Abstract
Context:
Direct pulp capping involves the placement of a biocompatible agent on pulp tissue that has been inadvertently exposed from traumatic injury or by iatrogenic means.
Aim:
To compare the human pulp response to calcium hydroxide and Biodentine as direct pulp-capping agents.
Materials and Methods:
Pulp exposures were performed on the pulpal floor of forty human permanent premolars. The exposure sites were dressed with either Dycal or Biodentine as pulp-capping materials. After 45 days, teeth were extracted and processed for histological examination.
Statistical Analysis:
The histological data were subjected to Wilcoxon rank-sum test.
Results:
The dentinal bridges in teeth that were capped with Biodentine were significantly thicker (P < 0.0001) and more continuous (P = 0.0001) with less pulpal inflammation (P = 0.0044) in comparison to Dycal.
Conclusion:
Based on the result of this study, Biodentine can be suggested as the material of choice for direct pulp capping procedure instead of Dycal. However, further long-term follow-up in vivo human studies using Biodentine on cariously exposed pulpal teeth are warranted to derive a definite conclusion.
Keywords: Biodentine, calcium hydroxide, direct pulp capping
INTRODUCTION
Direct pulp capping involves the placement of a biocompatible agent on pulp tissue that has been inadvertently exposed from traumatic injury or by iatrogenic means. The aim of the treatment is to maintain healthy pulp tissue by sealing the pulp against bacterial penetration and initiating a dentine bridge formation at the exposure site. Long-term clinical studies indicate that direct pulp capping can produce success rates of between 80% and 90%.[1,2] These figures are comparable to the success rate of root canal treatment which is expected to be 85% to 90%.[3]
Dentine bridge formation can occur under a myriad of pulp-capping materials such as Zinc oxide-eugenol cements, polycarboxylate cements, collagen, calcium hydroxide (Ca[OH]2), bonding agents, glass ionomer cements; to the more recent agents such as mineral trioxide aggregate (MTA), Biodentine, Emdogain, Propolis, and Endosequence.
Ca(OH)2 was introduced into dentistry by Hermann[4] and according to Schroder[5] Ca(OH)2 compounds are the gold standard for pulp capping in human teeth. A major disadvantage of Ca(OH)2 materials is that they do not seal the exposed pulp from the external environment. The inability of Ca(OH)2 to seal out bacteria might eventually lead to failure of the treatment.
Various animal and human studies postulated MTA to be superior to and a good substitute to Ca(OH)2 as a pulp-capping agent.[6,7,8,9,10,11] However, MTA has certain disadvantages such as, it is difficult to handle, it has a long setting time, and it has high material cost. To overcome these disadvantages, the recently introduced Biodentine has been proposed as a direct pulp-capping agent.
Biodentine was introduced in the dental practice as a restorative material and has recently been proposed for direct pulp capping. Biodentine is a new calcium silicate-based restorative cement with dentine-like mechanical properties. In direct contact with vital pulp tissue, it also promotes the formation of reparative dentine.[12]
Hence, the purpose of this clinical study was to evaluate the histological response of healthy pulp to Biodentine when used as a direct pulp-capping agent and to compare it with Ca(OH)2 (Dycal).
MATERIALS AND METHODS
This single-blinded, randomized, clinical study was conducted after gaining approval from the Institutional Ethics Committee. All the subjects were treated in accordance with the Helsinki Declaration. Consent forms were signed by patients and/or parents after receiving a detailed explanation about the experimental rationale, clinical procedures, and possible risks associated with the study. Forty healthy human premolar teeth scheduled for extraction for orthodontic reasons were selected from patients ranging from 15 to 25 years of age. A detailed case history was taken for all the patients, followed by clinical examination and standard intraoral periapical radiographs for each tooth; to assure the absence of caries, trauma, periapical, and periodontal lesions. The status of the pulp was assessed using electric pulp test (C Pulse, Foshan Coxo, China).
The procedure commenced with the administration of local anesthesia (2% lidocaine with 1:100,000 adrenaline; pharmacaine A/Pharmax Pvt. Ltd., India). Teeth were cleaned using 0.2% chlorhexidine solution (Hexidine, ICPA Health Products Ltd., India). After rubber dam application, standardized occlusal cavities were prepared by sterile diamond burs (SF-31; Mani, Inc., Japan) at high speed under air-water spray coolant. Pulp exposure of approximately 1 mm was performed in the center of the pulpal floor by means of a sterilized round diamond point (BR-45, Mani, Inc., Japan) under water cooling. Complete hemostasis was achieved by applying gentle pressure on the exposed site with cotton pellet moistened with sterile saline. The teeth were then randomly divided into two groups of twenty samples each by the coin flipping method. Those patients in whom there were 4 teeth for extraction, coin flipping method was done for each arch separately.
After hemostasis, the exposure site was dressed with pulp-capping materials directly in contact with the pulp tissue. In Group I, Dycal (Dentsply Caulk Milford, DE, USA; Batch no. 523899) base and catalyst were mixed on a paper pad with a plastic spatula according to the manufacturer's instructions. In Group II, Biodentine (Septodont, Saint-Maur-des-Fosses, France; Batch no. 05993) powder was mixed according to the manufacturer's instructions. The mixture was carried to the exposure site. After the placement of either pulp-capping materials, resin-modified glass ionomer cement (Glass Ionomer Light-Cured Universal Restorative 1-1 Mini Pack, GC Corporation, Tokyo, Japan) was placed as a liner followed by final restoration with composite resin (Medicept's Micro-Hybrid Composites Matrix).
On the 45th day after the procedure, electric sensitivity testing was performed to assess pulp health and then teeth were extracted as atraumatically as possible by a designated oral and maxillofacial surgeon.
The apical third of all teeth were sectioned to facilitate fixation in 10% formalin. After histopathological processing and staining with hematoxylin and eosin, all sections were blindly evaluated by two experienced oral and maxillofacial pathologists using the pentahead microscope (Labovision AXR 51) under ×40, ×100, and ×400 magnifications. In case of disagreement between the two examiners, the section was reexamined, and the more severe evaluation was chosen.[13]
All sections were evaluated for five histological features [Table 1]:
Table 1.
Grading used during histological examination

Dentine bridge formation at pulp medicament interface
Morphology of dentinal bridge
Inflammatory cell response
Continuity of dentinal bridge
Thickness of the dentinal bridge - The thickness of dentinal bridge was measured at the thickest, thinnest, and midmost point areas of the continuous dentinal bridge using the computer-assisted image analyzer. The average of the three values was calculated.[12]
Data thus collected was submitted for statistical analysis. Descriptive statistics such as mean, standard deviation, median, and range for each parameter were obtained. The comparison of two materials was carried out using Wilcoxon rank-sum test. The statistical significance was evaluated at 5% level, and all the analysis was carried out in R-3.0.0 programming package.
RESULTS
The pulp-capping procedure was completed on forty teeth from 14 patients; there were five male and nine female patients. Patients reported no particular symptoms during the experimental period. Before extraction, all teeth were electrosensitive and had vital pulp. There were no treatment failures and no dropouts.
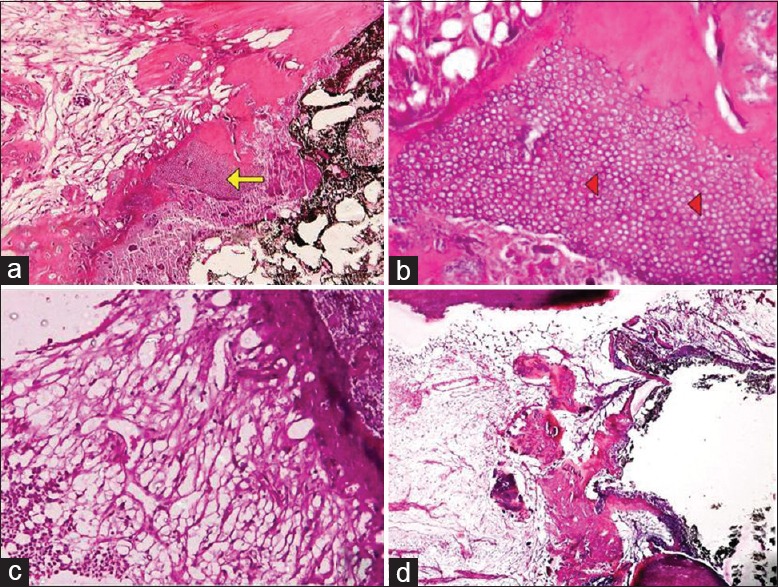
The results of this study as depicted in Table 2 showed no statistically significant difference between the Dycal and Biodentine samples in relation to the presence of dentinal bridge at the pulp medicament interface and morphology of dentinal bridge formed (P > 0.05). A dentinal bridge was formed directly underneath the capping materials at the pulp exposure site in all of the samples except one (5%) for both materials. In most of the samples in both groups, dentine was associated with an irregular hard tissue with odontoblast and odontoblast-like cells seen adjacent to the dentinal bridge with well-distinguishable dentine tubules [Figures 1a and b, 2a and b].
Table 2.
Comparison of histological features between both the materials
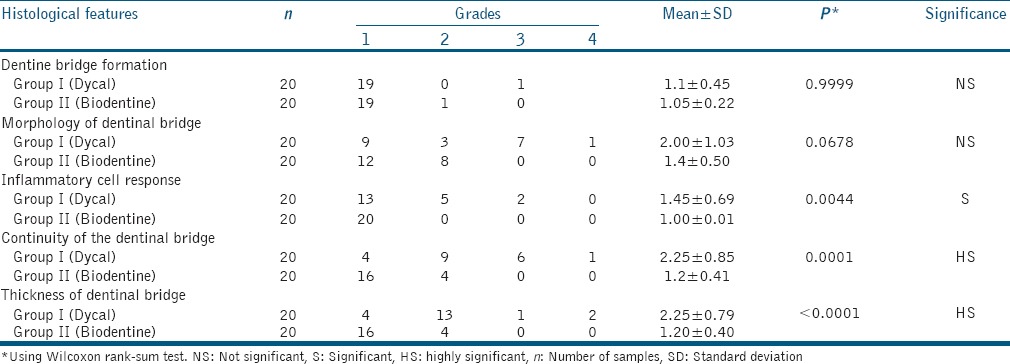
Figure 1.
Bridge formation under Dycal. (a) Dentine formation at ×100 magnification (arrow), (b) dentinal tubules and odontoblastic processes at ×400 magnification (arrowheads), (c) mild pulpal inflammation, (d) thin, discontinuous bridge
Figure 2.
Bridge formation under Biodentine. (a) Bridge lined by odontoblasts at ×100 magnification (arrows), (b) odontoblastic processes at ×400 magnification (arrowheads), (c) no pulpal inflammation, (d) thick, continuous bridge (arrow)
5 (25%) and 2 (10%) samples of Dycal showed the presence of mild and severe pulpal inflammation, respectively, [Figure 1c] whereas, there was no evidence of inflammation, abscess, or necrosis below the dentinal bridge in the Biodentine samples [Figure 2c].
Complete dentinal bridge formation was observed in 16 (80%) samples of Biodentine and 4 (20%) samples of Dycal, and the mean thicknesses of the dentinal bridge in the Biodentine and Dycal groups were 0.58 and 0.17 mm, respectively. Thus, the results of this study showed a significantly thicker and more continuous dentinal bridge in the Biodentine samples with an absence of inflammatory cells in the pulp as compared to Dycal [Figures 1d and 2d].
DISCUSSION
The results of the present study showed that pulps that were treated with Biodentine were essentially free of inflammation and led to the formation of a good quality bridge which was more predictable, uniform, thick, continuous, and completely sealed the pulp tissue in comparison to Dycal.
In similar studies of pulp-capping, 5–10 subjects per group were recruited due to ethical concerns and time constraints.[9,11,14] However, the previous studies have emphasized on having enough number of samples in each group to provide a reasonable condition for statistical analysis.[11,15] Therefore, in the present study, the sample size was twenty for each group.
Preventing microorganisms from entering the pulp and control of bleeding are key factors for successful direct pulp capping. Hence, in the present study, strict isolation with rubber dam application was done to prevent microbial leakage, and complete hemostasis was achieved by applying pressure with cotton pellet moistened with sterile saline. According to the studies, Sodium hypochlorite in concentrations of 0.25% was efficacious for hemorrhage control without being toxic. However, since sodium hypochlorite has a pH of 12, it may extract growth factors from the walls of dentine just as Ca(OH)2.[16] Thus, in the present study, saline was used to control hemorrhage to prevent the supplementary reparative dentinogenesis action of sodium hypochlorite.
Similar to the previous studies, in the present study, a period of 45 days was chosen to evaluate the effect of capping materials on pulp tissues.[12,14] In a study by Yoshiba et al.[17] it was found that after pulp capping with Ca(OH)2, the appearance of dentine-like tissue was observed in 4 weeks after the procedure. Other studies showed that extracts of tricalcium silicate were able to induce human dental pulp stem cells proliferation, differentiation, and mineralization at a faster rate than Ca(OH)2.[18,19] Hence, a period of 45 days was justifiable to study the dentine bridge formation histologically in both the materials.
In the present study, both materials induced the formation of a dentinal bridge at its interface with the pulp tissue. The mechanism of dentine bridge formation varies in the various formulations of Ca(OH)2, depending on the pH of the material. In the case of a high pH material, such as Pulpdent a necrotic zone is formed adjacent to the material, and the dentine bridge then forms between this layer and the underlying vital pulp. The necrotic tissue eventually degenerates and disappears, leaving a void between the capping material and the bridge. In the case of a material of lower pH, such as Dycal, a necrotic zone is formed adjacent to the material but is resorbed before the formation of the dentine bridge, which then comes to be formed directly against the capping material as was observed in the present study.[20,21]
During the time frame of this study, odontoblast-like cell formation and early tubule development were observed in most specimens of the Dycal and Biodentine capped teeth. The success of Ca(OH)2 as a pulp-capping agent is due to the stimulation of odontoblast activity and subsequent mineralization. Biodentine is a tricalcium silicate-based cement that releases Ca(OH)2 as a by-product of hydration.[22] Therefore, it is believed that the mechanism of action of Biodentine is similar to that of Ca(OH)2.
In accordance with the present study, Laurent et al.[23] demonstrated that direct pulp capping with Biodentine, MTA, and Ca(OH)2 using ex vivo human tooth culture model induced odontoblast-like cell differentiation and mineralization foci with a morphological appearance of osteodentine. This was confirmed by the presence of molecular markers of dentine and odontoblasts revealed by immunohistochemistry.[24]
Nowicka et al.[12] compared the pulpal response to MTA and Biodentine in human molars and found no inflammation in the samples of both materials. Similarly, the present study also demonstrated no pulpal inflammation in the Biodentine samples because Biodentine has excellent sealing properties which prevent microleakage and pulpal inflammation by providing a predictable secondary barrier under the surface seal. In accordance with the previous studies, samples that were capped with Dycal showed less satisfactory results.[6,7,9,11,25]
In terms of the continuity of the hard tissue bridge, the findings of the current study are in accordance with Tran et al.,[19] who showed that the reparative structures induced by both calcium silicate cements (MTA and Biodentine) were homogenous and in continuity with primary dentine. In contrast, the reparative tissue induced by Ca(OH)2 had a porous organization. This porosity may eventually facilitate bacterial ingress into the pulp, compromising the tooth vitality.
In a study by Peng et al.[18] extracts of tricalcium silicate showed enhanced human dental pulp stem cells proliferation, differentiation, and mineralization in comparison to Ca(OH)2 due to the silicon ions released from silicate-containing cements. Silicon was reported to promote osteoblast proliferation and gene expression by involvement in metabolism, collagen synthesis, bone mineralization, and connective tissue cross-linking. This study could possibly explain the increased thickness of the dentine bridge in the Biodentine group as compared to the Dycal group at the end of the stipulated time of 45 days as seen in the current study.
It must be noted that this study was performed in sound teeth. However, pulpal exposures are more likely to occur in carious teeth possibly complicated with pulpal inflammation which may alter the results of this investigation. Thus, to determine whether Biodentine can be used for direct pulp capping requires further study with a longer observation period on cariously exposed pulpal teeth.
CONCLUSION
Biodentine is an interesting and promising pulp-capping agent that has the potential to make major contributions to maintaining pulp vitality in patients judiciously selected for direct pulp capping. It shares both its indications and mode of action with Ca(OH)2 but does not have its drawbacks. Based on the results and within the limitations of the present study, Biodentine is superior to Ca(OH)2 for pulp capping of mechanically exposed human teeth.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baume LJ, Holz J. Long term clinical assessment of direct pulp capping. Int Dent J. 1981;31:251–60. [PubMed] [Google Scholar]
- 2.Horsted P, Sandergaard B, Thylstrup A, El Attar K, Fejerskov O. A retrospective study of direct pulp capping with calcium hydroxide compounds. Endod Dent Traumatol. 1985;1:29–34. doi: 10.1111/j.1600-9657.1985.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 3.Swartz DB, Skidmore AE, Griffin JA., Jr Twenty years of endodontic success and failure. J Endod. 1983;9:198–202. doi: 10.1016/S0099-2399(83)80092-2. [DOI] [PubMed] [Google Scholar]
- 4.Hermann BW. Dentine obliteration of the root canal after treatment with calcium. Zahnarztl Rundsch. 1930;39:888–9. [Google Scholar]
- 5.Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64:541–8. doi: 10.1177/002203458506400407. [DOI] [PubMed] [Google Scholar]
- 6.Pitt Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp capping material. J Am Dent Assoc. 1996;127:1491–4. doi: 10.14219/jada.archive.1996.0058. [DOI] [PubMed] [Google Scholar]
- 7.Faraco IM, Jr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163–6. doi: 10.1034/j.1600-9657.2001.170405.x. [DOI] [PubMed] [Google Scholar]
- 8.Dammaschke T, Stratmann U, Wolff P, Sagheri D, Schäfer E. Direct pulp capping with mineral trioxide aggregate: An immunohistologic comparison with calcium hydroxide in rodents. J Endod. 2010;36:814–9. doi: 10.1016/j.joen.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: A preliminary report. Int Endod J. 2003;36:225–31. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 10.Accorinte Mde L, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E, Jr, et al. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod. 2008;34:1–6. doi: 10.1016/j.joen.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int Endod J. 2008;41:128–50. doi: 10.1111/j.1365-2591.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 12.Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39:743–7. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Liu T, Li H, Pi G. Histological evaluation of direct pulp capping with a self-etching adhesive and calcium hydroxide on human pulp tissue. Int Endod J. 2008;41:643–50. doi: 10.1111/j.1365-2591.2008.01396.x. [DOI] [PubMed] [Google Scholar]
- 14.Parolia A, Kundabala M, Rao NN, Acharya SR, Agrawal P, Mohan M, et al. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal. Aust Dent J. 2010;55:59–64. doi: 10.1111/j.1834-7819.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 15.Olsson H, Petersson K, Rohlin M. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. Int Endod J. 2006;39:429–42. doi: 10.1111/j.1365-2591.2006.01116.x. [DOI] [PubMed] [Google Scholar]
- 16.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 17.Yoshiba K, Yoshiba N, Nakamura H, Iwaku M, Ozawa H. Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J Dent Res. 1996;75:1590–7. doi: 10.1177/00220345960750081101. [DOI] [PubMed] [Google Scholar]
- 18.Peng W, Liu W, Zhai W, Jiang L, Li L, Chang J, et al. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod. 2011;37:1240–6. doi: 10.1016/j.joen.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, et al. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res. 2012;91:1166–71. doi: 10.1177/0022034512460833. [DOI] [PubMed] [Google Scholar]
- 20.Tronstad L. Reaction of the exposed pulp to Dycal treatment. Oral Surg Oral Med Oral Pathol. 1974;38:945–53. doi: 10.1016/0030-4220(74)90348-x. [DOI] [PubMed] [Google Scholar]
- 21.Heys DR, Cox CF, Heys RJ, Avery JK. Histological considerations of direct pulp capping agents. J Dent Res. 1981;60:1371–9. doi: 10.1177/00220345810600071401. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri J. Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent Mater. 2014;30:709–15. doi: 10.1016/j.dental.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Laurent P, Camps J, About I. Biodentine (TM) induces TGF-ß1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012;45:439–48. doi: 10.1111/j.1365-2591.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 24.Téclès O, Laurent P, Aubut V, About I. Human tooth culture: A study model for reparative dentinogenesis and direct pulp capping materials biocompatibility. J Biomed Mater Res B Appl Biomater. 2008;85:180–7. doi: 10.1002/jbm.b.30933. [DOI] [PubMed] [Google Scholar]
- 25.Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, Torabi M, Parirokh M. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent. 2011;14:351–5. doi: 10.4103/0972-0707.87196. [DOI] [PMC free article] [PubMed] [Google Scholar]


