Abstract
This study sought to assess the pharmacokinetic (PK) changes of caffeine and its CYP1A2 metabolites across the 3 trimesters of pregnancy. A prospective, multicenter PK study was conducted among 59 pregnant women (93.2% white) who were studied once during a trimester. One beverage with 30–95 mg caffeine was consumed, and a blood/urine sample was collected within 1 hour postingestion. Concentrations of caffeine and its primary metabolites were quantified from serum and urine by LC-MS/MS. There was a significant increase in dose-normalized caffeine serum and urine concentrations between the first and third trimesters (P< .05 and P< .01, respectively). Normalized theophylline concentrations also increased significantly in the third trimester in serum (P < .001) and in urine (P <.05). The caffeine urine/serum concentration ratio also increased in the last trimester (P < .05). No significant difference was found in normalized paraxanthine or theobromine concentrations. This study identified decreased caffeine metabolism and an increase in the active metabolite theophylline concentrations during pregnancy, especially in the third trimester, revealing evidence of the large role that pregnancy plays in influencing caffeine metabolism.
Keywords: caffeine, pregnancy, metabolites, pharmacokinetics
Drug pharmacokinetics (PK) are frequently altered during pregnancy, which may necessitate modifications to standard dosing regimens for pregnant women.1 However, there are usually a limited number of patients or samples that can be ethically and practically obtained for PK studies among pregnant women. This situation was acknowledged at the American Conference of Pharmacometrics in 2011, which emphasized efficient study design and analysis for this special population.2
Caffeine is frequently consumed by pregnant women (75% as reported by a large-scale prospective cohort study3). It is extensively metabolized by hepatic cytochrome P450 1A2 (CYP1A2) to paraxanthine, theobromine, and theophylline, which are further metabolized into the secondary and tertiary products shown in Figure 1.4,5 Conversion to paraxanthine constitutes about 80% of the primary metabolic pathways in nonpregnant subjects and is exclusively catalyzed by CYP1A2.6 Thus, the molar ratio of caffeine/paraxanthine in plasma (at 4 hours) has been validated as a phenotypic indicator of CYP1A2 activity.7,8 CYP2E1 also contributes to the formation of theobromine and theophylline as well as other downstream metabolites.9–11 Caffeine and its primary metabolites are nonselective adenosine receptor antagonists and phosphodiesterase inhibitors that lead to stimulatory effects in the cardiorespiratory and central nervous systems. It is important to delineate the PK changes of caffeine together with its active primary metabolites to better understand the use of caffeine and other CYP1A2-mediated medications among pregnant women.
Figure 1.
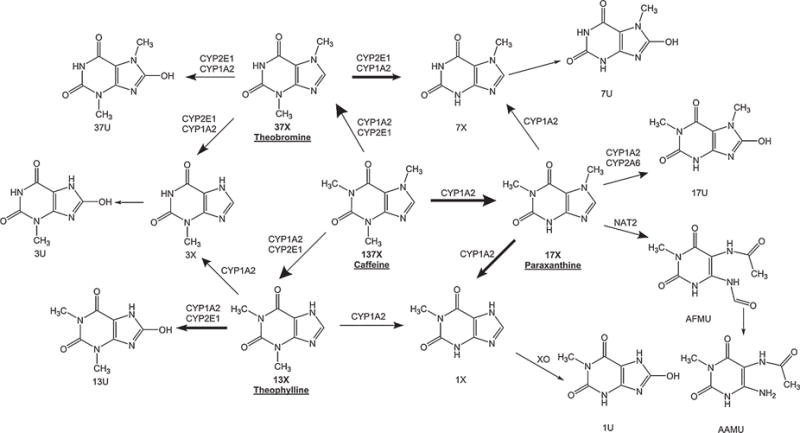
Chemical structure and metabolic pathways of caffeine and its metabolites.5,32 Parent compound, 1,3,7-trimethylxanthine (137X, caffeine); primary metabolites (pointed to by large arrow) 1,7-dimethylxanthine (17X, paraxanthine), 3,7-dimethylxanthine (37X, theobromine), 1,3-dimethylxanthine (13X, theophylline); secondary metabolites (pointed to by medium arrow) 1-methylxanthine (1X), 3-methylxanthine (3X), 7-methylxanthine (7X), 1,7-dimethyluracil (17U), 1,3-dimethyluracil (13U), 3,7-dimethyluracil (37U), 5-acetylamino-6-formylamino-3-methyluracil (AFMU); tertiary metabolites (pointed to by small arrow) 1-methyluracil (1U), 7-methyluracil (7U), 5-acetylamino-6-amino-3-methyluracil (AAMU); enzymes cytochrome P450 1A2(CYP1A2), cytochrome P450 2E1 (CYP2E1), cytochromeP450 2A6 (CYP2A6), N-acetyltransferase2 (NAT2), xanthine oxidase (XO). Major metabolic pathways of caffeine and its primary metabolites were highlighted by bold arrows.
CYP1A2 has been shown to play an important role in activating or eliminating many medications. It metabolizes approximately 9% of all hepatic P450-eliminated drugs among the top 200 most prescribed drugs in the United States.12 Pregnancy leads to temporal inhibition of CYP1A2 activity (–32.8% in the first trimester, −48.1% in the second trimester, and −65.2% in the third trimester), partially due to changing hormone levels.13 Although maternal physiology involves a series of renal function changes including an increase in the glomerular filtration rate and enlarged effective renal plasma flow, resulting in an increase in the of excretion of renally eliminated drugs,14 caffeine still exhibits an overall reduction in clearance due to decreased CYP1A2 activity.15 These physiological changes have the effect of increasing caffeine exposure following the same dose during pregnancy. This has led to recommendations that pregnant women who habitually consume caffeine-containing products reduce their regular caffeine intake. The American College of Obstetricians and Gynecologists states that daily caffeine consumption <200 mg is considered safe and does not appear to contribute to miscarriage or preterm birth.16
Due to the many challenges of clinical studies in the pregnant population, sparse sampling is commonly used in drug studies involving pregnant women.1 Typically 1–2 samples per dosing interval are collected from each patient for PK analysis. Fauchet et al used a population PK approach to describe abacavir PK in HIV-infected pregnant women (n = 36) with 1–2 samples collected per patient.17 Zheng et al reported tacrolimus maternal blood concentrations using 1 sample per patient, which was collected at the time of delivery.18 Our study also adopted a sparse sampling strategy that allowed us to coordinate the study visit with regularly scheduled prenatal visits.
The objective of this preliminary study was to evaluate the PK changes of caffeine and its metabolites across the 3 trimesters of pregnancy.
Methods
Patient Recruitment
A prospective, multicenter PK study was conducted among pregnant women (≥18 years). Pregnant women who were willing to drink a commercially available caffeinated beverage once during a trimester were included in this study; patients with any medical conditions that might interfere with caffeine absorption, distribution, and elimination were excluded. The patients were recruited at obstetrics/gynecology clinics at the University Hospital and South Main Clinic, which are both University of Utah Health Care facilities. Eligible women were approached, informed of the nature and objectives of this study, and offered the opportunity to consent to participate in the study. The study protocol was reviewed and approved by the University of Utah Institutional Review Board.
Sample Collection
Pregnant women were approached for recruitment once per trimester during their regular prenatal visits (maximum 3 study visits). They were advised by clinical practitioners not to smoke during the pregnancy. On the day of the study visit, patients were restricted from consuming any methylxanthine-containing products for at least 2 hours prior to the study visit (typically overnight abstinence). On arrival, patients urinated and emptied their bladders before taking the study beverage. One beverage with 30–95 mg caffeine was consumed within 30 minutes, and a blood and/or urine sample was collected within an hour of the cessation of drinking. Blood samples were collected into red-top serum tubes (BD Vacutainer, Franklin Lakes, New Jersey). The serum was separated within 2 hours of collection and kept frozen (−20 °C) until the samples were assayed. Patient demographic information was collected including race, ethnicity, age, pregnancy stage in weeks (confirmed by last menstrual period date or ultrasound examination per clinical determination), trimester, current weight, height, body mass index (BMI), blood pressure, and any comedications (dose, frequency, and timing of taking comedications).
Caffeine and Metabolite Assays
Caffeine and metabolite concentrations were measured by LC-MS/MS based on an in-house validated method at the Center for Human Toxicology at the University of Utah. The lower limit of quantitation (LLOQ) for caffeine, paraxanthine, theophylline, and theobromine was 30 ng/mL in both human serum and urine. The upper limit of quantitation (ULOQ) was 5000 ng/mL for caffeine, paraxanthine, theophylline, and theobromine in human serum and urine. The intra- and interassay imprecision were approximately 10%.
Descriptive Pharmacokinetic Analyses
The relationships between dose-normalized concentrations and patient characteristics were analyzed by multiple variable linear regression for continuous covariates (age, pregnancy stage in weeks, current weight, height, BMI) and categorical covariates (race, ethnicity, trimester, use of prenatal vitamins, use of CYP1A2-interacting comedications). Concentration changes in caffeine and its metabolites were compared for each trimester. Molar ratios of the parent to metabolite concentration (caffeine/paraxanthine, caffeine/total metabolites) as well as the molar ratio of urine/serum concentration of each compound were also evaluated across all trimesters.
Statistics
Data are presented as mean ± SD unless otherwise indicated. If the concentration fell below the LLOQ, its value was recorded as half of the corresponding LLOQ. The relationship between dose-normalized concentrations and patient covariates was evaluated by multiple-variable linear regression (SAS 9.3, SAS Institute Inc., Cary, North Carolina). Trends in concentration-time curves were fitted for each trimester using nonlinear second-order polynomials (Graph Pad Prism 5.04, La Jolla, California). Statistical comparisons were performed using 1-way ANOVA (SAS 9.3, SAS Institute Inc.), and differences were considered significant at P < .05.
Results
A total of 72 study visits were completed by 59 unique women (93.2% white) from February 2013 to June 2014. The mean age was 29.9 ± 5.0 years at the time of enrollment. Patient demographic characteristics are featured in Table 1. The body size measures (current weight, BMI) of the study participants, stratified by trimester, are recorded in Table 2. No increasing trend was observed in body size measures among the 3 trimesters, as most patients (81.4%) completed only 1 study visit (9 patients had a second visit in a different trimester, 2 patients completed 3 visits in 3 trimesters). Other patient demographics and clinical characteristics were similar among the 3 trimesters. All patients’ blood pressures were in the normal range. A total of 57 women (96.6%) were taking prenatal vitamins. Nine women (15.3%) had comedications that could potentially interact with CYP1A2, including ondansetron for nausea (6 cases), acetaminophen for headache (2 cases), and propranolol for ventricular tachycardia (1 case).
Table 1.
Baseline Demographics of the Pregnant Study Participants at the Time of Enrollment (n = 59 patients)
| Characteristics | Mean ±SD or Number (%) |
|---|---|
| Age (years) | 29.9 ± 5.0 |
| Height (cm) | 163.2 ± 6.2 |
| Race | |
| White | 55 (93.2) |
| Asian | 2 (3.4) |
| Black | 1 (1.7) |
| Other | 1 (1.7) |
| Ethnicity | |
| Not Hispanic or Latino | 44 (74.6) |
| Hispanic or Latino | 15 (25.4) |
| Prenatal vitamin consumption | |
| Yes | 57 (96.6) |
| No | 2 (3.4) |
| Comedication with potential CYP1A2 interaction | |
| Yes | 9 (15.3) |
| No | 50 (84.7) |
Table 2.
Patient Characteristics of the Pregnant Women (n = 72 Encounters)
| Characteristics | First Trimester (n = 10) |
Second Trimester (n = 26) |
Third Trimester (n = 36) |
|---|---|---|---|
| Week of pregnancya | 10 (6–13) | 19 (14–26) | 33 (27–37) |
| Weight (kg) | 76.2 ± 10.7 | 70.9 ± 14.0 | 78.4 ± 16.8 |
| Body mass index (kg/m2) | 29.1 ± 6.1 | 27.4 ± 5.7 | 29.1 ± 5.7 |
Data are presented as the mean ± SD.
Median (range).
The mean caffeine dose consumed by the study participants was 55 ± 20 mg per beverage. No covariates significantly influenced dose-normalized concentrations within the time window of 1 hour postingestion except trimester and pregnancy weeks. As shown in Figure 2, there was a significant increase in dose-normalized caffeine serum concentrations within 1 hour postingestion between the first and third trimesters (mean 1859 vs 3164 ng/mL, P <. 05). Dose-normalized theophylline concentrations also significantly increased in the third trimester in serum (first trimester 33 vs third trimester 193 ng/mL, P <.001; second trimester 119 vs third trimester 193 ng/mL, P < .05). No significant differences in normalized paraxanthine or theobromine serum concentrations were observed. The PK in urine closely mimicked the serum profiles; there was a significant increase in dose normalized caffeine concentrations within 1 hour postingestion between the first and third trimesters (mean 1587 vs 3601 ng/mL, P <. 01) and for theophylline (mean 213 vs 905 ng/mL, P <. 05) (Figure 3). No differences were found in normalized paraxanthine or theobromine concentrations in urine.
Figure 2.
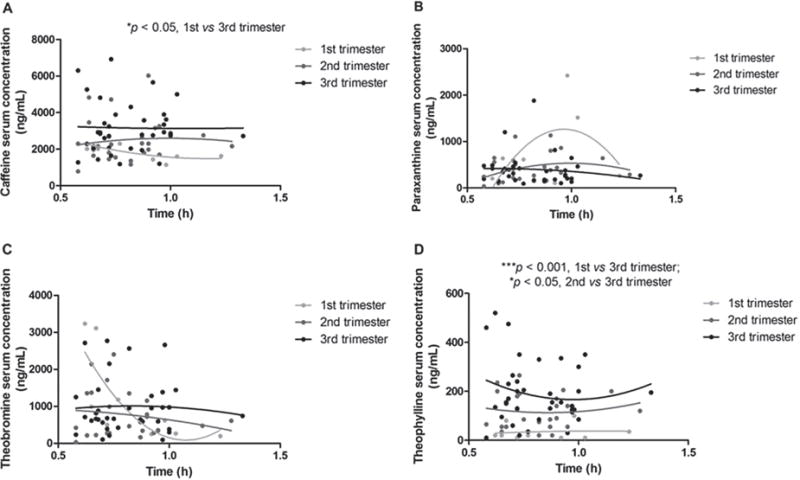
Concentration-time curves of (A) caffeine, (B) paraxanthine, (C) theobromine, and (D) theophylline in serum per trimester. Concentrations have been normalized to the median dose of 50 mg caffeine. Dots represent a single observation from each patient during a trimester; the line was fitted by a nonlinear second-order polynomial to illustrate the concentration-time trend for each trimester. *P < .05, significant difference in caffeine serum concentrations between first and third trimesters, or significant difference in theophylline serum concentration between second and third trimesters; ***P < .001, significant difference in theophylline serum concentration between first and third trimesters.
Figure 3.

Concentration-time curves of (A) caffeine, (B) paraxanthine, (C) theobromine, and (D) theophylline in urine per trimester. Concentrations have been normalized to the median dose of 50 mg caffeine. Dots represent single observations from each patient during a trimester; the line was fitted by a nonlinear second-order polynomial to illustrate the concentration-time trend for each trimester. **P < .01, significant difference in caffeine urine concentrations between first and third trimesters; *P <. 05, significant difference in theophylline urine concentration between first and third trimesters.
The molar ratios of parent to metabolite concentrations in serum within 1 hour postingestion were similar across all 3 trimesters. For each compound, the urine/serum concentration ratios were also similar among all 3 trimesters except for caffeine, which had an increased urine/serum ratio in the last trimester (first trimester 0.8 vs third trimester 1.1, P < .05; second trimester 0.9 vs third trimester 1.1, P < .05) (Table 3).
Table 3.
Urine-to-Serum Concentration Ratios of Caffeine and Its Primary Metabolites, Stratified by the Trimester of Pregnancy
| Urine/Serum Ratio | First Trimester (n = 8) |
Second Trimester (n = 19) |
Third Trimester (n = 27) |
|---|---|---|---|
| Caffeine | 0.8 ± 0.3 | 0.9 ± 0.2 | 1.1 ± 0.2* |
| Paraxanthine | 5.8 ± 4.5 | 8.2 ± 3.0 | 9.1 ± 5.1 |
| Theobromine | 4.2 ± 1.8 | 8.3 ± 4.2 | 7.2 ± 3.6 |
| Theophylline | 5.7 ± 3.5 | 5.0 ± 3.2 | 4.3 ± 2.5 |
Data are presented as the mean ± SD.
P <. 05, there was a significant difference in caffeine urine/serum concentration ratios between earlier trimesters (first, second) and the third trimester.
Discussion
Our descriptive analysis of the PK of caffeine and its metabolites showed that caffeine serum concentrations within 1 hour postconsumption are significantly increased in the third trimester of pregnancy, which is in agreement with previously published studies.19,20 Because caffeine is approximately 27–35% bound to serum proteins in nonpregnant subjects,21,22 it is likely that pregnancy-related hemodynamic changes (reduced albumin level) lead to a decrease in plasma protein binding and partially contribute to the change in total caffeine concentrations. The week of pregnancy (or trimester) significantly affects the PK of caffeine and its primary metabolite theophylline. In contrast, patient age, body size measures, and other demographic/clinical characteristics did not influence caffeine or theophylline concentrations obtained within the time window of 1 hour postingestion. The covariate of pregnancy weeks has been previously shown to influence the PK of hepatic metabolized drugs,23,24 suggesting that dose adjustments may be needed, especially during the last weeks of pregnancy.
During in the last trimester of pregnancy, increased caffeine urine/serum concentration ratios were observed. However, the proportion of the ingested dose eliminated through the renal route is relatively low because the urine/serum concentration ratio was less than 2.0 throughout pregnancy. Only a small fraction of the caffeine dose is excreted unchanged into urine; the bulk is eliminated via N-demethylation in the liver.25 The effect of pregnancy on caffeine metabolism is bidirectional: renal clearance is enhanced, while CYP1A2 activity reduces over the course of pregnancy. This decrease in CYP1A2 metabolism outcompetes the increase in renal function and leads to increased caffeine concentrations as observed in our study and results in increased caffeine exposure throughout pregnancy.
Our findings showed no significant differences in paraxanthine or theobromine concentrations across the 3 trimesters of pregnancy. As shown in Figure 1, CYP2E1, CYP2A6, NAT2, and XO are also involved in the metabolism of caffeine and its metabolites, aside from the primary metabolic enzyme CYP1A2. CYP2E1 facilitates the formation of theobromine and theophylline and their subsequent metabolism. In vitro tests showed that CYP2E1 expression was induced by placental lactogen in female human hepatocytes.26 Whether pregnancy affects CYP2E1 expression and activity remains to be determined in humans. CYP2A6 partially contributes to the metabolism of paraxanthine, which also exhibits induced expression in primary human hepatocytes treated with estradiol and progesterone at levels similar to those reached during pregnancy.27 NAT2 showed lower activity, as assessed by the urinary metabolic ratio, in the first trimester than after delivery.28 There was no significant difference in metabolic ratios for XO during pregnancy and after delivery.28 These enzymes are minor/indirect contributors to the formation and metabolism of the primary metabolites of caffeine,14 which may partially explain the lack of changes in paraxanthine or theobromine concentrations during pregnancy.
In this study we observed that the concentrations of theophylline, an active metabolite of caffeine, increased over the course of pregnancy. It could be due to the increase in renal clearance offset by significantly reduced nonrenal clearance in the third trimester as illustrated in a previous study on the PK of intravenous theophylline in pregnant women for the treatment of asthma.29 The causal relationship between pregnancy-related physiological changes and alterations in drug PK could be readily applied to other methylxanthines, as they are structurally close and share similar metabolic pathways that mainly involve CYP1A2.30 Even though the observed concentrations of theophylline fell below the therapeutic range of 5–15 mg/L, these concentrations may still exert effects on the cardiorespiratory and neurological systems.31 An aversion to coffee during pregnancy was reported by many of our patients, which may be related to the increased cumulative exposure of caffeine and theophylline, which both act as cardiovascular and neurological stimulants. Considering the changes in the PK of caffeine and its metabolites, caffeine use/dose reduction is deemed to be beneficial to avoid discomforts associated with increased exposure during pregnancy.
This study has several limitations. First, this study has limited statistical power to test the effect of covariates on caffeine PK and metabolism in pregnancy due to the small number of patients. Second, a limited number of samples were collected per patient according to local ethical and practical considerations. The timing and duration of the study visits for each patient coincided with their regular prenatal visits. Patients were given a caffeinated beverage before they entered the clinic room, where they ingested the beverage as instructed. Once out of the clinic room, they stayed for 1 blood draw and/or provided a urine sample. The blood/urine samples were obtained around the time during which caffeine concentrations peaked; however, there were no samples collected from the terminal elimination phase, thus limiting our ability to develop a caffeine metabolite model. Third, the abstinence from methyl-xanthine-containing food prior to the study visit was self-reported by the patients; however, contamination by previous methylxanthine consumption, if randomly occurring, should not change the directionality of the study results.
In conclusion, this preliminary study revealed marked PK changes in caffeine and its active metabolite theo-phylline over the course of a pregnancy.
Acknowledgments
The authors would like to acknowledge Kerri Pitcher for recruiting the study participants at the University Hospital Clinic 4. The authors also would like to thank Eric M. Brozek, PhD, and Diana G. Wilkins, PhD, for LC-MS/MS analyses used in this study. Chris Stockmann was supported by the American Foundation for Pharmaceutical Education’s Clinical Pharmaceutical Sciences Fellowship. Katherine Schoen received funding from NIH Medical Student Summer Research Program (T35 HL007744). The authors would also like to thank Stephen Duffull, PhD, at the University of Otago for the PK analysis consultation.
Funding resource: This study was supported by a Primary Children Hospital Foundation Early Career Development Research Grant awarded to Catherine M. T. Sherwin.
Footnotes
Conflicting Interests
The authors declare no conflict of interests.
References
- 1.Ke AB, Rostami-Hodjegan A, Zhao P, Unadkat JD. Pharmacometrics in pregnancy: an unmet need. Annu Rev Pharmacol Toxicol. 2014;54:53–69. doi: 10.1146/annurev-pharmtox-011613-140009. [DOI] [PubMed] [Google Scholar]
- 2.van Hasselt JG, Andrew MA, Hebert MF, Tarning J, Vicini P, Mattison DR. The status of pharmacometrics in pregnancy: highlights from the 3rd American conference on pharmacometrics. Br J Clin Pharmacol. 2012;74(6):932–939. doi: 10.1111/j.1365-2125.2012.04280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198(3):279 e1–8. doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- 4.Grant DM, Tang BK, Kalow W. Variability in caffeine metabolism. Clin Pharmacol Ther. 1983;33(5):591–602. doi: 10.1038/clpt.1983.80. [DOI] [PubMed] [Google Scholar]
- 5.Blake MJ, Abdel-Rahman SM, Pearce RE, Leeder JS, Kearns GL. Effect of diet on the development of drug metabolism by cytochrome P-450 enzymes in healthy infants. Pediatr Res. 2006;60(6):717–723. doi: 10.1203/01.pdr.0000245909.74166.00. [DOI] [PubMed] [Google Scholar]
- 6.Arnaud MJ. Pharmacokinetics and metabolism of natural methyl-xanthines in animal and man. Handb Exp Pharmacol. 2011;200:33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- 7.Fuhr U, Rost KL. Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics. 1994;4(3):109–116. doi: 10.1097/00008571-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Perera V, Gross AS, McLachlan AJ. Caffeine and paraxanthine HPLC assay for CYP1A2 phenotype assessment using saliva and plasma. Biomed Chromatog. 2010;24(10):1136–1144. doi: 10.1002/bmc.1419. [DOI] [PubMed] [Google Scholar]
- 9.Ha HR, Chen J, Freiburghaus AU, Follath F. Metabolism of theophylline by cDNA-expressed human cytochromes P-450. Br J Clin Pharmacology. 1995;39(3):321–326. doi: 10.1111/j.1365-2125.1995.tb04455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjia JF, Colbert J, Back DJ. Theophylline metabolism in human liver microsomes: inhibition studies. J Pharmacol Exp Ther. 1996;276(3):912–917. [PubMed] [Google Scholar]
- 11.Gates S, Miners JO. Cytochrome P450 isoform selectivity in human hepatic theobromine metabolism. Br J Clin Pharmacol. 1999;47(3):299–305. doi: 10.1046/j.1365-2125.1999.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanger UM, Turpeinen M, Klein K, Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem. 2008;392(6):1093–1108. doi: 10.1007/s00216-008-2291-6. [DOI] [PubMed] [Google Scholar]
- 13.Tracy TS, Venkataramanan R, Glover DD, Caritis SN, National Institute for Child Health and Human Development Network of Maternal-Fetal-Medicine U Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Brazier JL, Ritter J, Berland M, Khenfer D, Faucon G. Pharmacokinetics of caffeine during and after pregnancy. Dev Pharmacol Ther. 1983;6(5):315–322. doi: 10.1159/000457332. [DOI] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 462: Moderate caffeine consumption during pregnancy. Obstet Gynecol. 2010;116(2 Pt 1):467–468. doi: 10.1097/AOG.0b013e3181eeb2a1. [DOI] [PubMed] [Google Scholar]
- 17.Fauchet F, Treluyer JM, Preta LH, et al. Population pharmacokinetics of abacavir in pregnant women. Antimicrob Agents Chemother. 2014;58(10):6287–6289. doi: 10.1128/AAC.03469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng S, Easterling TR, Hays K, et al. Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br J Clin Pharmacol. 2013;76(6):988–996. doi: 10.1111/bcp.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutti R, Rothweiler H, Schlatter C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur J Clin Pharmacol. 1981;21(2):121–126. doi: 10.1007/BF00637512. [DOI] [PubMed] [Google Scholar]
- 20.Aldridge A, Bailey J, Neims AH. The disposition of caffeine during and after pregnancy. Semin Perinatol. 1981;5(4):310–314. [PubMed] [Google Scholar]
- 21.Blanchard J. Protein binding of caffeine in young and elderly males. J Pharm Sci. 1982;71(12):1415–1418. doi: 10.1002/jps.2600711229. [DOI] [PubMed] [Google Scholar]
- 22.Cherrah Y, Falconnet JB, Desage M, Brazier JL, Zini R, Tillement JP. Study of deuterium isotope effects on protein binding by gas chromatography/mass spectrometry. Caffeine and deuterated isotopomers. Biomed Environ Mass Spectrom. 1987;14(11):653–657. doi: 10.1002/bms.1200141115. [DOI] [PubMed] [Google Scholar]
- 23.Patel JP, Green B, Patel RK, Marsh MS, Davies JG, Arya R. Population pharmacokinetics of enoxaparin during the antenatal period. Circulation. 2013;128(13):1462–1469. doi: 10.1161/CIRCULATIONAHA.113.003198. [DOI] [PubMed] [Google Scholar]
- 24.Lebaudy C, Hulot JS, Amoura Z, et al. Changes in enoxaparin pharmacokinetics during pregnancy and implications for antithrombotic therapeutic strategy. Clin Pharmacol Ther. 2008;84(3):370–377. doi: 10.1038/clpt.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Axelrod J, Reichenthal J. The fate of caffeine in man and a method for its estimation in biological material. J Pharmacol Exp Ther. 1953;107(4):519–523. [PubMed] [Google Scholar]
- 26.Lee JK, Chung HJ, Fischer L, Fischer J, Gonzalez FJ, Jeong H. Human placental lactogen induces CYP2E1 expression via PI 3-kinase pathway in female human hepatocytes. Drug Metab Dispos. 2014;42(4):492–499. doi: 10.1124/dmd.113.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SY, Koh KH, Jeong H. Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos. 2013;41(2):263–269. doi: 10.1124/dmd.112.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutsumi K, Kotegawa T, Matsuki S, et al. The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activities in humans. Clin Pharmacol Ther. 2001;70(2):121–125. doi: 10.1067/mcp.2001.116495. [DOI] [PubMed] [Google Scholar]
- 29.Frederiksen MC, Ruo TI, Chow MJ, Atkinson AJ., Jr Theophylline pharmacokinetics in pregnancy. Clin Pharmacol Ther. 1986;40(3):321–328. doi: 10.1038/clpt.1986.183. [DOI] [PubMed] [Google Scholar]
- 30.Lelo A, Birkett DJ, Robson RA, Miners JO. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. Br J Clin Pharmacol. 1986;22(2):177–182. doi: 10.1111/j.1365-2125.1986.tb05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Self TH, Heilker GM, Alloway RR, Kelso TM, Abou-Shala N. Reassessing the therapeutic range for theophylline on laboratory report forms: the importance of 5–15 micrograms/mL. Pharmacotherapy. 1993;13(6):590–594. [PubMed] [Google Scholar]
- 32.Rybak ME, Pao CI, Pfeiffer CM. Determination of urine caffeine and its metabolites by use of highperformance liquid chromatog-raphy-tandem mass spectrometry: estimating dietary caffeine exposure and metabolic phenotyping in population studies. Anal Bioanal Chem. 2014;406(3):771–784. doi: 10.1007/s00216-013-7506-9. [DOI] [PubMed] [Google Scholar]


