Abstract
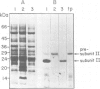
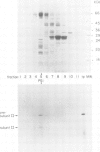
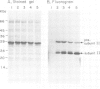
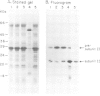
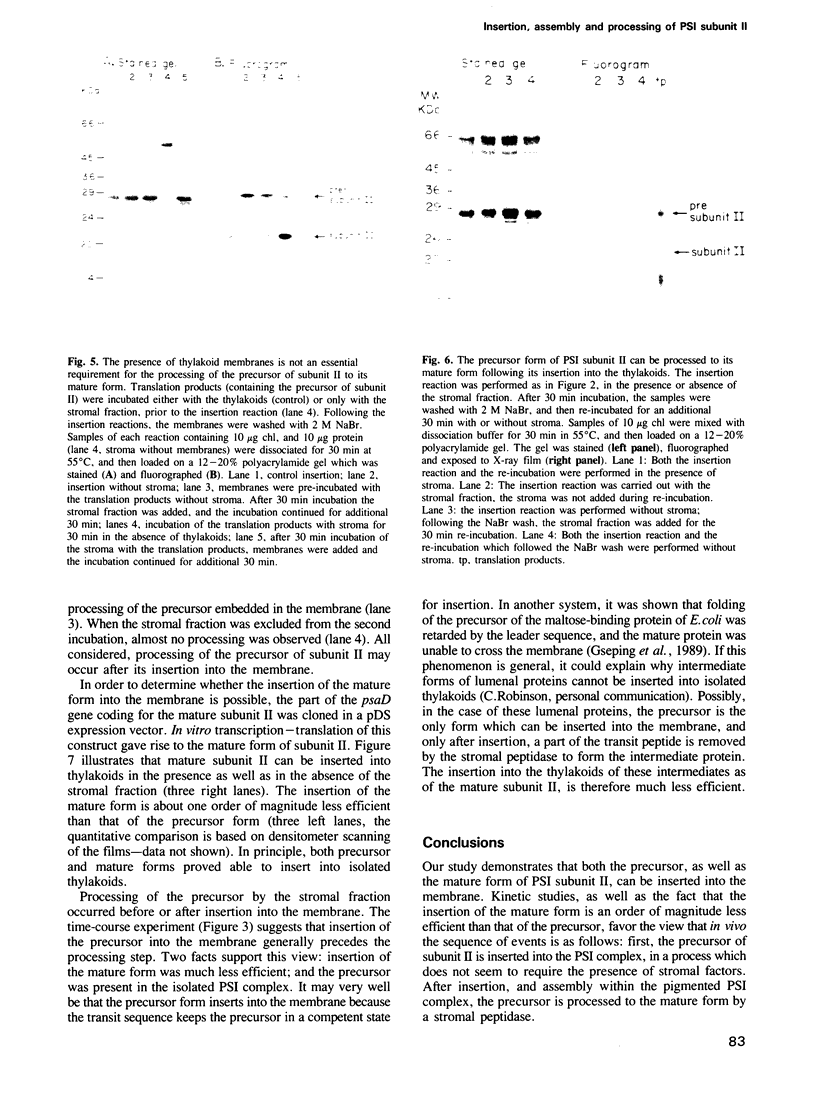
The biogenesis and assembly of subunit II of photosystem I (PSI) (psaD gene product) were studied and characterized. The precursor and the mature form were produced in vitro and incubated with intact plastids or isolated thylakoids. Following import of the precursor into isolated plastids, mostly the mature form of subunit II was found in the thylakoids. However, when the processing activity was inhibited only the precursor form was present in the membranes. The precursor was processed by a stromal peptidase and processing could occur before or after insertion of the precursor into the thylakoids. Following insertion into isolated thylakoids, both the precursor and the mature form of subunit II were confined to the PSI complex. Insertion of the mature form of subunit II was much less efficient than that of the precursor. Kinetic studies showed that the precursor was inserted into the membrane. Only at a later stage, the mature form began to accumulate. These results suggest that in vivo the precursor of subunit II is inserted and embedded in the thylakoids, as part of the PSI complex. Only later, it is processed to the mature form through the action of a stromal peptidase.
Full text
PDF
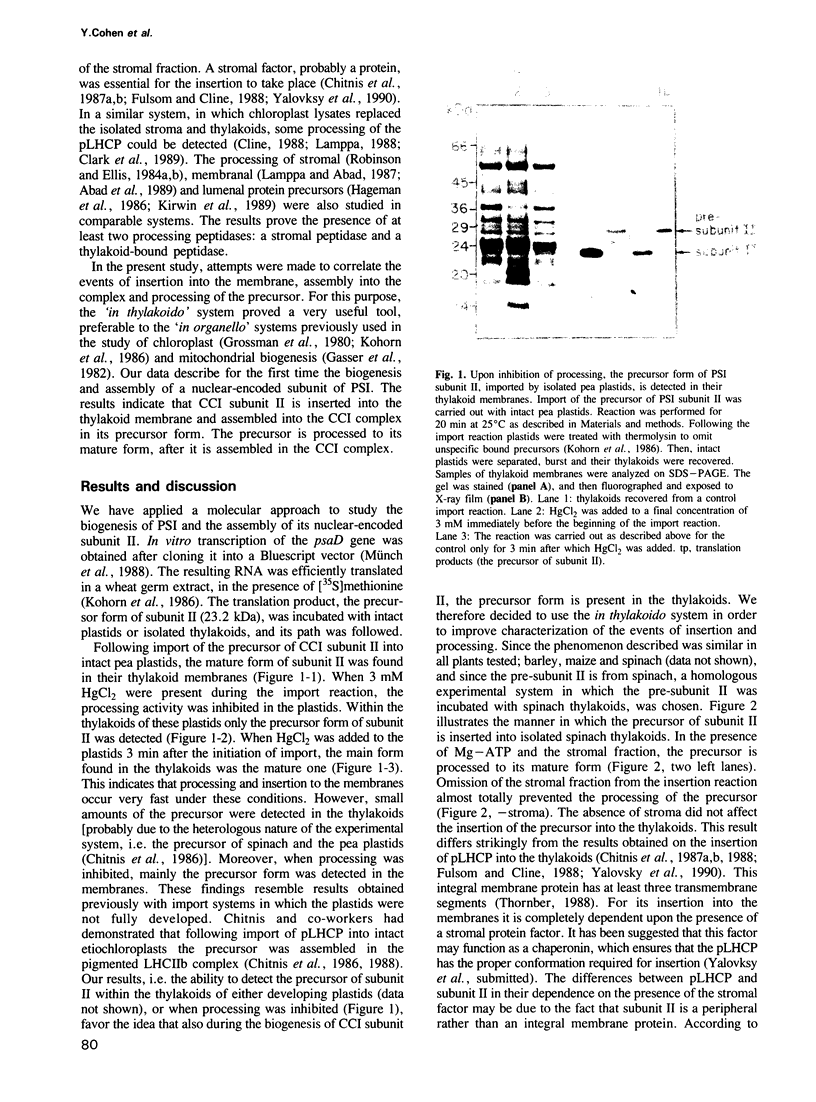
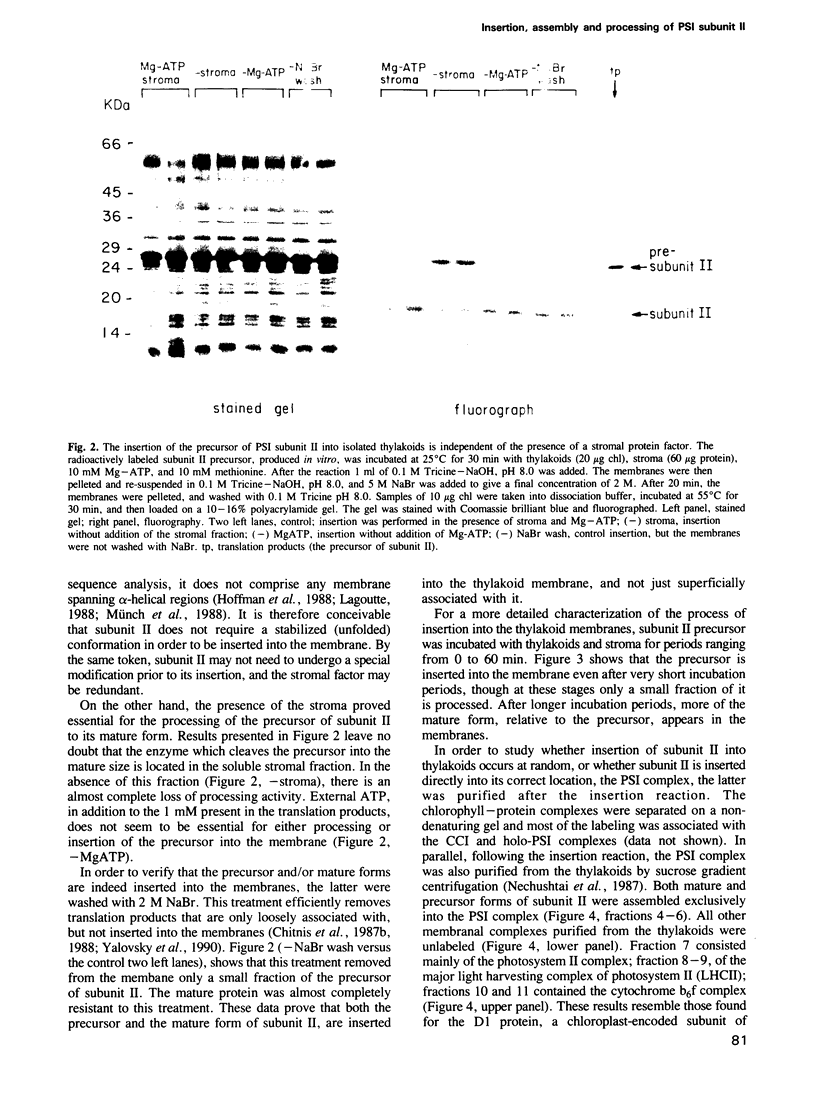



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adir N., Shochat S., Ohad I. Light-dependent D1 protein synthesis and translocation is regulated by reaction center II. Reaction center II serves as an acceptor for the D1 precursor. J Biol Chem. 1990 Jul 25;265(21):12563–12568. [PubMed] [Google Scholar]
- Chitnis P. R., Harel E., Kohorn B. D., Tobin E. M., Thornber J. P. Assembly of the precursor and processed light-harvesting chlorophyll a/b protein of Lemna into the light-harvesting complex II of barley etiochloroplasts. J Cell Biol. 1986 Mar;102(3):982–988. doi: 10.1083/jcb.102.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. E., Abad M. S., Lamppa G. K. Mutations at the transit peptide-mature protein junction separate two cleavage events during chloroplast import of the chlorophyll a/b-binding protein. J Biol Chem. 1989 Oct 15;264(29):17544–17550. [PubMed] [Google Scholar]
- Cline K. Light-Harvesting Chlorophyll a/b Protein : Membrane Insertion, Proteolytic Processing, Assembly into LHC II, and Localization to Appressed Membranes Occurs in Chloroplast Lysates. Plant Physiol. 1988 Apr;86(4):1120–1126. doi: 10.1104/pp.86.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulson D. R., Cline K. A Soluble Protein Factor is Required in Vitro for Membrane Insertion of the Thylakoid Precursor Protein, pLHCP. Plant Physiol. 1988 Dec;88(4):1146–1153. doi: 10.1104/pp.88.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Golbeck J. H. Structure, function and organization of the Photosystem I reaction center complex. Biochim Biophys Acta. 1987;895(3):167–204. doi: 10.1016/s0304-4173(87)80002-2. [DOI] [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Williams R. S., Robinson C. Transport of proteins into chloroplasts. Organization, orientation, and lateral distribution of the plastocyanin processing peptidase in the thylakoid network. J Biol Chem. 1988 Dec 5;263(34):18128–18132. [PubMed] [Google Scholar]
- Kohorn B. D., Harel E., Chitnis P. R., Thornber J. P., Tobin E. M. Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol. 1986 Mar;102(3):972–981. doi: 10.1083/jcb.102.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagoutte B. Cloning and sequencing of spinach cDNA clones encoding the 20 kDa PS I polypeptide. FEBS Lett. 1988 May 23;232(2):275–280. doi: 10.1016/0014-5793(88)80752-x. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K., Abad M. S. Processing of a wheat light-harvesting chlorophyll a/b protein precursor by a soluble enzyme from higher plant chloroplasts. J Cell Biol. 1987 Dec;105(6 Pt 1):2641–2648. doi: 10.1083/jcb.105.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G. K. The chlorophyll a/b-binding protein inserts into the thylakoids independent of its cognate transit peptide. J Biol Chem. 1988 Oct 15;263(29):14996–14999. [PubMed] [Google Scholar]
- Liu G., Topping T. B., Randall L. L. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch S., Ljungberg U., Steppuhn J., Schneiderbauer A., Nechushtai R., Beyreuther K., Herrmann R. G. Nucleotide sequences of cDNAs encoding the entire precursor polypeptides for subunits II and III of the photosystem I reaction center from spinach. Curr Genet. 1988 Nov;14(5):511–518. doi: 10.1007/BF00521277. [DOI] [PubMed] [Google Scholar]
- Nechushtai R., Nelson N. Purification properties and biogenesis of Chlamydomonas reinhardii photosystem I reaction center. J Biol Chem. 1981 Nov 25;256(22):11624–11628. [PubMed] [Google Scholar]
- Reed J. E., Cline K., Stephens L. C., Bacot K. O., Viitanen P. V. Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem. 1990 Nov 26;194(1):33–42. doi: 10.1111/j.1432-1033.1990.tb19423.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. The precursor of small subunit of ribulose bisphosphate carboxylase is processed to the mature size in two steps. Eur J Biochem. 1984 Jul 16;142(2):343–346. doi: 10.1111/j.1432-1033.1984.tb08292.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Siegenthaler P. A., Depéry F. Influence of unsaturated fatty acids in chloroplasts. Shift of the pH optimum of electron flow and relations to deltapH, thylakoid internal pH and proton uptake. Eur J Biochem. 1976 Jan 15;61(2):573–580. doi: 10.1111/j.1432-1033.1976.tb10052.x. [DOI] [PubMed] [Google Scholar]
- Yalovsky S., Schuster G., Nechushtai R. The apoprotein precursor of the major light-harvesting complex of photosystem II (LHCIIb) is inserted primarily into stromal lamellae and subsequently migrates to the grana. Plant Mol Biol. 1990 May;14(5):753–764. doi: 10.1007/BF00016508. [DOI] [PubMed] [Google Scholar]
- Zanetti G., Merati G. Interaction between photosystem I and ferredoxin. Identification by chemical cross-linking of the polypeptide which binds ferredoxin. Eur J Biochem. 1987 Nov 16;169(1):143–146. doi: 10.1111/j.1432-1033.1987.tb13591.x. [DOI] [PubMed] [Google Scholar]
- Zilber A. L., Malkin R. Ferredoxin Cross-Links to a 22 kD Subunit of Photosystem I. Plant Physiol. 1988 Nov;88(3):810–814. doi: 10.1104/pp.88.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]