Abstract
Aim:
Dental tissues such as enamel, dentinoenamel junction (DEJ), dentin, and root dentin can react differently to demineralization and remineralization. The aim of this study was to evaluate the remineralization ability of sodium fluoride on the microhardness of enamel, dentin, and dentinoenamel junction.
Materials and Methods:
Ten extracted third molar teeth were sectioned mesiodistally to form control and test groups. For the test group, initial demineralization was done with acetic acid for 24 h followed by remineralization for 28 days by application of sodium fluoride (226 ppm) for 2 min twice a day. Vickers microhardness test was done to control and test groups at different sites after initial demineralization and on the 3rd, 5th, 7th, 14th, and 28th day of remineralization.
Statistical Analysis Used:
Data were analyzed with one-way analysis of variance and post hoc test with a significance level of P < 0.001 with SPSS (21) software.
Results:
Microhardness values in the demineralization group were significantly lower than controls (P < 0.001). Evaluation of remineralization samples showed that microhardness similar to control values were achieved at the 3rd day in root predentin and on the 5th day in coronal dentin and coronal predentin. On the 7th day, remineralization coronal predentin was significantly higher than the control (P < 0.001). On the 14th day, DEJ axial zone and root dentin were similar to control and coronal dentin was significantly higher than the control (P < 0.001). Enamel was similar to control on the 28th day. Microhardness of DEJ-cusp tip and DEJ-center of the fissure was significantly lower than control even at the 28th day (P < 0.001).
Conclusion:
Long-term repeated application of sodium fluoride (226 ppm) can improve the microhardness of demineralized dental tissues on enamel, dentin, and DEJ-axial zone, except in the DEJ-cusp tip and DEJ-center of fissure.
Keywords: Dentinoenamel junction, microhardness, remineralization, sodium fluoride
INTRODUCTION
Enamel and dentin are functionally organized to provide an integrated mechanical system in the human tooth. Ectodermally derived enamel is hard, brittle, and will provide a masticatory surface, which is wear and acid resistant. Ectomesenchymally derived dentin is softer, flexible, and tough.[1] Dentin can absorb energy and resist fracture. Physical properties of enamel and dentin have been studied by many investigators.[2,3]
The functional success of teeth depends on the stable union of structurally diverse enamel and dentin by the dentinoenamel junction (DEJ).[1] It is also believed that the DEJ may help in preventing crack propagation from enamel to dentin, which may inhibit further catastrophic tooth fracture.[4]
Xu et al.[5] using Raman spectroscopy reported that there are changes in the width of the DEJ in different intratooth locations along with differences in the inorganic and organic components between occlusal and cervical DEJ sites.
Dental caries is defined as a pathological process of localized destruction and demineralization of enamel and dentin by microorganisms.[6] When it progresses, acid produced by bacterial action on dietary fermentable carbohydrates diffuses into the tooth and dissolves the carbonated hydroxyapatite mineral resulting in demineralization.[7]
Fluoride has a high affinity to calcified tissues.[8] Topical and/or systemic fluoride exposure is very common. Therefore, it is important to evaluate the influence of fluoride on remineralization and microhardness, both of which can affect tooth quality. Tooth quality is related to the tooth's ability to fulfill its function, including withstanding occlusal forces, and may be evaluated by the measurement of a tooth's material, structural, and mechanical properties.[8]
The aim of this study, therefore, was to investigate the microhardness of enamel, coronal dentin, root dentin, and the DEJ before and after demineralization and remineralization with sodium fluoride (NaF 226 ppm) using Vickers microhardness apparatus.
MATERIALS AND METHODS
Thirteen freshly extracted impacted noncarious third molars from individuals aged between 25 and 30 years, requiring such extractions as part of their dental treatment, were collected and ultrasonically cleaned to remove hard and soft deposits and then were stored in 10% buffered formalin for 14 days.[9] Transillumination was done to exclude possibilities of cracks and extraction damage. After exclusion of three samples, a total of 10 teeth (n = 10) were taken and randomly divided into two groups, Group A (n = 5) and Group B (n = 5). They were stored in filtered distilled water throughout the study.
All teeth samples were sectioned mesiodistally into two equal parts using a water-cooled low-speed Isomet diamond saw (Buehler Ltd., Lake Bluff, IL, USA) resulting in 10 samples per group. Group A samples were divided into Control A (n = 5) and test Group A (n = 5); similarly, Group B samples were divided into Control B (n = 5) and test Group B (n = 5). After sectioning, all the samples were mounted on cold resin filled in cylindrical molds, with the sectioned surface facing outward and then metallographically polished through a series of silicon carbide abrasive papers from 600 to 3000 grit and finely polished with water-based diamond paste of 1–0.25 μm (Buehler, Lake Bluff, IL, USA)[3] to provide a flat surface.
Microhardness testing was done with the Vickers microhardness tester with a standard load of 500 g force and a dwell time of 20 s, at 8 sites on each sample with 5 repetitions making 45 test sites per sample.[1] The mean value of five repetitions was taken as the microhardness of that particular site.
The control Groups A and B (n = 10) were initially tested for microhardness.
For the test samples, the test Group A and test Group B were demineralized for 24 h out of which the Test Group A samples alone were tested for Vickers microhardness (demineralized Group). The Test Group B samples were stored in a remineralizing solution and were remineralized for 28 days with NaF (226 ppm) applied for 2 min twice a day. They were tested for microhardness (remineralized Group) on the 3rd, 5th, 7th, 14th, and 28th day.
Microhardness sites for all the groups are as follows [Figure 1].
Figure 1.
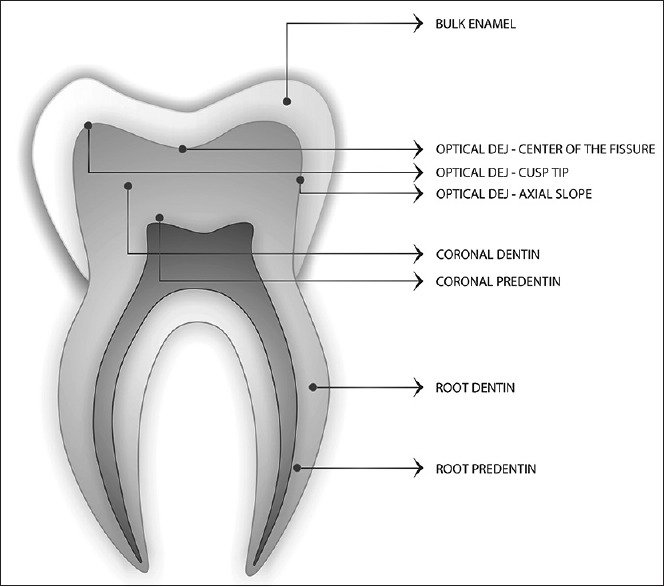
Tooth section showing the indentation sites
Enamel at least 200 μm from the DEJ
Optical DEJ-cusp tip (±5 μm)
Optical DEJ-axial zone (±5 μm)
Optical DEJ-occlusal fissure (±5 μm)
Coronal dentin at least 200 μm from the DEJ
Coronal predentin
Root dentin
Root predentin.
Interaction between neighboring indentations was minimized by maintaining a distance of at least two times the crack length in enamel and similarly two times the impression diagonal for dentin.[10]
The demineralization solution contained 2.2 mM CaCl2, 2.2 mM KH2PO4, and 0.05 M acetic acid and the pH was adjusted to 4.4 with 1M KOH[11] and the samples were demineralized for 24 h.
The remineralizing solution contained 1.5 mM CaCl2, 0.9 mM KH2PO4, and 0.13 mMKCl and the pH was adjusted to 7.0 KOH[11] along with NaF of 226 ppm application for 2 min twice a day and tested for microhardness on 3rd, 5th, 7th, 14th, and 28th day.[12]
Statistical analysis
Statistical analysis was performed with one-way analysis of variance and post hoc test with a significance level of P < 0.001 with SPSS (20.2, SPSS Inc., Chicago, II, USA) software.
RESULTS
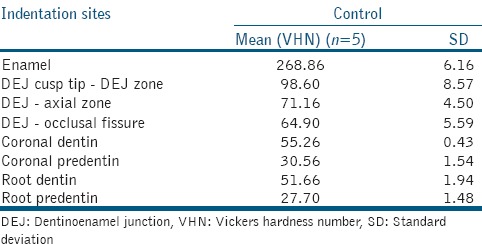
Microhardness values for Control A and Control B were compared and there was statistically no significant difference between them. The mean values [Table 1] were referred to as Control GrouP values.
Table 1.
Microhardness values of control group
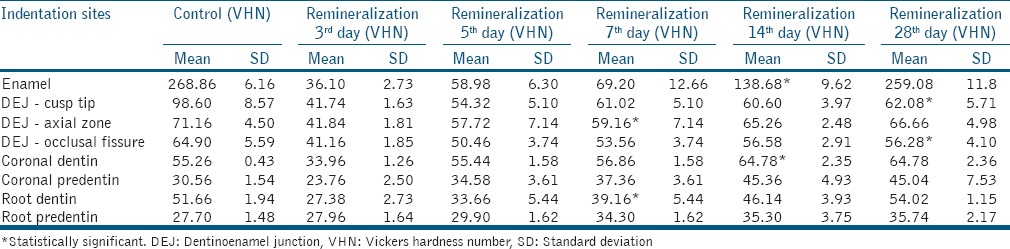
Microhardness values in the demineralization group [Table 2] were significantly lower than controls (P < 0.001). After the 3rd day of remineralization, only root predentin had microhardness values similar to control. At the 5th day, coronal dentin and coronal predentin also reached values similar to control. On the 7th day, coronal predentin was significantly higher than the control values (P < 0.001). On the 14th day, DEJ axial zone and root dentin reached values similar to control values and coronal dentin was significantly higher than the control values (P < 0.001). On the 28th day, enamel values were close to control, while DEJ-cusp tip and DEJ-occlusal fissure were still significantly lower than the control (P < 0.001) [Table 3].
Table 2.
Comparison of control and demineralization microhardness values
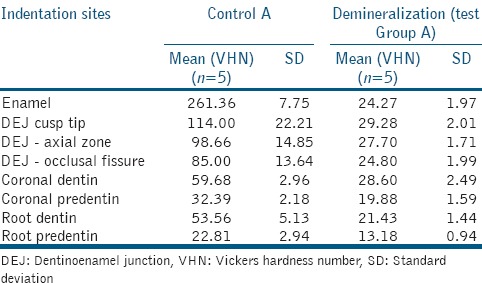
Table 3.
Microhardness of control with different remineralization time periods
DISCUSSION
The effect of fluoride on different dental tissues such as enamel and dentin may vary during remineralization.[13] In this in vitro study, tooth demineralization and remineralization was evaluated in enamel, coronal dentin, coronal predentin, root dentin, and root predentin by evaluating the Vickers microhardness. In addition, the DEJ was also evaluated at three different locations.
Fluoride has demonstrated caries preventive effects. Fluoride can get incorporated into hydroxyapatite forming fluorohydroxyapatite, which has a lower solubility.[14] Although Sh et al.[15] have reported that amine fluoride may markedly increase enamel microhardness in comparison to NaF, Lippert et al. 2009[16] have reported that the anticaries potential of NaF was better. Therefore, in this study, we have used NaF (226 ppm) as a remineralizing agent. Artificial saliva can mimic oral environment. However, remineralization may depend on localized concentrations of calcium and phosphate, rather than concentrations in saliva. Calcium and phosphate concentrations, and the corresponding calcium-to-phosphate ratios, in the remineralization solution may be higher than physiological saliva.[11] We have stored the specimens in a remineralizing solution of known composition. Thus, the concentration and ratio of the ions may determine the rate and the site of mineral deposition in the demineralized lesion.
Vickers microhardness test was used to evaluate demineralized and remineralized dental tissues, as it is a relatively simple, rapid, and nondestructive method and has been previously used.[15,17]
When control values were evaluated, enamel values showed the highest microhardness (268.38 Vickers hardness number [VHN]) and also showed marked decrease in microhardness values after demineralization to 23.64 VHN. Similar results were obtained by Diniz in 2009[18] who reported that the microhardness of bulk enamel was 286.77 VHN which reduced to 38.48 VHN after demineralization.
After remineralization with NaF (226 ppm), there was a gradual increase in microhardness values in Enamel to 251.08 VHN at the 28th day, which was similar to the control values (268.38 VHN).
These results were similar to a study by Salehzadeh Esfahani et al., 2015,[19] who compared the effects of casein phosphopeptide–amorphous calcium phosphate complex, Remin Pro, and 5% sodium fluoride varnish on remineralization of enamel lesions and reported that 1-month application of all compounds improved microhardness and achieved baseline values.
The change in properties across the DEJ has been termed its functional width. Studies using varying methods, for example, fracture studies, and indentation studies, have given different estimates of the functional width.[1] Marshall et al. in 2003[20] have reported that the functional width of the DEJ may vary from 1 to 11.8 micron based on the technique used for evaluation.
In this study, microhardness was evaluated at the optical DEJ ± 5 μm. Xu et al.[5] have suggested that the structure of the DEJ may vary depending on its intratooth location, which may be related to function.
The DEJ was evaluated at three sites. In the control samples, DEJ-cusp tip showed the highest microhardness values (98.60 VHN), followed by DEJ-axial zone (71.16 VHN) and DEJ-occlusal fissure (64.90 VHN). All these sites demonstrated a significant reduction in microhardness after demineralization.
After remineralization, all three DEJ locations showed an increase in microhardness until 28th day. However, the microhardness values for DEJ-cusp tip and DEJ-occlusal fissure were significantly lower than the controls even after 28 days of remineralization with NaF whereas DEJ-axial zone showed values similar to control. Similarly, Lechner et al.[21] in their AFM study of the demineralized DEJ have also reported that the remineralization process occurs much more slowly and in two phases. The attributed reason according to Lechner et al.[21] was erosion caused deeper clefts in DEJ whereas trenches were formed in enamel and dentin. Prolonged remineralization caused globular particles (presumably inorganic calcium phosphate) to refill the deep clefts. These results indicate that DEJ at the cusp tips and DEJ occlusal fissure may remineralize at a much slower rate than the DEJ axial zone, which may have clinical implications.
Microhardness values of coronal dentin increased to near normal values of 55.44 VHN on the 5th day of remineralization to significantly higher values than the control by the 14th day. Similarly, the remineralization values of coronal predentin increased to 34.58 VHN on the 5th day, which was close to the control values, and the values increased to 37.36 VHN on the 7th day, with further increase to 45.04 VHN on the 28th day which was significantly higher than the control.
Root dentin and root predentin values for the control group were 51.66 VHN and 27.70 VHN, respectively. Craig and Peyton in 1958[22] obtained similar microhardness values of 34 Knoop hardness number (KHN) on dentin adjacent to the root canal. Saleh and Ettman in 1999[23] also obtained similar results of 51.7–54.4 KHN for root dentin that was 1 mm away from the pulp. It has been reported by Ryge et al. in 1961[24] that VHN and KHN of dentin are comparable with loads of 500 g.
After demineralization, the microhardness values for root dentin decreased to 21.43 VHN and to 13.18 VHN for root predentin. With remineralization, the microhardness values for root dentin steadily increased reaching 46.14 VHN on the 14th day and 54.02 VHN on the 28th day, which were similar to the control values. Remineralization of root predentin was much faster with 3rd day values of 27.96 VHN, which was similar to the control root predentin value. The values further increased to 35.74 VHN that was significantly higher than the control on the 28th day.
In this study, it was seen that dentin remineralized at a much faster rate than enamel. Similar results were reported by Laheij et al. 2010[13] who evaluated in situ remineralization of enamel and dentin.
According to ten Cate in 2008,[25] the demineralized organic matrix of dentin may constitute a scaffold to enhance remineralization. Moreover, proteins such as dentin phosphoprotein may play a role in enhanced mineralization of dentin.[26]
Similarly van Strijp et al. 1999s[27] observed full remineralization of dentinal lesions after using fluoride toothpastes and observed that remineralization conditions for dentin lesions may be favorable compared to enamel lesions. They suggested that the demineralized organic matrix of dentin may constitute a scaffold to enhance remineralization.
CONCLUSION
Within the limitations of this in vitro study, it can be concluded that:
The fastest zones to get remineralized were root predentin which happened on the 3rd day, while coronal predentin and coronal dentin were on the 5th day
Remineralization values of DEJ-axial zone and root dentin were comparable to control on the 14th day
Enamel values were comparable to control values on the 28th day
Microhardness of DEJ-cusp tip and DEJ Center of the fissure did not reach control values by the 28th day. Further studies are required to assess the time duration needed to achieve optimal remineralization on the DEJ site
Clinical significance is that NaF (226 ppm) therapy should continue beyond 28 days to achieve optimal remineralization.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.White SN, Miklus VG, Chang PP, Caputo AA, Fong H, Sarikaya M, et al. Controlled failure mechanisms toughen the dentino-enamel junction zone. J Prosthet Dent. 2005;94:330–5. doi: 10.1016/j.prosdent.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen ST, Patchin RE. Fracture properties of human enamel and dentin in an aqueous environment. J Dent Res. 1984;63:1362–8. doi: 10.1177/00220345840630120501. [DOI] [PubMed] [Google Scholar]
- 3.Braly A, Darnell LA, Mann AB, Teaford MF, Weihs TP. The effect of prism orientation on the indentation testing of human molar enamel. Arch Oral Biol. 2007;52:856–60. doi: 10.1016/j.archoralbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CP, Douglas WH. Structure-property relations and crack resistance at the bovine dentin-enamel junction. J Dent Res. 1994;73:1072–8. doi: 10.1177/00220345940730050901. [DOI] [PubMed] [Google Scholar]
- 5.Xu C, Yao X, Walker MP, Wang Y. Chemical/molecular structure of the dentin-enamel junction is dependent on the intratooth location. Calcif Tissue Int. 2009;84:221–8. doi: 10.1007/s00223-008-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds EC. Calcium phosphate – Based remineralization systems: Scientific evidence? Aust Dent J. 2008;53:268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 7.Vieira AP, Hanocock R, Eggertsson H, Everett ET, Grynpas MD. Tooth quality in dental fluorosis genetic and environmental factors. Calcif Tissue Int. 2005;76:17–25. doi: 10.1007/s00223-004-0075-3. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council. Health Effects of Ingested Fluoride. Washington, DC: National Academy Press; 1993. [Google Scholar]
- 9.Secilmis A, Dilber E, Ozturk N, Yilmuz FG. The effect of storage solutions on mineral content of enamel. Mater Sci Appl. 2013;4:439–45. [Google Scholar]
- 10.Xu HH, Smith DT, Jahanmir S, Romberg E, Kelly JR, Thompson VP, et al. Indentation damage and mechanical properties of human enamel and dentin. J Dent Res. 1998;77:472–80. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- 11.Exterkate RA, Damen JJ, ten Cate JM. A single-section model for enamel de- and remineralization studies 1. The effects of different Ca/P ratios in remineralization solutions. J Dent Res. 1993;72:1599–603. doi: 10.1177/00220345930720121201. [DOI] [PubMed] [Google Scholar]
- 12.Dogan F, Civelek A, Oktay I. Effect of different fluoride concentrations on remineralization of demineralized enamel: An in vitro pH cycling study. OHDMBSC. 2004;1:20–6. [Google Scholar]
- 13.Laheij AM, van Strijp AJ, van Loveren C. In situ remineralization of enamel and dentin after the use of an amine flouride mouthrinse in addition to twice daily brushings with amine flouride toothpaste. Caries Res. 2010;44:260–6. doi: 10.1159/000314673. [DOI] [PubMed] [Google Scholar]
- 14.ten Cate JM. Fluorides in caries prevention and control: Empiricism or science. Caries Res. 2004;38:254–7. doi: 10.1159/000077763. [DOI] [PubMed] [Google Scholar]
- 15.Sh P, Raghu R, Shetty A, Gautham P, Reddy S, Srinivasan R. Effect of organic versus inorganic fluoride on enamel microhardness: An in vitro study. J Conserv Dent. 2013;16:203–7. doi: 10.4103/0972-0707.111314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippert F, Newby EE, Lynch RJ, Chauhan VK, Schemehorn BR. Laboratory assessment of the anticaries potential of a new dentifrice. J Clin Dent. 2009;20:45–9. [PubMed] [Google Scholar]
- 17.Lata S, Varghese NO, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phosphor peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent. 2010;13:42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diniz MB, Paes Leme AF, Cardoso Kde S, Rodrigues Jde A, Cordeiro Rde C. The efficacy of laser fluorescence to detect in vitro demineralization and remineralization of smooth enamel surfaces. Photomed Laser Surg. 2009;27:57–61. doi: 10.1089/pho.2007.2230. [DOI] [PubMed] [Google Scholar]
- 19.Salehzadeh Esfahani K, Mazaheri R, Pishevar L. Effects of treatment with various remineralizing agents on the microhardness of demineralized enamel surface. J Dent Res Dent Clin Dent Prospects. 2015;9:239–45. doi: 10.15171/joddd.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall SJ, Balooch M, Habelitz S, Balooch G, Gallagher R, Marshall GW. The dentin-enamel junction – A natural, multilevel interface. J Eur Ceram Soc. 2003;23:2897–904. [Google Scholar]
- 21.Lechner BD, Röper S, Messerschmidt J, Blume A, Magerle R. Monitoring demineralization and subsequent remineralization of human teeth at the dentin-enamel junction with atomic force microscopy. ACS Appl Mater Interfaces. 2015;7:18937–43. doi: 10.1021/acsami.5b04790. [DOI] [PubMed] [Google Scholar]
- 22.Craig RG, Peyton FA. The micro-hardness of enamel and dentin. J Dent Res. 1958;37:661–8. doi: 10.1177/00220345580370041301. [DOI] [PubMed] [Google Scholar]
- 23.Saleh AA, Ettman WM. Effect of endodontic irrigation solutions on microhardness of root canal dentine. J Dent. 1999;27:43–6. doi: 10.1016/s0300-5712(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 24.Ryge G, Foley DE, Fairhurst CW. Micro-identa-tion hardness. J Dent Res. 1961;40:1116–26. [Google Scholar]
- 25.ten Cate JM. Remineralization of deep enamel dentine caries lesions. Aust Dent J. 2008;53:281–5. doi: 10.1111/j.1834-7819.2008.00063.x. [DOI] [PubMed] [Google Scholar]
- 26.Milan AM, Sugars RV, Embery G, Waddington RJ. Adsorption and interactions of dentine phosphoprotein with hydroxyapatite and collagen. Eur J Oral Sci. 2006;114:223–31. doi: 10.1111/j.1600-0722.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 27.van Strijp AA, Buijs MJ, ten Cate JM. In situ fluoride retention in enamel and dentine after the use of an amine fluoride dentifrice and amine fluoride/sodium fluoride mouthrinse. Caries Res. 1999;33:61–5. doi: 10.1159/000016496. [DOI] [PubMed] [Google Scholar]


