Abstract
Aim:
The purpose of this study was to evaluate the influence of chlorhexidine (CHX), grape seed extract (GSE), riboflavin/chitosan modification on microtensile bond strength (μTBS) of composite resin to dentin after polymerase chain reaction (PCR) thermocycling.
Materials and Methods:
Forty extracted human molars were used and a flat surface was then prepared by removing the occlusal one-third. The teeth were randomly assigned into four groups - Group I in which self-etch adhesive (Adper Easy One) was applied and Groups II, III, IV were pretreated with 2% CHX, 6.5% GSE, and 1% riboflavin/chitosan, respectively, before the application of self-etch adhesive. Composite build-ups were constructed, and PCR thermocycling (5000 cycles) was performed. The μTBS was evaluated using the universal testing machine. Data were analyzed using one-way analysis of variance and Tukey's test.
Results:
The mean μTBS values for Group I (control), Group II (CHX), Group III (GSE), and Group IV (riboflavin/chitosan modification) were 30.81, 43.15, 38.79, and 35.07 MPa, respectively.
Conclusion:
Pretreatment with CHX and GSE leads to a significant increase in μTBS of composite resin to dentin.
Keywords: Chlorhexidine, grape seed extract, microtensile bond strength, polymerase chain reaction thermocycling, riboflavin/chitosan
INTRODUCTION
The use of composite restorations has transfigured today's dental practice by being able to replace the lost tooth tissue in an invisible and conservative way with immense success.[1] Achieving efficient and stable bond between composite and dentin still remains a challenge in restorative dentistry.[2]
While bonding to enamel has been shown to be authentic over time, bonding to dentin is a great challenge. The major limitations of dentin as a bonding substrate are its heterogeneous composition and hydrophilic nature.[3]
An important challenge to the dentin bond durability is degradation of collagen from the matrix-bound proteases, namely matrix metalloproteinases (MMPs) and cysteine cathepsins. Pretreatment of the bonding substrate with agents that inhibit the activity of MMPs might improve bond durability. Chlorhexidine (CHX) strongly inhibits the proteolytic activities of MMP-2, -8, and -9.[4]
Several chemicals, both natural and synthetic, which have the ability to increase the collagen cross-links are used to improve the bond durability. Proanthocyanidins (PAs) are oligomeric flavonoids found in high concentrations in grape seed, pine bark, cranberries, lemon tree bark, and hazelnut tree leaves.[5] Very few studies have been done to find the role of grape seed extract (GSE) in improving the bonding characteristics of dental adhesives.
Riboflavin helps in collagen cross-linking by its ability to produce free radicals when photoactivated with spectral range from ultraviolet to visible light.[6] In addition to cross-linking, reinforcement of the collagen can be achieved by incorporating biopolymers such as chitosan that can be cross-linked with collagen fibrils. Incorporation of chitosan improves the biological and mechanical properties of collagen.[7,8,9]
The aim of this study was to evaluate the effects of aging and pretreatment using CHX, GSE, riboflavin/chitosan modification on microtensile bond strength (μTBS) of composite resin bonded to dentin with self-etch adhesive after polymerase chain reaction (PCR) thermocycling.
MATERIALS AND METHODS
Preparation of solutions
6.5% Grape seed extract solution
A quantity of 6.5 g of GSE in the form of powder (Zenith Nutrition, Medizen Labs Pvt. Ltd., India) was collected from the capsules and dissolved in 100 mL of distilled water.
Riboflavin and chitosan modification
One gram of riboflavin in the form of powder was collected from the capsules and dissolved in 100 mL of distilled water.
One gram of chitosan in the form of powder (Pelican Biotech and Chemicals Labs Pvt. Ltd., Kerala, India) was dissolved in 100 mL of distilled water. Then, chitosan was added to riboflavin at 20% v/v ratio.
Specimen preparation
Forty noncarious extracted human permanent molars were selected for the study. The extracted teeth were stored in 0.1% (w/v) thymol immediately after extraction at room temperature for not more than 1 month. The roots of the teeth were removed using a water-cooled low-speed cutting saw with micromotor handpiece. A flat surface was then prepared by removing the occlusal one-third of the tooth crowns to expose the mid-coronal dentin. The dentin surface was polished using a 600-grit silicon carbide paper to create a standardized smear layer. The teeth were randomly divided into four groups, the control group of ten teeth and the experimental groups of thirty teeth.
Group I (n = 10, control)
No pretreatment was done on the exposed dentin surface. The self-etch adhesive, Adper Easy One, was used according to the manufacturer's instructions. Adhesive was applied to tooth surface for a total of 20 s and then gently air dried for 5 s and light cured (Blue phase LED light-curing unit, Monitex GT 1200, Taiwan) for 10 s. Composite build-up was done by placing two increments of 2-mm-thick composite resin (Filtek Z 250 XT, Universal restorative, A2 shade, 3M ESPE, St. Paul, MN, USA) with each increment being light cured for 20 s.
The experimental specimens (n = 30) were randomly divided into three groups based on the surface treatment of dentin as follows.
Group II (n = 10)
2% CHX solution pretreatment. A composition of 2% CHX digluconate solution (Neelkanth Healthcare Pvt. Ltd., Safe Plus, Rajasthan, India) was applied to the dentin for 30 s, and then dried with absorbent paper.
Group III (n = 10)
6.5% GSE solution pretreatment. The specimens were pretreated with 6.5% GSE solution for 10 min and rinsed with distilled water.
Group IV (n = 10)
1% riboflavin/chitosan pretreatment. Dentin surface was pretreated with 1% riboflavin/chitosan for 5 min and photoactivated by conventional dental blue light-curing unit of 600 mW/cm2 output for 20 s.
Then, dentin bonding and composite restoration were done as described in the control group.
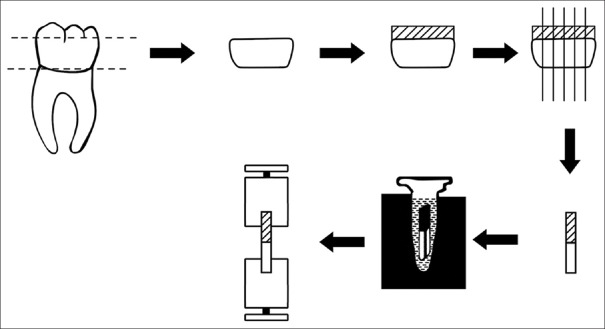
Teeth are sectioned as shown in Figure 1 across the adhesive interface to obtain forty resin-dentin beams with cross-sectional areas of about 1 mm × 1 mm with the IsoMet saw (Low-speed Buehler, USA).
Figure 1.
Experimental diagram of polymerase chain reaction thermocycling. Thermocycling was performed for 5000 cycles on the polymerase chain reaction thermal cycler heat block
Thermocycling using polymerase chain reaction thermal cycler
The resin-dentin beams were placed into PCR tubes with artificial saliva as medium, and then placed in heat blocks. Thermocycling (5000 cycles) was performed through a PCR program at two different temperatures (5°C and 55°C) using a PCR thermal cycler (Eppendorf Mastercycler Pro Thermal Cyclers, Applied Biosystems, Foster City, CA, USA).
In conventional thermocycling, two different water baths are used and specimens are transferred alternatively at 5°C and 55°C. Nakata et al.[10] developed simplified thermocycling method using PCR thermal cycler. In the present study, artificial saliva was used as inner fluid in PCR tubes to better simulate clinical conditions.[11]
Microtensile bond strength test
Each resin-dentin beam was attached to the testing apparatus with a cyanoacrylate adhesive and loaded until failure under tension using universal testing machine (DL 2000; Emic Sao Jose de Pinhas, PR, Brazil) at a cross-head speed of 1 mm/min. The μTBS was calculated as the maximum load at failure divided by the cross-sectional area, and was expressed in MPa.
Statistical analysis
The data presented as mean ± standard deviations were calculated using SPSS version 16.0 (SPSS, Chicago, IL, USA) software. One-way analysis of variance was applied to evaluate the μTBS values and post hoc multiple comparison tests were conducted using Tukey's test at 5% significance level.
RESULTS
The results of this study are shown in Table 1. The results showed that the mean μTBS values (MPa) of Group II (43.15 ± 3.69), III (38.79 ± 2.64), IV (35.07 ± 4.71) were significantly higher than the mean μTBS value in the control group (30.81 ± 5.98) (P < 0.001).
Table 1.
Means±standard deviations of microtensile bond strengths in MPa of different study groups

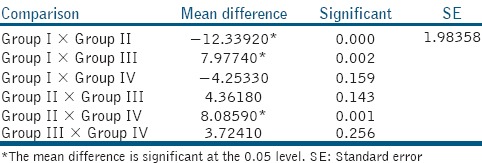
Intergroup comparisons are shown in Table 2. Mean μTBS of Group II and III showed a difference of −12.33 and −7.97 MPa, respectively, when compared with Group I which was statistically significant (P < 0.05). Group IV when compared with Group I showed a difference of −4.25 MPa (P > 0.05), which was statistically not significant.
Table 2.
Post hoc multiple comparisons using Tukey's test
DISCUSSION
Dentin collagen reinforcement and strengthening through inter- and intra-molecular collagen cross-linking help increase bond strength and durability of the resin/dentin interface with time against enzymatic and/or hydrolytic degradation.
After performing PCR thermocycling for 5000 times, CHX, GSE, and riboflavin/chitosan groups showed higher bond strength values compared to the control group.
Group II (2% CHX) showed a significantly higher bond strength to dentin compared with Group I (control), Group III (GSE), and Group IV (riboflavin/chitosan). It can be due to its MMP-inhibitory properties which prevent the binding of metal ions, such as zinc or calcium, to MMPs, thus inhibiting its catalytic activity.[12] Not only MMPs but also evidence of inhibition of dentinal cysteine cathepsins B, K, and L by CHX had recently been demonstrated.[13]
Group III (6.5% GSE) showed significantly higher bond strength to dentin compared with Groups I and IV (P < 0.001). This is in accordance with the findings of Srinivasulu et al.,[3] who showed that the application of 6.5% GSE to deep dentin significantly improved the shear bond strength values of composite to dentin compared with the use of 10% sodium ascorbate. The increase in bond strength may be due to the greater number of collagen cross-links which improved collagen stability. Unique characteristics of PAs include presence of four monomers molecules (catechin, ent-catechin, epicatechin, ent-epicatechin) and different types of interflavonoid bonds.[14] PAs bind to proline-rich proteins, such as collagen, and facilitate the enzyme proline hydroxylase activity, essential for collagen biosynthesis.[15,16]
Castellan et al.[17] showed that when demineralized dentin was treated with PA, it resulted in improved mechanical properties and reduced water absorption due to the formation of dense collagen network. The proposed mechanisms for interaction include covalent, ionic, hydrogen bonding, and hydrophobic interactions.
Group IV (1% riboflavin/chitosan) showed higher bond strength to dentin compared with Group I but was not statistically significant. Riboflavin is a strong free radical-producing agent when activated by light with maximum absorption peaks at wavelengths of 270, 366 and 445 nm. Although the use of ultraviolet light activation was proven effective as a photoactivation method, the safety issues regarding the use of ultraviolet A (UVA) and its practicality for dental use should be considered. Conventional blue light-curing units might be a possible alternative owing to its ready availability and its safe use in dentistry.[6]
The free radicals (O2 and O2−) are released when riboflavin is photoactivated forming covalent cross-links between adjacent collagen molecules. Reduction in histidine and tyrosine during cross-linking and the formation of dityrosine is a possible mechanism in collagen aggregation mediated through riboflavin.[18]
Cova et al.[19] showed that riboflavin/UVA pretreatment can also inactivate MMPs, particularly MMP-9 through direct cross-linking. Telopeptidase activity of osteoclast-derived MMP-9, eliminating collagen molecule telopeptides, is considered essential for collagenase activity against insoluble bone collagen. The riboflavin-induced MMP-9 inhibition may be responsible for the increased durability of hybrid layers, through reduced MMP-9 telopeptidase activity.
Fawzy et al.[20] showed that modification with riboflavin/chitosan increased the mechanical properties, enhanced the mechanical stability of demineralized dentin substrates against hydrolytic and/or collagenolytic degradation challenges and decreased hydroxyapatite release with collagenase exposure. When chitosan was added to riboflavin at 20% v/v ratio, significant improvement in bond strength at 24 h and 6 months in distilled water was found indicating the positive dual effect on bonding to dentin.
The use of riboflavin and chitosan/riboflavin formulations to modify dentin collagen-matrix, with the defined ratios, stabilizes the collagen fibrillar network and enhances resin infiltration and hybrid layer formation.[21]
Thermal stresses, generated through the repeated contracting expanding process during low–high temperature cycling, are also one of the key factors that influence bond strength.
The PCR thermal cycler, which is used for DNA amplification, can perform reactions simultaneously in many samples with a high degree of precision. The PCR cycler is repeatedly cycled from a high temperature for melting to lower temperature for annealing and extension during thermocycling. Character of the temperature change was gradual warming up and cooling.[12]
Yi Liu et al. found that different concentrations of PA (0%, 2.5%, 5%, and 10%) hamper the monomer conversion and alters the polymerization kinetics of bis-GMA/HEMA model adhesive, but within acceptable limits.[22] GSE may stain dentin brown and the durability of long-term bond strength need to be examined.[23]
However, future research should more fully investigate the role of cross-linking agents on dentinal MMPs.
CONCLUSION
Within the limitations of this in vitro study, significant improvement in the bond strength of resin composite to dentin bonded using a self-etch adhesive was obtained when the dentin surface was pretreated with 2% CHX, 6.5% GSE, 1% riboflavin/chitosan compared with the control group.
Hence, dentin pretreatment with collagen cross-linkers can be safely recommended as an effective chairside procedure to improve μTBS of composite resin to dentin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Poptani B, Gohil KS, Ganjiwale J, Shukla M. Microtensile dentin bond strength of fifth with five seventh-generation dentin bonding agents after thermocycling: An in vitro study. Contemp Clin Dent. 2012;3(Suppl 2):S167–71. doi: 10.4103/0976-237X.101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: Aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasulu S, Vidhya S, Sujatha M, Mahalaxmi S. Effect of collagen cross-linkers on the shear bond strength of a self-etch adhesive system to deep dentin. J Conserv Dent. 2013;16:135–8. doi: 10.4103/0972-0707.108194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekambaram M, Yiu CK, Matinlinna JP, King NM, Tay FR. Adjunctive application of chlorhexidine and ethanol-wet bonding on durability of bonds to sound and caries-affected dentine. J Dent. 2014;42:709–19. doi: 10.1016/j.jdent.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Fine AM. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern Med Rev. 2000;5:144–51. [PubMed] [Google Scholar]
- 6.Fawzy AS, Nitisusanta LI, Iqbal K, Daood U, Neo J. Riboflavin as a dentin crosslinking agent: Ultraviolet A versus blue light. Dent Mater. 2012;28:1284–91. doi: 10.1016/j.dental.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Everaerts F, Torrianni M, van Luyn M, van Wachem P, Feijen J, Hendriks M. Reduced calcification of bioprostheses, cross-linked via an improved carbodiimide based method. Biomaterials. 2004;25:5523–30. doi: 10.1016/j.biomaterials.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 8.Madhavan K, Belchenko D, Motta A, Tan W. Evaluation of composition and crosslinking effects on collagen-based composite constructs. Acta Biomater. 2010;6:1413–22. doi: 10.1016/j.actbio.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha A, Friedman S, Kishen A. Photodynamically crosslinked and chitosan-incorporated dentin collagen. J Dent Res. 2011;90:1346–51. doi: 10.1177/0022034511421928. [DOI] [PubMed] [Google Scholar]
- 10.Nakata T, Fujita M, Nagano F, Noda M, Sano H. Effect of a new thermal cycling method on bond strength of two-step self-etching adhesive systems. Dent Mater J. 2007;26:635–41. doi: 10.4012/dmj.26.635. [DOI] [PubMed] [Google Scholar]
- 11.Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B Appl Biomater. 2007;80:268–72. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 12.Deng D, Huang X, Huang C, Yang T, Du X, Wang Y, et al. Effects of chlorhexidine on bonding durability of different adhesive systems using a novel thermocycling method. Aust Dent J. 2013;58:148–55. doi: 10.1111/adj.12038. [DOI] [PubMed] [Google Scholar]
- 13.Scaffa PM, Vidal CM, Barros N, Gesteira TF, Carmona AK, Breschi L, et al. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J Dent Res. 2012;91:420–5. doi: 10.1177/0022034511435329. [DOI] [PubMed] [Google Scholar]
- 14.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003;65:118–24. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 15.Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256:4494–7. [PubMed] [Google Scholar]
- 16.Ku CS, Sathishkumar M, Mun SP. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere. 2007;67:1618–27. doi: 10.1016/j.chemosphere.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater. 2010;26:968–73. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato Y, Uchida K, Kawakishi S. Aggregation of collagen exposed to UVA in the presence of riboflavin: A plausible role of tyrosine modification. Photochem Photobiol. 1994;59:343–9. doi: 10.1111/j.1751-1097.1994.tb05045.x. [DOI] [PubMed] [Google Scholar]
- 19.Cova A, Breschi L, Nato F, Ruggeri A, Jr, Carrilho M, Tjäderhane L, et al. Effect of UVA-activated riboflavin on dentin bonding. J Dent Res. 2011;90:1439–45. doi: 10.1177/0022034511423397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fawzy AS, Nitisusanta LI, Iqbal K, Daood U, Beng LT, Neo J. Chitosan/Riboflavin-modified demineralized dentin as a potential substrate for bonding. J Mech Behav Biomed Mater. 2013;17:278–89. doi: 10.1016/j.jmbbm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Daood U, Iqbal K, Nitisusanta LI, Fawzy AS. Effect of chitosan/riboflavin modification on resin/dentin interface: Spectroscopic and microscopic investigations. J Biomed Mater Res A. 2013;101:1846–56. doi: 10.1002/jbm.a.34482. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Wang Y. Effect of proanthocyanidins and photo-initiators on photo-polymerization of a dental adhesive. J Dent. 2013;41:71–9. doi: 10.1016/j.jdent.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability – A literature review. Dent Mater. 2016;32:e41–53. doi: 10.1016/j.dental.2015.11.007. [DOI] [PubMed] [Google Scholar]