Abstract
TERT promoter mutations (TERT-mut) are detectable in the majority of urothelial carcinomas. The detection of TERT-mut in urine is under investigation as a potential urine-based molecular-screening assay for bladder cancer. A small but significant number of bladder carcinomas are pure squamous cell carcinoma. We sought to assess the incidence of TERT-mut in squamous cell carcinoma of the urinary bladder. A retrospective search of the institutional pathology archives yielded 15 cystectomy specimens performed for squamous cell carcinoma (2000–2014). Histologic slides were reviewed by a senior urologic pathologist to confirm the diagnosis and select a representative formalin-fixed paraffin-embedded tissue block for mutational analysis. All cases yielded adequate material for DNA analysis. Sequencing for TERT-mut was performed using previously described SafeSeq technique. We detected TERT-mut in 12/15 (80%) of bladder squamous cell carcinomas. TERT promoter mutations, commonly found in conventional urothelial carcinoma, are also highly prevalent in urinary bladder squamous cell carcinoma suggesting a common tumorigenesis and potential utility as a molecular urine-based-screening assay.
Maintenance of telomeres is a critical function for cell survival and continued replication, and one mechanism by which cells maintain telomeres is through the action of an extension complex that adds telomeric repeats to the ends of chromosomes.1 This complex includes the protein product of the telomerase reverse transcriptase (TERT) gene, (5p15.33). High rates of activating mutations in the upstream promoter of the TERT gene (TERT-mut) have been found in several solid tumor types.2–5 Mutations tend to occur in ‘hot spots’, particularly g.1295228C> T and g.129525OC> T. These mutations generate a CCGGAA/T or GGAA/T motif, thereby altering the binding site for Ets transcription factor, thereby increasing TERT promoter activity.2,6,7
Our group and others have demonstrated TERT-mut to be the most common genetic alterations in urothelial (transitional cell) carcinoma of the bladder and upper urinary tract.8–13 In the study by Kinde et al,12 66% of muscle invasive and 74% of non-muscle invasive bladder lesions were shown to harbor these alterations.
Greater than 90% of bladder carcinomas are urothelial type. Squamous cell carcinoma, adenocarcinoma, and small cell carcinoma represent the remaining most common types.14,15 Recently, we investigated TERT-mut incidence in small cell carcinomas of the urinary bladder.16 In the current study, we sought to determine the prevalence of TERT-mut in primary squamous cell carcinoma of the urinary bladder.
Materials and methods
A retrospective review was performed and the archives of a single institution were searched for cases of pure squamous cell carcinoma primary to the bladder treated by cystectomy, cystoprostatectomy, or other radical excision between the years 2000 and 2014. To ensure the inclusion of only ‘pure’ squamous cell carcinoma cases that would satisfy the World Health Organization/International Society of Urologic Pathology (WHO/ISUP 2004) definition of the entity, we limited our study to cystectomy and cystoprostatectomy specimens. All histologic slides were reviewed by a senior urologic pathologist to confirm the diagnosis of squamous cell carcinoma and select a representative tumor formalin-fixed paraffin-embedded block for mutational analysis. A total of 15 cases were included in the study based on the availability of formalin-fixed paraffin-embedded blocks with sufficient tumor for sampling. The tumor areas were cored with a sterile 16-gauge needle and the fraction of neoplastic cells was estimated from adjacent sections. The cores were placed in 1.5 ml sterile tubes for DNA purification.
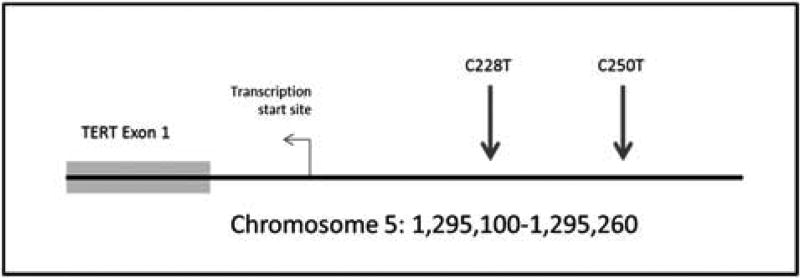
Following DNA purification, samples were analyzed with Safe-SeqS, a sequencing error-reduction technology described previously,17 which better discriminates genuine TERT-mut from artefactual sequencing variants introduced during the sequencing process. Safe-SeqS amplification primers were designed to amplify segments containing the region of the TERT promoter previously shown to harbor mutations in melanomas and other tumors (Figure 1).12 The forward and reverse amplification primers contained the TERT-specific sequences at their 3′ ends and a universal priming site at their 5′ end. The reverse primer additionally contained a 14-base unique identifier comprised of 14 degenerate N bases (equal likelihood of being an A, C, T, or G) between the universal priming site and gene-specific sequences. The sequences of the forward and reverse primers were either 5′-CACACAGGAAACAGCTATGACCATGGGCCGCGGAAAGGAAG and 5′-CGACGTAAAACGACGGCCAGTNNNNNNNNNNNNNNCG TCCTGCCCCTTCACC, or 5′CACACAGGAAACAGCTATGACCATGGCGGAAAGGAAAGGGAG and 5′-CG ACGTAAAACGACGGCCAGTNNNNNNNNNNNNNNCCGTCCCGACCCCTC. PCR products were purified with AMPure and sequenced on a MiSeq instrument. Data were analyzed as previously described.17 Tumor samples were considered positive if the fraction of mutations exceeded 1% of alleles (which was a frequency at least 5 × higher than found in control DNA templates). All sequencing assays scored as positive were confirmed in at least one additional, independent PCR and sequencing experiment.
Figure 1.
Two mutational ‘hotspots are repeatedly seen in the TERT promoter, at position 250 anal position228. Both of the mutations are a C-> T base substitution mutation.
Two sets of negative control samples were also analyzed. Ninety-four peripheral blood samples from healthy population were tested for TERT-mut as negative PCR procedure controls. Eight formalin-fixed paraffin-embedded benign transurethral bladder biopsy samples were also used as negative tissue controls.
Results
Patient Demographics, Clinicopathologic Features, and Outcome
This cohort included six females and nine males. The mean patient age was 65 years (range 51–83). None of the patients had associated schistosomiasis. The tumors were primarily centered in the bladder wall (12 patients) with the remaining in the trigone, bladder neck urethral region, and bladder dome. of note, one patient had a history of extrophy of the bladder and had a tumor that extended from the bladder wall to involve the dermis of the overlying abdominal wall. Two patients (13%) had lymph node metastases identified at the time of resection. Mean follow up time was 1564 days with a median follow up interval of 981 days (range 22–5459 days). Two patients (13%) died from disease at 337 and 169 days post surgery, the remaining 13 patients are presently disease free (Table 1). Morphologically, all tumors demonstrated typical feature of conventional squamous cell carcinoma. Papillary architecture was observed in one squamous cell carcinoma, and focal sarcomatoid features were found in another case.
Table 1.
Patient demographics, clinicopathologic features, and outcome
| Case | Age | Gender | Histologic type | Tumor gradea |
Tumor location | Tumor stage | Recurrences | Progression | Outcomeb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | Conventional | G3 | Bladder wall | T3aN0 | No | No | NED |
| 2 | 73 | M | Conventional | G3 | Bladder wall | T2N0 | No | No | UNK |
| 3 | 71 | M | Conventional | G3 | Bladder wall | T3aN0 | No | No | UNK |
| 4 | 53 | F | Conventional | G3 | Bladder wall and skin (extrophy) | T4bNX | No | No | NED |
| 5 | 53 | M | Conventional | G2 | Bladder trigone | T3aN0 | Yes | Yes | DOD |
| 6 | 79 | F | Conventional | G2 | Bladder wall | T3N0 | No | No | NED |
| 7 | 73 | F | Conventional | G2 | Bladder wall | T3Nx | No | No | NED |
| 8 | 56 | M | Conventional | G2 | Bladder wall | T3bN2 | No | No | NED |
| 9 | 51 | F | Conventional | G3 | Bladder wall | T4NX | No | No | NED |
| 10 | 72 | M | Conventional | G2 | Bladder wall | T2bN0 | No | No | NED |
| 11 | 63 | F | Conventional | G2 | Bladder wall | T3aN0 | No | No | NED |
| 12 | 70 | M | Papillary squamous cell carcinoma | G3 | Bladder wall | T3bN2 | No | No | NED |
| 13 | 73 | M | Squamous cell carcinoma with focal sarcomatoid features | G1 | Bladder wall | T1NX | No | No | NED |
| 14 | 54 | M | Conventional | G2 | Bladder trigone and bulbar urethra | T2N0 | No | No | NED |
| 15 | 83 | M | Conventional | G2 | Bladder wall | T3NX | Yes | Yes | DOD |
Tumor grade: well differentiated (G1), moderately differentiated (G2), poorly differentiated (G3).
Outcome: died of disease (DOD), died with disease (DWD), alive with disease (AWD), no evidence of disease (NED), unknown (UNK).
TERT Promoter Analysis
TERT-mut were identified in 12 out of 15 (80%) cases of squamous cell carcinoma. The majority of these (83%) were the conventional morphology, however both the papillary squamous cell carcinoma and squamous cell carcinoma with sarcomatoid features also had TERT-mut. Of all the patients with TERT-mut, 2 (17%) were g.129525OC> T and the remaining 10 (83%) were a g.1295228C> T alteration (Table 2).
Table 2.
TERT promoter mutations in squamous cell carcinoma by histologic type
| Histologic | Mutation | Mutation type | |
|---|---|---|---|
|
| |||
| TERT g.1295250C> T | TERT g.1295228T> C | ||
| Classical (n = 13) | 10/13 (77%) | 2/10 (20%) | 8/10 (80%) |
| Papillary (n = 1) | 1/1 (100%) | 0/1 | 1/1 (100%) |
| Sarcomatoid features (n = 1) | 1/1 (100%) | 0/1 | 1/1 (100%) |
| All (n = 15) | 12/15 (80%) | 2/12 (17%) | 10/12 (83%) |
All of the blood and formalin-fixed paraffin-embedded samples used as negative controls tested negative for TERT-mut.
Discussion
Bladder carcinoma is the most common malignancy of the urinary tract, and the fourth most common carcinoma in men in the Western world. Among bladder cancers, urothelial carcinoma is by far the most prevalent histologic type, with squamous cell carcinoma and adenocarcinoma making up ○ 5% of cases.18 Per patient, the cost of bladder cancer management is the highest among all tumors, owing to the need for long-term monitoring with regular cystoscopy, imaging, and urine cytology.19–21
TERT-mut were first reported in melanoma.2 Subsequently, the same mutations were discovered by our group and others in numerous solid cancers, including urothelial carcinoma, gliomas and hepato-cellular carcinoma.3–5 High rates of TERT-mut have been conspicuously absent in colorectal and lung carcinomas.3,22 More recently, high rates of TERT-mut have been demonstrated in small cell carcinoma of the urinary bladder, urothelial carcinoma with squamous differentiation, and nested variant of urothelial carcinoma.13,16,22 These consistent genetic alterations, shared by urothelial carcinoma and several of its variants, offer further support for a common oncogenic pathways.
Our study is the first to evaluate the presence of TERT-mut in conventional squamous cell carcinoma of the urinary bladder. As noted above, TERT-mut have been described in several tumor types in the bladder with urothelial differentiation; however, the absence of urothelial differentiation in all 12 cases makes this study novel. All mutations were from previously published hotspots, with the majority of cases (83%) having the g.1295228C> T mutation, whereas the remainder had a g.1295250C> T mutation. Histologically, all 12 TERT-mut-positive tumors were invasive conventional squamous cell carcinoma and with one tumor showing focal sarcomatoid differentiation and a second demonstrating a papillary architecture. Of note, one of the three tumors that lacked TERT-mut was the only case in our cohort that was centered in the bulbar urethra. Evaluation of prognostic significance of TERT-mut was not feasible in this limited study.
Our found rate of 80% prevalence of TERT-mut in squamous cell carcinoma of bladder is comparable to the rate previously demonstrated in conventional urothelial carcinoma including those with only focal squamous differentiation.9,11,12,22 This high rate is in stark contrast to the lack of TERT-mut in cervical and pulmonary squamous carcinomas.22 If further confirmed, the latter suggests a potential diagnostic role for identifying TERT-mut in assigning a primary urinary tract origin in cases where the differential include secondary involvement of bladder from such sites or cases of unknown primary. Finally, chronic bladder irritation (eg, stones, extrophy, and endemic Schistosomiasis) is associated with higher incidence of bladder squamous cell carcinoma.18 To our knowledge, the presence of TERT-mut in squamous cell carcinoma tumors arising in the later setting has not been specifically examined. None of our current patients had associated schistosomiasis. One of our TERT-mut-positive tumors occurred in a patient with a history of bladder extropliy.
In summary, TERT promoter mutations, commonly found in conventional urothelial carcinoma, are also highly prevalent in urinary bladder squamous cell carcinoma, suggesting a common tumorigenesis and potential utility as a molecular urine-based-screening assay.
Acknowledgments
Supported by grants from The Johns Hopkins Green-berg Bladder Cancer Institute, The Virginia and DK Ludwig Fund for Cancer Research, The Common-wealth Fund, The Conrad R Hilton Foundation, and The Sol Goldman Sequencing Facility at Johns Hopkins.
Footnotes
Disclosure/conflict of interest
KWK, NP, and BV are founders of Personal Genome Diagnostics, PapGene, and advise Sysmex-Inostics. These companies and others have licensed technologies from Johns Hopkins, of which BV, KWK, and NP are inventors and receive royalties from these licenses. The terms of these arrangements are being managed by the university in accordance with its conflict of interest policies.
References
- 1.Lit W, Zhang Y, Liu D, et al. Telomeres-structure, function, and regulation. Exp Cell Res. 2013;319:133–141. doi: 10.1016/j.yexcr.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived front cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott Ga, Laughlin TS, Rothberg PG. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod Pathol. 2014;27:516–523. doi: 10.1038/modpathol.2013.167. [DOI] [PubMed] [Google Scholar]
- 5.Qu Y, Shi L, Wang D, et al. Low frequency of TERT promoter mutations in a large cohort of gallbladder and gastric cancers. Int J Cancer. 2014;134:2993–2994. doi: 10.1002/ijc.28633. [DOI] [PubMed] [Google Scholar]
- 6.Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 7.Huang D-S, Wang Z, He X-J, et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur J Cancer. 2015;51:969–976. doi: 10.1016/j.ejca.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allory Y, Beukers W, Sagrera A, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65:360–366. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 9.Wu S, Huang P, Li C, et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: a genomic and molecular study. Eitr Urol. 2014;65:274–277. doi: 10.1016/j.eururo.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Rachakonda PS, Hosen I, de Verdier PJ, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci USA. 2013;110:17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borah S, Xi L, Zaug AJ, et al. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347:1006–1010. doi: 10.1126/science.1260200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinde I, Munari E, Faraj SF, et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013;73:7162–7167. doi: 10.1158/0008-5472.CAN-13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong M, Tian W, Zhuge J, et al. Distinguishing nested variants of urothelial carcinoma front benign mimickers by TERT promoter mutation. Am J Surg Pathol. 2015;39:127–131. doi: 10.1097/PAS.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 14.Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol. 2005;13:143–153. doi: 10.1177/106689690501300203. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Beltran A, Sauter G, Gasser T, et al. Tumors of the urinary system. In: Eble JN, Sauter GJIE, Sesterhenn I, editors. Pathol Genet Tumours Urin Syst Male Genit Organs. Lyon, France: IARC Press; 2004. pp. 89–123. [Google Scholar]
- 16.Zheng X, Zhuge J, Bezerra SM, et al. High frequency of TERT promoter mutation in small cell carcinoma of bladder, but not in small cell carcinoma of other origins. J Hematol Oncol. 2014;7:47. doi: 10.1186/s13045-014-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinde I, Wu J, Papadopoulos N, et al. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:9530–9535. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen SM, Shirai T, Steineck G. Epidemiology and etiology of premalignant and malignant urothelial changes. Scand J Uro1 Nephrol Suppl. 2000:105–115. doi: 10.1080/00365590050509869. [DOI] [PubMed] [Google Scholar]
- 19.Anastasiadis A, Cordeiro E, Bus MT, et al. Follow-up procedures for non-muscle-invasive bladder cancer: an update. Expert Rev Anticancer Ther. 2012;12:1229–1241. doi: 10.1586/era.12.98. [DOI] [PubMed] [Google Scholar]
- 20.Anastasiadis A, de Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Uro1. 2012;4:13–32. doi: 10.1177/1756287211431976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avritscher EBC, Cooksley CD, Grossman HB, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549–553. doi: 10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 22.Cheng KA, Kurtis B, Babayeva S, et al. Heterogeneity of TERT promoter mutations status in squamous cell carcinomas of different anatomical sites. Ann Diagn Pathol. 2015;19:146–148. doi: 10.1016/j.anndiagpath.2015.03.005. [DOI] [PubMed] [Google Scholar]