as in Figure 1.
To investigate potential cardiomyocyte-independent properties of pharmacological Gβγ-GRK2 inhibition, gallein was initiated 1 week post-I/R by intraperitoneal injection to GRK2fl/fl x αMHCMCM mice (Online Figure 3A). Significant improvement in GRK2fl/fl x αMHCMCM mice treated with gallein post-I/R compared with GRK2fl/fl control mice was observed by cardiac percent ejection fraction and fractional shortening (Figures 3C and 3D). Furthermore, there was a trend toward improvement in peak strain percentage (Online Figure 3C) along with a reduction in overall ventricular dyssynchrony (Online Figure 3D) when mice were assessed by strain analysis. Post-I/R gallein treatment offered numerical functional improvement to GRK2fl/fl x αMHCMCM mice after injury. Assessment of cardiomyocyte-specific GRK2 knockout mice treated with gallein post-I/R revealed a significant decrease in pathological fibrotic expansion and periostin secretion (Figures 3E through 3G). Collectively, these data validated Gβγ-GRK2 as a therapeutic target and suggested potential functional significance of Gβγ-GRK2 inhibition in cell types beyond the cardiomyocyte.
Activated Fibroblast-Specific GRK2 Ablation Post-I/R
Based on the beneficial effects of post-I/R gallein treatment observed in cardiomyocyte-specific GRK2 KO mice, the therapeutic potential of activated fibroblast restricted GRK2 ablation was investigated post-I/R with or without gallein treatment. Knockdown of GRK2 was achieved in GRK2fl/fl x PostnMCM mice through tamoxifen administration via the chow. These mice provided a powerful method by which to inducibly ablate GRK2 only in the pathologically activated fibroblast (Online Figure 5A) (12). Previous reports have demonstrated protein knockdown may not occur until 4 to 6 days after tamoxifen administration via the chow (12), which coincides with initiation of gallein treatment 1 week post-injury. To demonstrate successful gene knockdown, flow cytometry was utilized to select for CD31/CD45 negative and platelet-derived growth factor receptor α positive cells (Online Figure 5B); qPCR analysis revealed a significant reduction in the transcript expression of GRK2 (Adrbk1) in this cell population following myocardial injury and tamoxifen administration (Online Figure 5C).
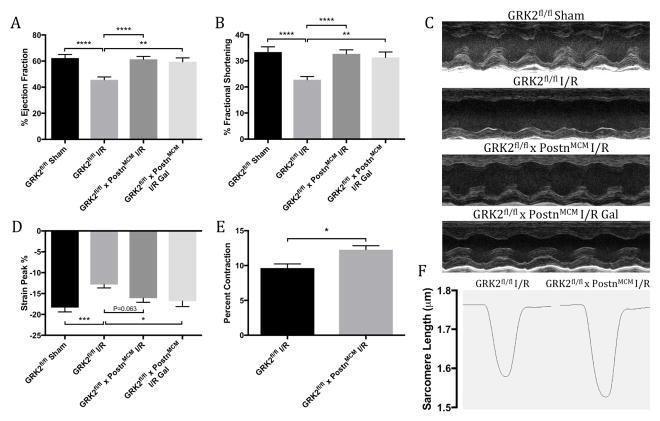
Following injury, GRK2fl/fl control mice presented with significant cardiac dysfunction 4 weeks after injury, demonstrating reductions in functional parameters by echocardiography compared with mice receiving sham surgery (Figures 4A through 4C). Mice in which GRK2 was ablated post-I/R, solely in activated cardiac fibroblasts, maintained nearly normal contractile performance, including significant improvements in percent ejection fraction (Figure 4A) and fractional shortening (Figure 4B) versus GRK2fl/fl control mice. Post-I/R GRK2 ablation in periostin-expressing cells also significantly normalized cardiac dimensions following injury. Surprisingly, addition of the small molecule Gβγ-GRK2 inhibitor gallein 1 week post-I/R offered no observable further cardioprotection over fibroblast-specific GRK2 ablation alone as assessed by m-mode echocardiography (Figures 4A and 4B). However, while GRK2fl/fl x PostnMCM mice trended towards improvement in peak percentage by echocardiographic strain analysis, addition of gallein did induce a significant improvement over control animals (Figure 4D). Overall, these findings demonstrated that inducible, post-I/R GRK2 ablation in activated cardiac fibroblasts was cardioprotective in an I/R model of HF and that addition of a small molecule Gβγ-GRK2 inhibitor provided no further functional protection as assessed by standard echocardiographic measures (Online Table 2). Interestingly, gallein improved advanced strain-based imaging measures, suggesting a possible role in attenuating diastolic dysfunction. These data suggested that pathological Gβγ-GRK2 signaling plays a significant role in the activated cardiac fibroblast and contributes to cardiac remodeling and dysfunction.
Figure 4. Cardiac Function and Cardiomyocyte Contractility in Activated Fibroblast GRK2 Knockout Mice Post-I/R.
Cardiac function assessed by echocardiography is shown by (A) percent ejection fraction and (B) fractional shortening 4 weeks post-I/R, with (C) representative long-axis m-mode images at level of papillary muscle. (D) Peak percentage determined by strain analysis. Sarcomeric contractility was assessed in primary cardiomyocytes 4 weeks post-I/R; shown are (E) percent sarcomeric contractility and (F) representative sarcomeric length traces over a single contraction. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Abbreviations as in Figure 1.
Despite specifically targeting activated cardiac fibroblasts, the effect of this fibroblast-specific GRK2 ablation on cardiomyocyte contractility was explored. Cardiomyocytes were isolated from the hearts of GRK2fl/fl x PostnMCM mice and GRK2fl/fl controls 4 weeks post-I/R and sarcomeric shortening was evaluated. Interestingly, cardiomyocytes isolated from mice in which GRK2 was deleted solely in activated cardiac fibroblasts possessed significantly enhanced contractility compared with controls (Figure 4E), with representative sarcomeric length traces shown in Figure 4F. These data suggested that GRK2 signaling in the activated fibroblast can affect resident cardiomyocyte contractility.
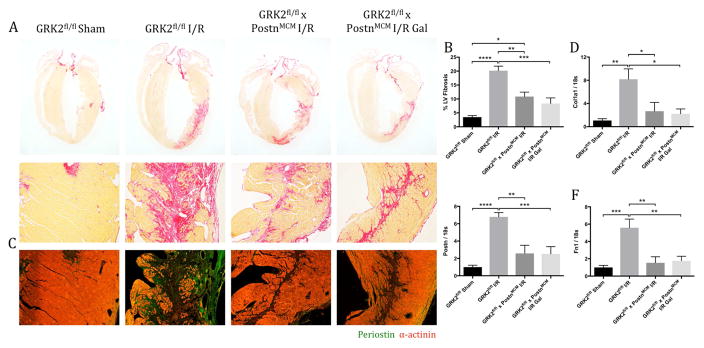
The hearts of GRK2fl/fl x PostnMCM mice treated with or without gallein were evaluated histologically to determine the effects of post-I/R Gβγ-GRK2 inhibition and targeted GRK2 ablation in activated fibroblasts on pathological cardiac remodeling following establishment of the initial infarct immediately post-I/R. Picrosirius red staining showed a significant reduction in collagen deposition and infarct expansion in mice in which GRK2 was ablated in activated cardiac fibroblasts to a similar degree regardless of gallein treatment (Figures 5A and 5B). Immunofluorescence staining for periostin revealed an expression pattern similar to that of collagen, which was reduced in mice in which GRK2 was ablated post-I/R in activated cardiac fibroblasts compared with GRK2fl/fl controls following injury (Figure 5C). To confirm these results, the expression of several fibrotic markers was assessed in the LV tissue; qPCR analysis revealed significant reductions in the mRNA levels of collagen type I α1 (Col1a1), Postn, and fibronectin (Fn1) (Figures 5D through 5F). Taken together, these findings suggest that improvements observed in overall cardiac function were potentially due to restored ventricular compliance as the result of a lessened post-I/R fibrotic burden caused by attenuation of pathological Gβγ-GRK2 signaling in activated fibroblasts.
Figure 5. Fibrosis and Fibrotic Marker Gene Expression in Activated Fibroblast GRK2 Knockout Mice Following Injury.
Fibrotic scar formation assessed by (A) picrosirius red staining, with (B) quantification expressed as proportion of fibrotic region over total LV area. (C) Representative images of periostin expression within the ventricular free wall. Transcript expression of fibrosis markers (D) Col1a1, (E) Postn, and (F) Fn1, measured by qPCR and normalized to 18s. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Abbreviations as shown in Figures 1 and 3.
Pharmacological Gβγ-GRK2 Inhibition in Vitro
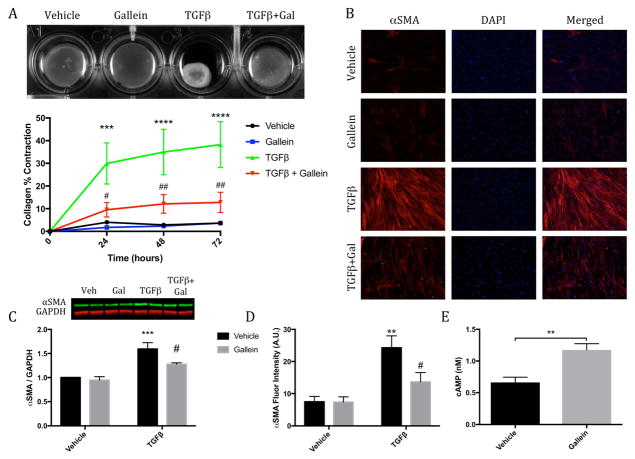
To determine the functional effects of attenuating Gβγ-GRK2 signaling ex vivo, primary cardiac fibroblasts were isolated from the ventricles of adult mice to assess the efficacy of Gβγ-GRK2 inhibition in preventing pathological fibroblast activation. These cells were cultured in collagen gels, stimulated with transforming growth factor β (TGF-β) to induce activation, and treated with either gallein or vehicle. Time-dependent contraction of the collagen gels was observed in fibroblasts treated with TGF-β, which was significantly attenuated in cells treated with gallein (Figure 6A). Furthermore, expression of the contractile protein smooth muscle α-actin (α-SMA), which confers contractile properties to activated fibroblasts, was evaluated in vitro; gallein treatment significantly reduced the expression of α-SMA both by immunofluorescence and Western blotting in response to TGF-β stimulation (Figures 6B through 6D). To explore a possible mechanism for this reduction in fibroblast activation, the effect of pharmacological Gβγ-GRK2 inhibition on modulating cyclic adenosine monophosphate (cAMP) production in response to β-AR stimulation was investigated, as cAMP has previously been described as antifibrotic (15). Interestingly, gallein treatment in mouse cardiac fibroblasts significantly increased cAMP production in response to stimulation by isoproterenol (Figure 6E).
Figure 6. Effect of Gβγ-GRK2 Inhibition on Pathological Myofibroblast Activation.
(A) Collagen contractility assay with representative collagen gels showing contraction 72 h after gel release, with the percent collagen gel contraction quantified over a 72-h period. ***p < 0.001 vs. Veh; ****p < 0.0001 vs. Veh; #p < 0.05 vs. transforming growth factor-β (TGF-β); ##p < 0.01 vs. TGF-β. (B) Representative smooth muscle α actin (α-SMA) immunofluorescence images. (C) Western blot analysis of α-SMA expression normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (D) Quantification of relative α-SMA fluorescence intensity. **p < 0.01 vs. Veh; ***p < 0.001 vs. Veh; #p < 0.05 vs. TGF-β. (E) Production of cyclic adenosine monophosphate (cAMP) measured following 10 min of isoproterenol stimulation. **p < 0.01. Other abbreviations as in Figure 1.
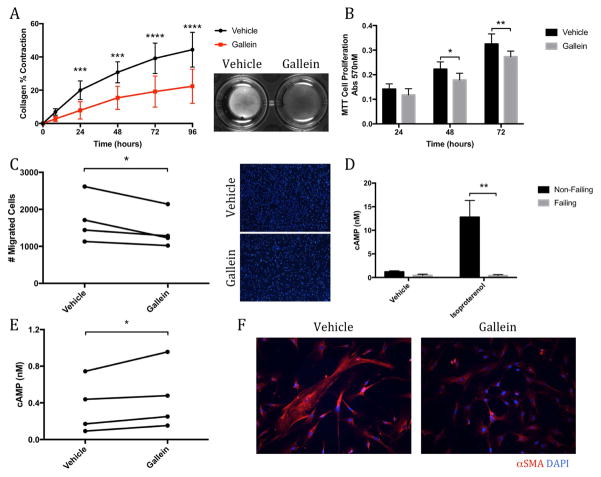
Failing human cardiac fibroblasts were isolated from the hearts of end-stage HF patients undergoing LV assist device implantation. Approximately 98% of these cells stained positive for vimentin, which offered some reassurance that these failing human cells consisted of a relatively pure population of cardiac fibroblasts (Online Figures 6A and 6B). As with murine cardiac fibroblasts, small molecule Gβγ-GRK2 inhibition significantly attenuated the ability of these human HF fibroblasts to contract collagen gels (Figure 7A). Gallein treatment also significantly reduced the proliferative rate of the failing human cardiac fibroblasts (Figure 7B), along with their ability to migrate across a cell-permeable membrane (Figure 7C). Importantly, gallein was shown to have no negative impact on fibroblast integrity (Online Figure 6C). These cells also possessed a blunted response to adrenergic stimulation compared with nonfailing controls, as seen by a significant attenuation in cAMP production following stimulation with isoproterenol (Figure 7D). Gallein treatment significantly restored cAMP production induced by adrenergic stimulation in cardiac fibroblasts isolated from patients with end-stage HF (Figure 7E). Finally, while no significant alternations in the overall expression of α-SMA were observed (data not shown), gallein treatment appeared to reduce the formation of prominent α-SMA stress fibers (Figure 7F).
Figure 7. Failing Human Cardiac Fibroblasts: Reduced Activation State.
Gallein is shown to reduce the activation state of failing human cardiac fibroblasts. Analysis of (A) collagen gel contraction and (B) cell proliferation by MTT assay in failing human cardiac fibroblasts treated with gallein. *p < 0.05 vs. Veh; **p < 0.01 vs. Veh; ***p < 0.001 vs. Veh; ****p < 0.0001 vs. Veh. (C) Cell migration was measured across a cell-permeable transwell membrane; representative membrane images are shown from which migrated cells were quantified. *p < 0.05. Production of cAMP (D) assessed in normal and failing human cardiac fibroblasts following isoproterenol stimulation. (**p < 0.01 vs. non-failing) and (E) in failing human cardiac fibroblasts treated with or without gallein (*p < 0.05). (F) Representative images of α-SMA immunofluorescence. DAPI = 4',6-diamidino-2-phenylindole; other abbreviations as in Figure 6.
Discussion
The transformation of cardiac fibroblasts to activated myofibroblasts plays a key role in myocardial fibrosis and remodeling, which contribute significantly to HF progression (2). Currently, no clinical interventions effectively target this important cell population and its pathological contributions to disease progression; development of such a therapy would significantly enhance current approaches in HF treatment.
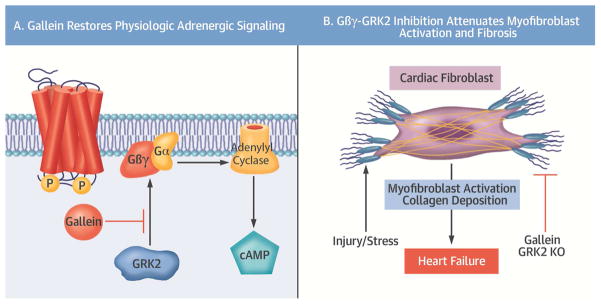
The adrenergic system plays a fundamental role in the physiological regulation of the myocardium; however, chronic overstimulation can induce both cardiac hypertrophy and fibrosis (3). This is also associated with an upregulation in the expression of GRK2 in human HF, in which this protein is known to play a central role in the uncoupling and desensitization of β-ARs (4). The therapeutic potential of pathological Gβγ-GRK2 signaling inhibition has been extensively explored in various animal models of HF. We recently described a small molecule known as gallein that targets specific Gβγprotein interactions, including its association with GRK2 (7,10). Disruption of pathological Gβγ-GRK2 signaling, using various inhibitory peptides (6) and small molecules (7–9), has proven highly successful. We recently demonstrated the therapeutic efficacy of gallein-mediated Gβγ-GRK2 inhibition in cardiorenal syndrome, where it directly attenuated both myocardial and renal dysfunction and fibrosis (16). However, the cellular specificity of this systemic inhibition had not previously been thoroughly investigated, particularly regarding the cardiac fibroblast. In this study, we examined the therapeutic potential for small molecule Gβγ-GRK2 inhibition administered following myocardial I/R, including whether it would offer further protection over the previously established beneficial effects of GRK2 deletion in cardiomyocytes. GRK2 ablation post-I/R in activated cardiac fibroblasts, in the presence or absence of systemic post-I/R small molecule Gβγ-GRK2 inhibition, was also investigated for its therapeutic potential in treating extant HF (Central Illustration).
Central Illustration. Attenuation of Myofibroblast Activation by Gβγ-GRK2 Inhibition.
(A) The cardioprotective effects of gallein are mediated through disruption of the interaction between active Gβγsubunits and G protein-coupled receptor kinase 2 (GRK2), thus reducing desensitization and downregulation of β-adrenergic receptors. This restoration of β-adrenergic receptor signaling potentiated production of the second messenger cyclic adenosine monophosphate (cAMP) (A) to reduce fibroblast activation and the resulting fibrotic remodeling, thereby attenuating the progression of heart failure (B). KO = knockout.
The present study utilized murine cardiac I/R injury, a more clinically relevant mouse model of ischemic HF that is initially characterized by a robust inflammatory response and extensive cardiomyocyte necrosis within the immediate infarction region (3). To prevent cardiac rupture, this area of ischemic injury is restored by fibrotic tissue deposition (replacement fibrosis) through elevated secretion of extracellular matrix components by activated cardiac fibroblasts. Fibroblast persistence then allows for the development of pathological infarct expansion beyond the original infarct region leading to ventricular noncompliance and HF (17). Disruption of pathological Gβγ-GRK2 signaling by the small molecule inhibitor gallein was initiated 1 week post-injury with dose titration to assess its therapeutic efficacy in the setting of existing cardiac damage (8).
Gallein treatment initiated 1 week post-I/R injury offered significant protection against myocardial dysfunction and attenuated cardiac dilation compared with vehicle-treated animals. These mice were also assessed by echocardiographic strain analysis, which provided a global assessment of ventricular wall function and a valuation of dyssynchrony between various wall segments (18). Significant rescue of peak strain percentage confirmed the preservation of ventricular wall integrity with gallein treatment. Additionally, staining for collagen deposition revealed a significant amelioration of fibrotic infarct expansion and a concomitant reduction in fibrotic marker expression in gallein-treated mice, suggesting interference in the persistent activation of cardiac fibroblasts. These data were consistent with our prior report of pharmacological Gβγ-GRK2 inhibition with gallein in both pressure-overload HF (8) as well as high-dose paroxetine in myocardial infarction (9). Since gallein is delivered systemically, it was imperative to investigate the cellular specificity of pharmacological Gβγ-GRK2 inhibition by exploring the effects of GRK2 ablation in various resident myocardial cells.
Targeting of the cardiomyocyte for the treatment of cardiovascular disease has been at the forefront of cardiac research for several decades. Abundant evidence has suggested salutary properties of cardiomyocyte-specific GRK2 inhibition both in improving contractility in isolated cardiomyocytes and in rescuing cardiac function following cardiac injury (13,14,19). The aim of this study was to evaluate potential cardiomyocyte-independent properties of gallein when given to inducible, cardiomyocyte-specific GRK2 knockout mice subjected to I/R injury. In the present study, GRK2 ablation in cardiac myocytes was induced following I/R to assess the potential of treating extant HF as described previously (13). Tamoxifen administration via the chow was limited to the duration required to induce gene ablation, while attempting to avoid potential off-target effects. Of note, high doses of tamoxifen, in combination with various inducible mouse lines, can induce focal cardiac fibrosis; additionally, several reports indicate that high levels of Cre expression itself can be cytotoxic (20). Successful knockdown was confirmed in isolated cardiomyocytes by reduced GRK2 transcript and protein expression following 2 weeks of tamoxifen chow administration.
Following injury, a modest level of cardioprotection was observed in cardiomyocyte-specific GRK2 knockout mice, in both functional parameters and fibrotic remodeling. These mice possessed numerically improved cardiac performance with what appeared to be reduced cardiomyocyte apoptosis and subsequent replacement fibrosis. Interestingly, gallein administration initiated 1 week post-I/R in GRK2fl/fl x α-MHCMCM mice provided significant cardioprotection, suggesting that cardiomyocyte-independent properties of pharmacological Gβγ-GRK2 inhibition were likely important for its salutary effects. To that end, an inducible system was utilized to ablate GRK2 under the control of the promoter for periostin (12), a secreted extracellular matrix protein expressed by activated fibroblasts within areas of cardiac injury (21), allowing us to assess the effects of inducible, post-I/R GRK2 ablation in activated cardiac fibroblasts.
Remarkably, mice in which GRK2 was inducibly ablated post-I/R in activated cardiac fibroblasts demonstrated nearly complete preservation of cardiac function. This included a significant decrease in pathological fibrotic expansion along with concomitant reductions in the transcript expression levels of several pro-fibrotic markers. Altogether, these findings suggested that improvements observed in overall cardiac function were potentially due to restored ventricular compliance as the result of a decreased fibrotic burden. As an unexpected consequence, the addition of pharmacological Gβγ-GRK2 inhibition 1 week post-I/R to the GRK2fl/fl x PostnMCM mice offered no further improvements to cardiac function by m-mode echocardiography, nor further reductions in fibrotic expansion or fibrotic marker gene expression. However, a significant improvement was detected when fibroblast-specific GRK2 knockout mice treated with gallein were assessed by strain analysis, which was not achieved by GRK2 knockdown alone, suggesting a potential reduction in diastolic dysfunction through the addition of small molecule Gβγ-GRK2 inhibition. These findings suggested that the cardioprotection proffered by small molecule Gβγ-GRK2 inhibition may be substantially mediated through its effects in the pathologically activated cardiac fibroblast. However, considering the salutary effect observed with post-I/R GRK2 ablation in the myofibroblast, we cannot rule out the possibility that gallein might elicit salutary effects in other cells beyond the fibroblast.
To assess potential extra-fibroblast effects that might have contributed to the observed functional protection in activated fibroblast specific GRK2 knockout mice following injury, the contractility of isolated cardiomyocytes from these animals was quantified; cardiomyocytes from these mice demonstrated enhanced levels of sarcomeric contractility. This might be the result of enhanced crosstalk between cardiac fibroblasts and myocytes or simply that cardiomyocytes were isolated from healthier hearts. While the exact mechanism behind this preserved cardiomyocyte function remains unclear, the enhanced contractility of cardiomyocytes in mice with post-I/R activated fibroblast-specific GRK2 ablation likely contributed to the improvement observed in overall cardiac function following injury.
Cardiac fibroblasts respond to pathological stress and environmental stimuli by transforming into activated fibroblasts that express elevated levels of various pro-fibrotic factors, possess enhanced migratory and proliferative capacities, and acquire increased contractile properties (2). These features, which can be evaluated in vitro, lead to the development of adverse changes in ventricular structure and compliance. Primary cardiac fibroblasts isolated from the ventricles of adult mice were stimulated with TGF-β to recapitulate the activation state of fibroblasts following a cardiac event in vivo. As the contraction of the collagen network following myocardial injury is a key function of the activated fibroblast, measuring this phenomenon in culture can provide important information regarding cellular activation status. The decrease in the extent of collagen gel contraction mediated by cells treated with gallein suggested a reduction in their activation state. The protein α-SMA, which has been utilized extensively to identify myofibroblasts in vitro (in addition to its other functions) (2), grants contractile properties to activated fibroblasts allowing for fibrotic scar contraction. In association with the reduction in collagen gel contraction, a reduction in α-SMA protein expression was also observed by immunofluorescence and Western blotting, potentially accounting for this phenomenon. Collectively, these findings suggested that pharmacological Gβγ-GRK2 inhibition could attenuate fibroblast activation characteristics in vitro. The efficacy of small molecule Gβγ-GRK2 inhibition in reducing fibroblast activation characteristics was also assessed in cardiac fibroblasts isolated from patients with end-stage HF. In a similar fashion, gallein considerably reduced the contractile properties of these cells, evidenced by a reduction in their ability to contract collagen gels in which they were cultured. Furthermore, both the proliferative and migratory capacities of these cells were significantly attenuated, suggesting a reduction in their pathological activation state.
A recent study explored the effects of GRK2 ablation in collagen1-α-expressing cells initiated 3 weeks prior to ischemic myocardial injury; animals were then evaluated up to 72 h post-I/R (22). The authors described preventative maintenance of cardiac function and a relative reduction in replacement fibrosis. Interestingly, no alterations in α-SMA expression were described as a result of pre-I/R GRK2 ablation upon gross histological observation; however, no further characterization of the myofibroblast transition with established methods was reported in vivo or ex vivo (12,20).
As mentioned previously, the adrenergic system plays an important role in regulating the myocardium. Although several subtypes of the β-AR exist, the β2-AR appears to be the form that is predominantly expressed by cardiac fibroblasts (23). Chronic stimulation of this receptor, as would occur in HF, leads to Gβγ-GRK2-mediated β2-AR desensitization and has been shown to induce fibroblast proliferation, collagen secretion, and other characteristics of the activated fibroblast phenotype (23). It is well-established that acute (but not chronic) stimulation of the β2-AR directly increases the levels of cAMP, which has been shown to attenuate the proliferation of fibroblasts (23) and inhibit the synthesis and secretion of collagen (24). Furthermore, elevated levels of cAMP can inhibit activation of fibroblasts induced by stimulation with TGF-β (24,25). Here, it has been demonstrated that failing human cardiac fibroblasts have lost the ability to respond to adrenergic stimulation, as seen by an attenuation of isoproterenol-induced cAMP production compared with nonfailing cardiac fibroblasts. However, treatment of either primary adult mouse fibroblasts or failing human ventricular fibroblasts with the small molecule Gβγ-GRK2 inhibitor gallein increased the production of cAMP following stimulation by isoproterenol. This restoration of adrenergic responsiveness potentially accounted for the reduction in fibroblast activation observed in isolated cells treated with gallein, and on the reduction in fibroblast activation following cardiac injury in vivo in which there is chronically elevated catecholamine exposure (Central Illustration).
Study Limitations
Although this study has provided evidence of the therapeutic efficacy of Gβγ-GRK2 inhibition in cardiac myofibroblasts after ischemic myocardial injury, it does not preclude the possibility that gallein may exert beneficial effects in myocardial cell types beyond the fibroblast. Further investigation will help determine the specific molecular mechanisms responsible for enhancing isolated cardiomyocyte contractility in the setting of fibroblast-specific GRK2 ablation. Additionally, further exploration of off-target effects of gallein will be important to fully characterize its salutary properties.
Conclusions
These findings demonstrated the therapeutic efficacy of the selective small molecule Gβγ-GRK2 inhibitor gallein initiated 1 week post-I/R in preserving cardiac function and attenuating pathological cardiac remodeling in a more clinically relevant ischemic model of HF. Furthermore, we reported the cardioprotective properties of inducible post-I/R GRK2 ablation in activated cardiac fibroblasts, suggesting a paradigm shift in our understanding of the therapeutic role of Gβγ-GRK2 inhibition in the treatment of HF. This work added further evidence for the beneficial effects of Gβγ-GRK2 inhibition in treating cardiovascular disease. This small molecule and fibroblast-targeted approach might lead to refinement of existing targets and compounds, and possibly development of novel therapeutics for HF treatment.
Methods
Experimental Animals
All animal experiments were performed in accordance with the guidelines of the Department of Laboratory Animal Medicine and the University Committee on Animal Resources at Cincinnati Children’s Hospital Medical Center. Mice were maintained in our animal care facility under the care of professional veterinary technicians. Animals were fed a standard diet and water and were maintained on a 12hr light/dark cycle.
GRK2fl/fl mice(1) (Jackson Labs Stock #012458) were crossed with mice expressing tamoxifen-inducible Cre recombinase under the control of the α myosin heavy chain promotor (αMHCMCM)(2) to generate inducible cardiomyocyte-specific GRK2 knockout mice (GRK2fl/fl x αMHCMCM). GRK2fl/fl mice were also crossed with mice expressing inducible Cre recombinase under the control of the Periostin promoter (PostnMCM),(3) to generate inducible activated fibroblast-specific GRK2 knockout mice (GRK2fl/fl x PostnMCM). GRK2fl/fl (Cre negative) littermates served as controls. Animals were fed tamoxifen chow (400mg/kg; Harlan TD.130860) for two weeks following injury to induce GRK2 ablation. Mice received a once daily intraperitoneal injection of either vehicle or gallein (Tocris Biosciences 3090) beginning 7 days post-I/R. Gallein was titrated from 2.5 to 10 mg/kg weekly over a three week period.
Murine model of ischemia/reperfusion injury
Male ten to twelve week old mice were subjected to myocardial ischemia/reperfusion (I/R) injury as described previously.(4) Briefly, mice were anesthetized with isoflurane, ventilated with a rodent ventilator (MiniVent Type 845, Harvard Apparatus), and a left thoracotomy was performed. The left circumflex coronary artery was ligated for 30 minutes, followed by four weeks of reperfusion. Mouse body temperature was maintained at 37 °C for the duration of ischemia, which was confirmed by the observation of ST wave elevation by electrocardiogram. Sham animals, which served as controls, received identical surgical procedures excluding the final coronary artery ligation.
Echocardiography
Transthoracic echocardiographic analysis using the VisualSonics Vevo 2100 was utilized to assess cardiac function in anesthetized mice 4 weeks post-injury as described previously.(5) Two-dimensional m-mode echocardiograms and strain imaging were obtained along the long axis of the left ventricle at the level of the papillary muscles. All measurements were performed in a blinded fashion by the Cincinnati Children’s Medical Center echocardiography core.
Cell Culture
Adult murine cardiomyocytes and cardiac fibroblasts were prepared from the ventricles of mice with minor modifications to the protocol described previously.(6) Mice were anesthetized with isoflurane and given 100μL heparin (100U/mL) via intraperitoneal injection. Heart was excised and immediately suspended on a Langendorff apparatus by cannulation of the aortic root, and perfused at a constant rate of 4mL/min at 37 °C starting with 4 minutes of perfusion buffer. Subsequently, enzymatic digestion was achieved by 3 minutes perfusion with calcium-free digestion buffer (600 units/mL of collagenase II in perfusion buffer; Worthington LS004177) followed by 8 minutes of perfusion with digestion buffer containing 12.5μM CaCl2. Hearts were removed from the perfusion apparatus, atria were removed and ventricles placed in stopping buffer I (10% fetal bovine serum and 12.5 μM CaCl2 in perfusion buffer). Ventricles were gently mechanically disrupted using transfer pipettes until tissue was sufficiently digested. Cell suspension was filtered through a 200 μm mesh and cardiomyocytes were allowed to settle by gravity for 15 minutes. The supernatant was collected and centrifuged at 500 xg for 7 minutes to collect the first non-myocyte fraction. Cardiomyocytes were resuspended in 10 mL stopping buffer II (5% fetal bovine serum and 12.5μM CaCl2 in perfusion buffer) and subsequently allowed to settle for 15 minutes. Supernatant was collected, centrifuged and combined with the first non-myocyte fraction. Non-myocytes were resuspended and plated in growth media on a 10 cm plate coated with 1% gelatin. Cardiomyocytes were resuspended in stopping buffer II and CaCl2 was added incrementally to a final concentration of 1mM. The final cardiomyocyte pellet was resuspended in plating media (M199 media with FBS, glutamine, BSA, ITS and BDM) and plated onto laminin-coated glass chamber slides for 1hr in a 37 °C incubator at 5% CO2, or alternatively directly lysed for RNA/protein extraction.
Human Fibroblast Isolation
Failing human cardiac fibroblasts were isolated from ventricular tissue excised during left ventricular assist device implantation surgery. Tissue was washed in DMEM (HyClone SH30022.01) and cut into 1mm cubed pieces with scissors. Digestion mixture, consisting of 100mg collagenase (Worthington CLS-2), 1mg trypsin (Worthington TLR3) and 15mg BSA in DMEM, was applied to dissected tissue, and mixture was placed in shaker at 37°C for 20min. After incubation, a 10mL pipette was used to gently dissociate cells, and supernatant was collected and combined with neutralization media containing 10% FBS; this process was then repeated. The combined supernatant was centrifuged at 1300rpm for 7 minutes to collect the non-myocyte pellet, which was then resuspended in growth media and plated on gelatin-coated dishes. Media was changed every 48hr until cells reached confluency.
Flow Cytometry
Flow cytometry analysis was performed on the isolated non-cardiomyocyte fractions from GRK2fl/fl and GRK2fl/fl x PostnMCM hearts 15 days after I/R injury using a BD five-laser FACSAria II running FACSDiva software with the following configuration: 405 nm laser for Alexa405 (CD45), 561 nm laser for 7AAD and PE-Cy7 (CD31) and 640 nm laser for APC (PDGFRα). Single suspension cardiac cells were stained with conjugated primary antibodies as indicated: 405-conjugated rat anti-CD45 (BD Biosciences), PE-Cy7-conjugated rat anti CD31 (BD Biosciences), APC (allophycocyanin)-conjugated rat anti- PDGFRα (eBioscience). Briefly, cells were incubated for 15 min on ice with 1% bovine serum in HBSS containing antibodies at a 1:100 dilution. Cells were then washed three times with HBSS and stained with 7AAD (1:100, Biolegend) to eliminate dead cells. Labeled cells were sorted and gating was determined using single-strain and fluorescence minus one (FMO) controls and data analysis was performed using FlowJo v10.
Myocyte Contractility
Cardiomyocyte sarcomeric contractility was assessed as described previously.(6) Briefly, glass chamber slides were placed on a microscope stage (Olympus IX71) connected to a field stimulator (MyoPacer, IonOptix). Cardiomyocytes were stimulated at 0.5 Hz and imaged with a variable field-rate camera (MYO100 MyoCam, IonOptix). Peak contraction, the percentage of peak cell shortening, and maximum rates of contraction and relaxation were determined. Each data point represents cardiomyocytes isolated from a single animal with at least 10 individual cardiomyocytes measured.
Histology and Immunohistochemistry
To perform histological and immunohistochemical analyses, whole hearts were excised, fixed in a 10% formalin solution and embedded in paraffin. Sections were cut at thickness of 7 μm. For picrosirius red staining, sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Sections were incubated in 0.1% sirius red in saturated picric acid for 1hr, then washed in acidified water, cleared in ethanol and xylene, and mounted. The level of fibrosis was quantified as the ratio of the Sirius red-stained area to the total LV area using Image J software (National Institutes of Health).
For immunofluorescence analysis of cardiac sections, slides were deparaffinized and antigen retrieval was performed using antigen unmasking solution (Vector Laboratories H-3300). For cells grown on coverslips, cells were fixed in 4% paraformaldehyde and stained using an antibody for αSMA (Sigma A5228). Slides were incubated with primary antibodies directed against mouse periostin (Biovendor RD181045050), vimentin (Santa Cruz sc5565), actin (fluorescently labeled phalloidin; Life Technologies A12379) and sarcomeric α-actinin (Sigma A7811) to stain cardiomyocytes. This was followed by incubation with AlexaFluor secondary antibodies (Life Technologies); negative controls utilized only secondary antibodies. Sections were also stained with 4’, 6-diamidino-2-phenylindole (DAPI) for visualization of nuclei and mounted (Life Technologies P36962). TUNEL staining was performed using the FragEL™ DNA Fragmentation Detection Kit (MilliporeSigma QIA39) according to the manufacturer’s protocol.
Real-time qPCR Analysis
RNA was isolated and gene expression was analyzed by quantitative PCR. Total RNA was extracted from left ventricular tissue using the RNeasy mini kit (Qiagen) according to manufacturer protocols. cDNA construction was performed using the iScript cDNA synthesis kit (BioRad) per manufacturer’s instructions. Real-time qPCR was performed on the Applied Biosystems 7500 Fast Real-Time PCR system using TaqMan Master Mix (Life Technologies 4370074). Verified primer-probe sets were purchased from Applied Biosystems. The reaction conditions consisted of an initial denaturing step of 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds followed by 60°C for 1 minute. Gene expression was normalized to the expression of the 18S ribosomal subunit as an internal control, and is expressed as fold change calculated using the ΔΔCt comparative method.
Protein Preparation and Immunoblotting
Protein samples were collected using RIPA buffer (25mM Tris HCl, 150mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton-X, proteinase/phosphatase inhibitors, pH 7.6). Lysates were centrifuged at 14,000xg for 15 minutes at 4°C, and supernatant was removed and used for immunoblotting. Protein concentrations were determined by BCA Assay (Pierce 23227) using manufacturer’s protocols. Equal amounts of protein lysate were loaded onto pre-cast NuPAGE 4–12% Bis-Tris gels (Invitrogen), and proteins were transferred to PVDF membranes. Membranes were blocked using LiCOR blocking buffer (927–40000) and blotted using primary antibodies for GRK2 (Santa Cruz sc562), αSMA (Abcam ab5694) and GAPDH (Millipore MAB374) as an internal control. Primary antibodies were detected using fluorescently labeled secondary antibodies (Li-COR Biosciences). Blots were imaged and quantified using the Odyssey CLX Imaging System.
Collagen Contractility Assay
The ability of fibroblasts to contract collagen was assessed using the Cell Contraction Assay (Cell Biolabs CBA-201) per the manufacturer’s protocol. Fibroblasts were combined with a collagen solution, which was then allowed to polymerize. The collagen gel was detached from the plate and collagen gel size change (contraction index) was measured at various times and quantified with ImageJ (NIH).
MTT Proliferation Assay
Cell proliferation was determined using the MTT Cell Growth Assay (Millipore CT02) per manufacturer’s protocols. Briefly, fibroblasts were grown in a 96-well plate, and cell number was assessed in 24hr increments. The MTT substrate is cleaved in living cells by active mitochondria into a dark blue formazan crystal; absorbance is measured on a plate reader at 570nm and is proportional to the number of live cells present.
Transwell Migration Assay
A 24-well transwell plate containing an 8.0 μm polycarbonate membrane (Corning 3421) was utilized to assess cell migratory capacity. 10,000 fibroblasts were seeded into the upper chamber in serum-free media in the presence of gallein, and media with FBS was added to the well. The plate was incubated at 37°C for 24hr, at which time the membrane was fixed with 4% paraformaldehyde and migrated cells were stained with DAPI. Four images were taken per membrane, and number of migrated cells were counted using ImageJ. Results demonstrate the average number of cells per field.
cAMP Assay
Cardiac fibroblasts were plated in 12-well plates and treated with gallein or vehicle. Cells were incubated with Krebs-Ringers Solution containing IBMX for 30 minutes to inhibit endogenous phosphodiesterase activity, then stimulated with 10 μM isoproterenol for 10 minutes at 37°C to induce cAMP production. Cellular lysates were assayed for cAMP using the Cell Signaling Cyclic AMP XP® Assay Kit according to manufacturer’s instructions.
Statistical Analysis
Data is reported as mean ± standard error of the mean (SEM). For single biochemical and physiological observations, student t-test was applied when comparing treatments. Multiple responses were analyzed by one-way or two-way ANOVA. Where significant heterogeneity of variances was identified, the results of statistical analysis following logarithmic transformation of the data are shown. Post-hoc analysis was performed as described if statistical significance (P ≤ 0.05) was achieved. Calculations were performed using Graphpad Prism 6.0.
Supplementary Material
Supplemental Figure 1. Cardiac function and fibrotic scar tissue formation post I/R in WT mice treated with gallein. Echocardiographic analysis of wildtype mice four weeks after receiving either I/R injury or sham surgery, with representative long axis m-mode images (A); cardiac function is assessed by percent fractional shortening (B) and diastolic ventricular volume (C). Sham n = 7; I/R Vehicle n = 10; I/R Gallein n = 8. *P < 0.05; **P < 0.01 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis). Fibrotic scar remodeling revealed by Masson’s Trichrome staining showing collagen deposition in blue (D).
Supplemental Figure 2. Heart failure and fibrotic marker mRNA expression in gallein treated mice post I/R. Transcript expression of heart failure marker Nppa (A; statistical analysis following log transformation) and fibrotic markers Col3a1 (B) and Fn1 (C) normalized to 18s ribosomal subunit expression as quantified by qPCR. n = 6 per group (Nppa – GRK2fl/fl I/R n = 5 due to statistical outlier by Grubb’s Test). *P < 0.05; **P < 0.01 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 3. Cardiac function and GRK2 expression in cardiomyocyte specific GRK2 knockout mice. Timeline of surgery and drug administration (A). Tamoxifen is administered via the chow for two weeks following injury; gallein is initiated one week post-I/R and cardiac function is assessed four weeks after injury by echocardiography. Western blot and quantification of GRK2 protein expression in primary cardiomyocytes isolated from GRK2fl/fl x αMHCMCM mice following tamoxifen administration (B)..GRK2fl/fl x αMHCMCM n = 4; GRK2fl/fl x αMHCMCM + tamoxifen n = 3. **P < 0.01 (mean ± SEM; student t test). Cardiac function was assessed by strain analysis; the parameters peak percentage (C) and ventricular dyssynchrony as quantified by maximum opposing wall delay (D) were determined. GRK2fl/fl Sham n = 13; GRK2fl/fl I/R n = 24; GRK2fl/fl x αMHCMCM I/R n = 13; GRK2fl/fl x αMHCMCM I/R Gal n = 12. **P < 0.01 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 4. Cardiac function and pathologic remodeling in cardiomyocyte-specific GRK2 knockout mice +/− gallein. Cardiac functional assessment by echocardiography shown by percent ejection fraction (A), percent fractional shortening (B) and left ventricular volume (C), with representative long axis m-mode images (D). GRK2fl/fl Sham n = 13; GRK2fl/fl I/R n = 24; αMHCMCM I/R / GRK2fl/fl x αMHCMCM I/R n = 13; GRK2fl/fl x αMHCMCM I/R Gal n = 12. Fibrotic scar formation, by picrosirius red staining, and periostin expression within the ventricular free wall (F); fibrosis quantification is expressed as the proportion of fibrotic region over total left ventricular area (E). GRK2fl/fl Sham n = 4; GRK2fl/fl I/R n = 6; αMHCMCM I/R n = 8; GRK2fl/fl x αMHCMCM I/R ± Gal n = 5. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 5. GRK2 knockdown and fibrotic gene expression in activated fibroblast specific GRK2 knockout mice. Timeline of surgery and drug administration (A). Tamoxifen is administered via the chow for two weeks following injury; gallein or vehicle is initiated one week after injury and cardiac function is assessed four weeks post I/R by echocardiography. To assess GRK2 knockdown, CD31/45 negative and PDGFRα-positive cells were selected for using flow cytometry (B); GRK2 transcript expression was quantified by qPCR normalized to 18s (C; statistical analysis following log transformation). GRK2fl/fl I/R n = 24; GRK2fl/fl x PostnMCM I/R n = 3. *P < 0.01 (mean ± SEM; student t test). Percent fractional shortening as determined by echocardiography (D). GRK2fl/fl Sham n = 13; GRK2fl/fl x PostnMCM Sham n = 5; GRK2fl/fl I/R n = 24; PostnMCM I/R n = 5. Transcript expression of Col3a1 (E) and Nppb (F; statistical analysis following log transformation) was assessed by qPCR from ventricular lysates. GRK2fl/fl Sham / GRK2fl/fl I/R n = 6; GRK2fl/fl x PostnMCM Gal ± Gal n = 5. *P < 0.05 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 6. Failing human cardiac fibroblast purity and integrity assessment. Failing human cardiac fibroblasts were co-stained for the relatively specific fibroblast marker vimentin and actin using fluorescently labeled phalloidin (A). Relative fibroblast purity was assessed by determining the percent of cells isolated from failing human cardiac tissue that had positive vimentin expression (B). TUNEL staining was performed to investigate the level of cellular apoptosis in vehicle- and gallein-treated cardiac fibroblasts; the positive control consists of HL-60 cells treated with actinomycin D (C).
Supplemental Table 1. Echocardiographic parameters of cardiomyocyte-specific GRK2 KO mice post-I/R
Supplemental Table 2. Echocardiographic parameters of fibroblast-specific GRK2 KO mice treated with gallein
Table 1.
Echocardiographic Parameters: Mice Treated with Gallein Post-I/R
| Heart Rate (beats/min) | Fractional Shortening (%) | Ejection Fraction (%) | Diastolic LVID (mm) | Diastolic LV Vol (μl) | LVPW (mm) | Peak Percentage (%) | |
|---|---|---|---|---|---|---|---|
| GRK2fl/fl Sham | 519 ± 17 | 33.38 ± 1.99 | 62.31 ± 2.70 | 3.87 ± 0.13 | 66.12 ± 5.34 | 1.23 ± 0.04 | −18.37 ± 1.04 |
| GRK2fl/fl I/R | 505 ± 12 | 22.75 ± 1.23* | 45.63 ± 2.20* | 4.34 ± 0.11† | 86.92 ± 5.63† | 0.98 ± 0.04* | −12.84 ± 0.81* |
| GRK2fl/fl I/R Gal | 506 ± 20 | 34.09 ± 2.83‡ | 62.64 ± 3.81‡ | 3.85 ± 0.14§ | 65.27 ± 4.95§ | 1.21 ± 0.04|| | −17.83 ± 1.13|| |
Values are mean ± SEM.
p < 0.001 vs. sham.
p < 0.05 vs. sham.
p < 0.001 vs. I/R vehicle.
p < 0.05 vs. I/R vehicle.
p < 0.01 vs. I/R vehicle.
Gal = gallein; I/R = ischemia-reperfusion; LVID = left ventricular internal dimension; LVPW = left ventricle posterior wall thickness; LV Vol = left ventricular volume.
Table 2.
Echocardiographic Parameters: GRK2fl/fl x PostnMCM Mice Treated with Gallein Post-I/R
| Heart Rate (beats/min) | Fractional Shortening (%) | Ejection Fraction (%) | Diastolic LVID (mm) | Diastolic LV Vol (μl) | LVPW (mm) | Peak Percentage (%) | |
|---|---|---|---|---|---|---|---|
| GRK2fl/fl Sham | 520 ± 17 | 33.38 ± 1.99 | 62.31 ± 2.70 | 3.87 ± 0.13 | 66.12 ± 5.34 | 1.23 ± 0.04 | −18.37 ± 1.04 |
| GRK2fl/fl I/R | 505 ± 12 | 22.75 ± 1.23* | 45.63 ± 2.20* | 4.34 ± 0.11† | 86.92 ± 5.63† | 0.98 ± 0.04‡ | −12.84 ± 0.81‡ |
| GRK2fl/fl x PostnMCM I/R | 522 ± 14 | 32.69 ±1.52§ | 61.38 ± 2.19§ | 3.89 ± 0.10† | 66.58 ± 4.08|| | 1.21 ± 0.04¶ | −16.12 ± 0.98 |
| GRK2fl/fl x PostnMCM I/R Gal | 508 ± 26 | 31.30 ± 2.08# | 59.50 ± 2.94# | 3.88 ± 0.14 | 66.41 ± 6.10 | 1.07 ± 0.03 | −16.81 ± 1.30|| |
Values are mean ± SEM.
p < 0.0001 vs. sham.
p < 0.05 vs. sham.
p < 0.001 vs. sham.
p < 0.0001 vs. I/R vehicle.
p < 0.05 vs. I/R vehicle.
p < 0.001 vs. I/R vehicle.
p < 0.01 vs. I/R vehicle.
Abbreviations as in Table 1.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
Cardiac fibrosis represents a quintessential component of adverse myocardial remodeling and HF progression. However, no current clinical interventions specifically target the cardiac fibroblast or its adverse contributions to disease progression. Here, we report a novel potential therapeutic approach for combatting pathological cardiac remodeling.
TRANSLATIONAL OUTLOOK
These findings demonstrated the efficacy of small molecule or activated fibroblast targeting of the Gβγ-GRK2 interface after myocardial ischemic injury, and provide further evidence for the therapeutic potential of novel treatments directed against this signaling pathway for HF treatment.
Acknowledgments
This work was funded by NIH R01 HL132551, R01 HL133695, R01 HL134312 (BCB), P01 HL069779 (BCB and JDM), an AHA postdoctoral fellowship (FAK), and a predoctoral fellowship from the Pharmaceutical Research and Manufacturers of America Foundation (JGT). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- α-MHC
α-myosin heavy chain
- α-SMA
smooth muscle α-actin
- β-AR
β-adrenergic receptor
- cAMP
cyclic adenosine monophosphate
- GRK2
G protein-coupled receptor kinase 2
- I/R
ischemia-reperfusion
- LV
left ventricle
- qPCR
quantitative polymerase chain reaction
- TGF-β
transforming growth factor β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res. 2016;118:1021–40. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y, Weber KT. Animal models of cardiac fibrosis. Methods Mol Med. 2005;117:273–90. doi: 10.1385/1-59259-940-0:273. [DOI] [PubMed] [Google Scholar]
- 4.Kamal FA, Travers JG, Blaxall BC. G protein-coupled receptor kinases in cardiovascular disease: why “where” matters. Trends Cardiovasc Med. 2012;22:213–9. doi: 10.1016/j.tcm.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Philip JL, Xu X, Theccanat T, Abdur Razzaque M, Akhter SA. beta-Arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J Mol Cell Cardiol. 2014;76:73–83. doi: 10.1016/j.yjmcc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raake PW, Schlegel P, Ksienzyk J, et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–47. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey LM, Pistner AR, Belmonte SL, et al. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res. 2010;107:532–9. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamal FA, Mickelsen DM, Wegman KM, et al. Simultaneous adrenal and cardiac g-protein-coupled receptor-gbetagamma inhibition halts heart failure progression. J Am Coll Cardiol. 2014;63:2549–57. doi: 10.1016/j.jacc.2014.02.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher SM, Gao E, Zhu W, et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7:277ra31. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonacci TM, Mathews JL, Yuan C, et al. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–6. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 11.Sohal DS, Nghiem M, Crackower MA, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–5. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 12.Kanisicak O, Khalil H, Ivey MJ, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Q, Chen M, Zuo L, et al. Myocardial Ablation of G Protein-Coupled Receptor Kinase 2 (GRK2) Decreases Ischemia/Reperfusion Injury through an Anti-Intrinsic Apoptotic Pathway. PLoS One. 2013;8:e66234. doi: 10.1371/journal.pone.0066234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raake PW, Vinge LE, Gao E, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–22. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Insel PA, Murray F, Yokoyama U, et al. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol. 2012;166:447–56. doi: 10.1111/j.1476-5381.2012.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamal FA, Travers JG, Schafer AE, Ma Q, Devarajan P, Blaxall BC. G Protein-Coupled Receptor-G-Protein betagamma-Subunit Signaling Mediates Renal Dysfunction and Fibrosis in Heart Failure. J Am Soc Nephrol. 2017;28:197–208. doi: 10.1681/ASN.2015080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39:60–76. doi: 10.1016/s0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 18.Ram R, Mickelsen DM, Theodoropoulos C, Blaxall BC. New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol. 2011;301:H1765–80. doi: 10.1152/ajpheart.00559.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams ML, Hata JA, Schroder J, et al. Targeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109:1590–3. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 20.Swonger JM, Liu JS, Ivey MJ, Tallquist MD. Genetic tools for identifying and manipulating fibroblasts in the mouse. Differentiation. 2016;92:66–83. doi: 10.1016/j.diff.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9:548–55. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac Fibroblast GRK2 Deletion Enhances Contractility and Remodeling Following Ischemia/Reperfusion Injury. Circ Res. 2016;119:1116–27. doi: 10.1161/CIRCRESAHA.116.309538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner NA, Porter KE, Smith WH, White HL, Ball SG, Balmforth AJ. Chronic beta2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res. 2003;57:784–92. doi: 10.1016/s0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 24.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A. 2005;102:437–42. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70:1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
References
- 1.Matkovich SJ, Diwan A, Klanke JL, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 2.Sohal DS, Nghiem M, Crackower MA, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–5. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 3.Kanisicak O, Khalil H, Ivey MJ, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawaguchi M, Takahashi M, Hata T, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 5.Ram R, Mickelsen DM, Theodoropoulos C, Blaxall BC. New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol. 2011;301:H1765–80. doi: 10.1152/ajpheart.00559.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey LM, Pistner AR, Belmonte SL, et al. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res. 2010;107:532–9. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cardiac function and fibrotic scar tissue formation post I/R in WT mice treated with gallein. Echocardiographic analysis of wildtype mice four weeks after receiving either I/R injury or sham surgery, with representative long axis m-mode images (A); cardiac function is assessed by percent fractional shortening (B) and diastolic ventricular volume (C). Sham n = 7; I/R Vehicle n = 10; I/R Gallein n = 8. *P < 0.05; **P < 0.01 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis). Fibrotic scar remodeling revealed by Masson’s Trichrome staining showing collagen deposition in blue (D).
Supplemental Figure 2. Heart failure and fibrotic marker mRNA expression in gallein treated mice post I/R. Transcript expression of heart failure marker Nppa (A; statistical analysis following log transformation) and fibrotic markers Col3a1 (B) and Fn1 (C) normalized to 18s ribosomal subunit expression as quantified by qPCR. n = 6 per group (Nppa – GRK2fl/fl I/R n = 5 due to statistical outlier by Grubb’s Test). *P < 0.05; **P < 0.01 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 3. Cardiac function and GRK2 expression in cardiomyocyte specific GRK2 knockout mice. Timeline of surgery and drug administration (A). Tamoxifen is administered via the chow for two weeks following injury; gallein is initiated one week post-I/R and cardiac function is assessed four weeks after injury by echocardiography. Western blot and quantification of GRK2 protein expression in primary cardiomyocytes isolated from GRK2fl/fl x αMHCMCM mice following tamoxifen administration (B)..GRK2fl/fl x αMHCMCM n = 4; GRK2fl/fl x αMHCMCM + tamoxifen n = 3. **P < 0.01 (mean ± SEM; student t test). Cardiac function was assessed by strain analysis; the parameters peak percentage (C) and ventricular dyssynchrony as quantified by maximum opposing wall delay (D) were determined. GRK2fl/fl Sham n = 13; GRK2fl/fl I/R n = 24; GRK2fl/fl x αMHCMCM I/R n = 13; GRK2fl/fl x αMHCMCM I/R Gal n = 12. **P < 0.01 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 4. Cardiac function and pathologic remodeling in cardiomyocyte-specific GRK2 knockout mice +/− gallein. Cardiac functional assessment by echocardiography shown by percent ejection fraction (A), percent fractional shortening (B) and left ventricular volume (C), with representative long axis m-mode images (D). GRK2fl/fl Sham n = 13; GRK2fl/fl I/R n = 24; αMHCMCM I/R / GRK2fl/fl x αMHCMCM I/R n = 13; GRK2fl/fl x αMHCMCM I/R Gal n = 12. Fibrotic scar formation, by picrosirius red staining, and periostin expression within the ventricular free wall (F); fibrosis quantification is expressed as the proportion of fibrotic region over total left ventricular area (E). GRK2fl/fl Sham n = 4; GRK2fl/fl I/R n = 6; αMHCMCM I/R n = 8; GRK2fl/fl x αMHCMCM I/R ± Gal n = 5. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 5. GRK2 knockdown and fibrotic gene expression in activated fibroblast specific GRK2 knockout mice. Timeline of surgery and drug administration (A). Tamoxifen is administered via the chow for two weeks following injury; gallein or vehicle is initiated one week after injury and cardiac function is assessed four weeks post I/R by echocardiography. To assess GRK2 knockdown, CD31/45 negative and PDGFRα-positive cells were selected for using flow cytometry (B); GRK2 transcript expression was quantified by qPCR normalized to 18s (C; statistical analysis following log transformation). GRK2fl/fl I/R n = 24; GRK2fl/fl x PostnMCM I/R n = 3. *P < 0.01 (mean ± SEM; student t test). Percent fractional shortening as determined by echocardiography (D). GRK2fl/fl Sham n = 13; GRK2fl/fl x PostnMCM Sham n = 5; GRK2fl/fl I/R n = 24; PostnMCM I/R n = 5. Transcript expression of Col3a1 (E) and Nppb (F; statistical analysis following log transformation) was assessed by qPCR from ventricular lysates. GRK2fl/fl Sham / GRK2fl/fl I/R n = 6; GRK2fl/fl x PostnMCM Gal ± Gal n = 5. *P < 0.05 (mean ± SEM; one-way ANOVA with Tukey post-hoc analysis).
Supplemental Figure 6. Failing human cardiac fibroblast purity and integrity assessment. Failing human cardiac fibroblasts were co-stained for the relatively specific fibroblast marker vimentin and actin using fluorescently labeled phalloidin (A). Relative fibroblast purity was assessed by determining the percent of cells isolated from failing human cardiac tissue that had positive vimentin expression (B). TUNEL staining was performed to investigate the level of cellular apoptosis in vehicle- and gallein-treated cardiac fibroblasts; the positive control consists of HL-60 cells treated with actinomycin D (C).
Supplemental Table 1. Echocardiographic parameters of cardiomyocyte-specific GRK2 KO mice post-I/R
Supplemental Table 2. Echocardiographic parameters of fibroblast-specific GRK2 KO mice treated with gallein