SUMMARY
Here we describe a multiplexed immunohistochemical platform, with computational image processing workflows including image cytometry, enabling simultaneous evaluation of 12 biomarkers in one formalin-fixed paraffin-embedded tissue section. To validate this platform, we used tissue microarrays containing 38 archival head and neck squamous cell carcinomas, and revealed differential immune profiles based on lymphoid and myeloid cell densities, correlating with human papilloma virus status and prognosis. Based on these results, we investigated 24 pancreatic ductal adenocarcinomas from patients who received neoadjuvant GVAX vaccination, and revealed that response to therapy correlated with degree of mono-myelocytic cell density, and percentages of CD8+ T cells expressing T cell exhaustion markers. These data highlight the utility of in situ immune monitoring for patient stratification, and provide digital image processing pipelines (https://github.com/multiplexIHC/cppipe) to the community for examining immune complexity in precious tissue sections, where phenotype and tissue architecture are preserved to thus improve biomarker discovery and assessment.
Keywords: Cancer immunology, multiplex imaging, Immunohistochemistry, Histopathology
INTRODUCTION
Therapies targeting critical aspects of T cell regulation have revolutionized cancer therapy for some patients with highly antigenic cancer types (Palucka and Coussens, 2016); however, many patients still fail to respond and/or develop resistance to immune-based therapy. Moreover, tumors possessing low mutational burdens and/or those with limited antigenicity present unique therapeutic obstacles as response rates for these remain low. Given that increased numbers of patients are now receiving some form of immune therapy, a major goal is to identify either in situ or circulating biomarkers to aid patient stratification for precision immune therapy such that efficacy can be increased and expanded across tumor types, as well as biomarkers for longitudinal response monitoring, e.g., remission and resistance.
Profiling immune contexture has emerged as a powerful metric for tumor subclassification, as well as predicting clinical outcome (DeNardo et al., 2011; Fridman et al., 2012; Galon et al., 2006; Ruffell et al., 2014; Zhang et al., 2003). Based on these insights, we predicted that effective auditing of tumor leukocyte biomarkers in situ might provide sufficient stratification metrics with which to improve the success of immune-based therapies. A major obstacle for deployment of such a strategy is based on limited quantities of precious tumor-derived biopsy material for in situ prospective monitoring.
Herein we describe an optimized sequential immunohistochemistry (IHC) approach, utilizing either biopsy or surgical specimens in formalin-fixed paraffin embedded (FFPE) tissue sections, and panels of antibodies enabling comprehensive phenotyping of immune complexity, together with computational image processing workflows that support multiparameter cytometric quantification strategy. Together, these enable assessment of multiple lineage-selective and phenotypic biomarkers in three FFPE tissue sections quantitatively evaluated with three 12-antibody biomarker panels, that audit lymphoid and myeloid lineages, and the functional status of T cells.
Using this platform in a head and neck squamous cell carcinoma (HNSCC) cohort, we revealed that differential immune complexity, representing either lymphoid- or myeloid-inflamed tumors, correlated with clinical outcomes and tumor subclassification. In addition, by appending geometrical mapping analysis on top of leukocyte density, immune complexity status was linked to therapeutic response to vaccination therapy in pancreatic ductal adenocarcinoma (PDAC), where myeloid-inflamed and T cell exhaustion status correlated with shorter overall survival.
As a service to the community, all required pipelines for digital image processing and related computational manuals are available at https://github.com/multiplexIHC/cppipe. Since this multiplex IHC platform is based on conventional digital IHC examination without requiring additional instrumentation, this method is technically and economically equivalent to standard IHC, thus enabling feasibility for large-scale studies without significant cost. These advancements will lead to expanding biomarker-based discovery and deployment in oncoimmunology research, and improved ability to stratify and monitor patients receiving diverse immune-based therapeutics.
RESULTS
Optimized sequential IHC and digital image processing enable evaluation of 12 biomarkers in one FFPE tissue section
To develop an IHC workflow enabling simultaneous evaluation of multiple biomarkers in one FFPE sections, we built upon sequential IHC methodology originally reported for a 5-plex protocol (Glass et al., 2009), and subsequently expanded to enable 12 sequential IHC with iterative labeling and stripping steps, facilitating analysis of more than 12 proteins on one tissue section, regardless of detecting antibody species of origin (Figures 1A, B, and S1). Briefly, after standard IHC preparation and primary antibody incubation, antibodies are detected by a F(ab′) fragment-specific secondary antibody labeled polymer-based peroxidase. Following detection, slides are visualized by alcohol-soluble peroxidase substrate 3-amino-9-ethylcarbazole (AEC), followed by whole slide digital scanning. Iterative staining is achieved by AEC washing slides in ethanol (Glass et al., 2009; Tramu et al., 1978), followed by antibody stripping in heated citrate buffer (pH 6.0) (Lan et al., 1995). Slides are then washed and equilibrated in binding buffer, and readied for a subsequent round of primary antibody incubation. Complete stripping of antibodies and signals throughout all cycles was confirmed (Figure S2A). IHC sensitivity was equivalent to standard IHC throughout 11-repeated antibody-stripping rounds (Figures S2B and S2C).
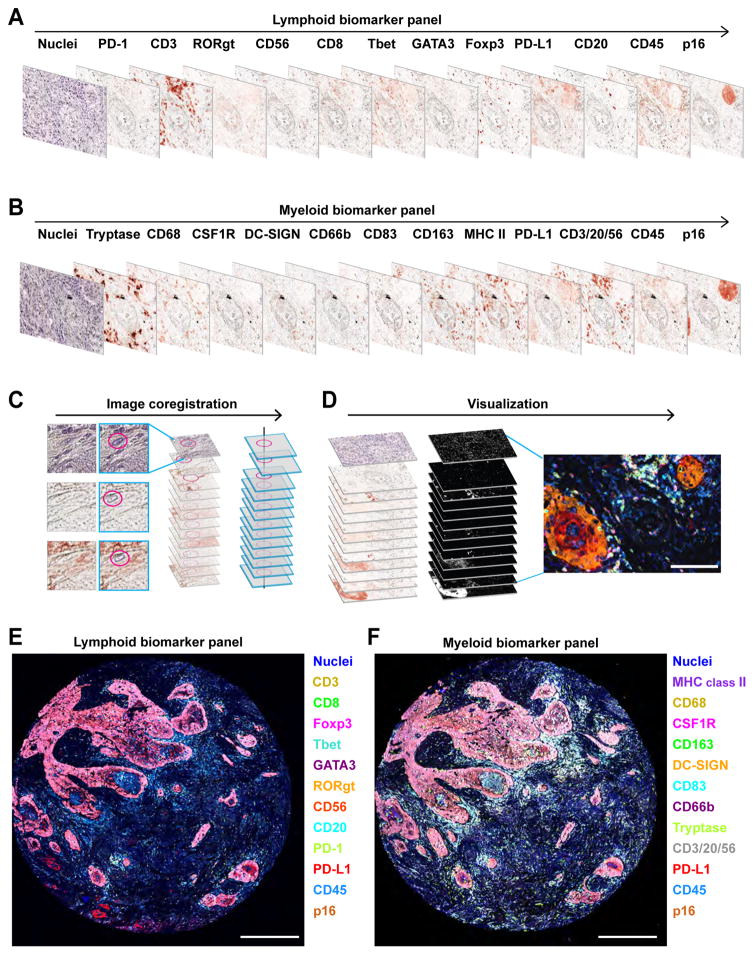
Figure 1. Schematic overview of 12-color sequential IHC and image visualization.
(A, B) Digital scans representing bright field sequential IHC of one formalin-fixed paraffin embedded (FFPE) section of human head and neck squamous cell carcinoma (HNSCC) tissue enable assessment of 12 different lymphoid (A) and myeloid (B) biomarkers. Primary antibodies were visualized with horseradish peroxidase-conjugated polymer and 3-amino-9-ethylcarbazole (AEC) detection followed by whole slide digital scanning. Following destaining in an alcohol gradient and a heat-based antibody stripping using citrate pH 6.0 (see Experimental Procedures and Table S1), samples were restained sequentially with the indicated antibodies. (C) Following manual selection of single cell or structure indicated by magenta circles, the XY coordinates of scanned images were calculated and utilized for adjustment of alignment in CellProfiler (see Experimental Procedures). (D) AEC color signals were extracted from each digitized single marker image by color deconvolution, followed by pseudo-coloring. Scale bar = 100 μm. (E, F) Two serial FFPE sections of HNSCC were stained with the lymphoid (E) and myeloid biomarker (F) panels by pseudo-coloring. Biomarkers and colors are shown on right. Corresponding single marker images are shown in Figures S1A and S1B. Scale bars = 500 μm.
After completing multiple rounds of sequential IHC, serially scanned and digitized images are processed with a computational image analysis workflow (see Supplemental Experimental Procedures). Briefly, sets of serial images are aligned based on a semi-automated coregistration pipeline utilizing CellProfiler software as a backbone (Carpenter et al., 2006) (Figure 1C). Coregistered images are subsequently transferred to ImageJ (Schneider et al., 2012), and AEC/hematoxylin-color information is extracted by color deconvolution algorithms (Ruifrok et al., 2003), wherein images are converted to gray scale, and then visualized as pseudo-colored images (Figures 1D–F).
Two 12-panels of lineage-selective antibodies identify and phenotype lymphoid and myeloid cells evaluating expression of 19-distinct biomarkers
To specifically audit complexity and phenotype of resident and infiltrating leukocytes in tumors where geographic distribution can be preserved, we established two panels of 12-biomarkers each, encompassing 19-distinct epitopes to phenotype lymphoid and myeloid lineage cells (Figures 1A, 1B, 2, S1, and Table S1). The lymphoid biomarker panel depicts CD8+ T cells, TH0-, TH1-, TH2-, and TH17-T cells, Regulatory T cells (TREG), B cells, and natural killer cells (NK), while the myeloid biomarker panel visualizes CD163+ vs − tumor-associated macrophages (TAM), immature (DC-SIGN+) versus mature (CD83+) dendritic cells (DC), CD66b+ granulocytes (Gr) including neutrophils and eosinophils, and tryptase+ mast cells (Figures 2A, 2B, S1A, S1B, S3A and S3B) thus enabling quantitative evaluation of 14-different immune cell populations (Figure 2C).
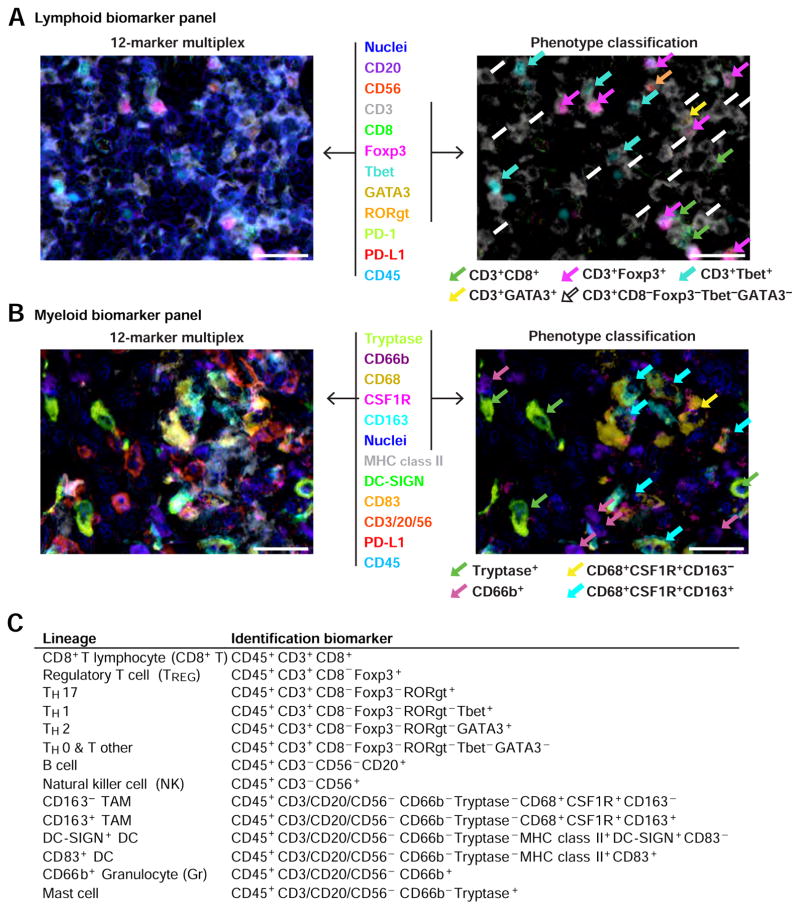
Figure 2. 12-color multiplex IHC to visualize lymphoid and myeloid immune cell phenotypes in FFPE sections.
(A, B) FFPE sections of human HNSCC tissues were analyzed by the two 12-marker panels of lineage-selective antibodies to identify lymphoid (A) and myeloid (B) lineages (left panels). Figure S3 shows single-color images for composites shown in (A) and (B). Colocalization of multiple markers enabled discernment of immune cell phenotypes including CD3+CD8+ T cells, regulatory T cells (TREG), TH0, TH1, TH2 and TH17 lymphocytes, CD163+ and CD163− macrophages, CD66b+ granulocytes (Gr), and mast cells (right panels with colored arrows). Biomarkers and colors are shown in the center. Scale bars = 25 μm. (C) Lineages and corresponding identification markers utilized in this study are shown.
Multiparameter image cytometry enables quantitative assessment of 14-different cell lineages in multiplexed IHC images
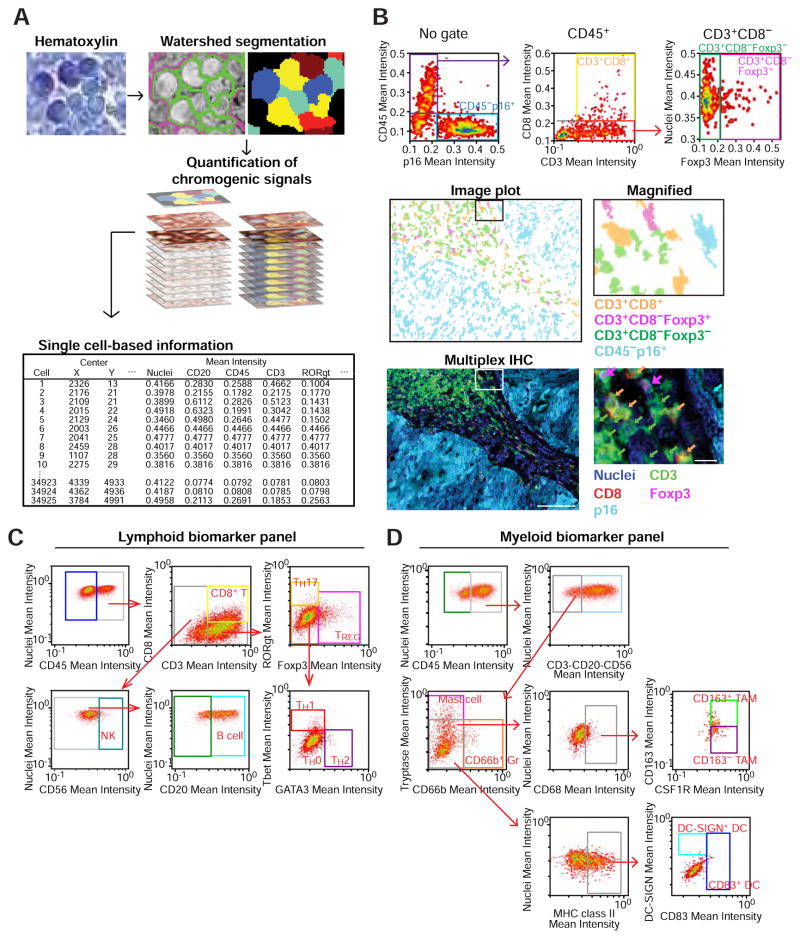
To enable quantitative evaluation of leukocyte features with regional and proximity analytics, we developed a multiparameter cytometric quantification approach via evaluation of single cell-based chromogenic intensities using single cell segmentation algorithms using CellProfiler (see Supplemental Experimental Procedures). We utilized hematoxylin-stained images for cell segmentation based on watershed algorithms (Padmanabhan et al., 2010; Wählby et al., 2004), followed by quantification of chromogenic signals in serial AEC-stained images, providing multiparametric information including cell size, compactness, and location with chromogenic intensity for each protein biomarker (Figure 3A). Single cell-based information, including pixel intensity and shape-size measurements were visualized and analyzed with qualitative assessment of signal intensities, analogous to flow cytometry (FACS) data (Figure 3B). Thresholds for qualitative identification were determined based on distribution of plots for each marker in negative control slides (Figure S4A). Importantly, gated cells in dot plots were visualized in the original image together with distribution in the tissue context, e.g., stromal versus within a neoplastic cell nest (Figure 3B). XY coordinates of selected single cells were also depicted in the original image, enabling positioning of each cell in the corresponding image (Figure S4A).
Figure 3. Multiparameter cytometric image analysis for quantification of multiplex IHC.
(A) A hematoxylin-stained image used for automated cell segmentation based on watershed segmentation algorithms by CellProfiler is shown (see Experimental Procedures). Segmentation results were utilized as templates for quantification of serially scanned AEC images, and pixel intensities of chromogenic signals and area-shape measurements were extracted and recorded by single cell-analysis together with location in original images. (B) Obtained single cell-based chromogenic signal intensity, cell size/area, and location were utilized for density plots similar to flow cytometry by using a cytometry analysis software, FCS Express 5 Image Cytometry (De Novo Software). Three dot plots shown at top represent image cytometric analysis in a p16+ HNSCC tissue. Gated cell populations of CD45+CD3+CD8+ T cells, CD45+CD3+CD8−Foxp3+, and CD45+CD3+CD8−Foxp3−, CD45−p16+ cells are shown (middle) as an image plot with coloring of orange, magenta, green, and cyan, respectively. A 5-color multiplex IHC image corresponding to the image plot is shown at bottom, revealing matched identification between image cytometry and visualized images. The boxes depict magnified areas. Scale bars = 100 μm (low magnification) and 10 μm (high magnification). (C, D) Image cytometry-based cell population analyses for the lymphoid and myeloid biomarker panels are shown in (C) and (D), respectively. The markers used for identification of cell lineages are shown in Figure 2C. Gating thresholds for qualitative identification were determined based on data in negative controls (Figures S4B and S4C). The x and y axes are shown on a logarithmic scale.
To achieve quantitative data analogous to multiparametric 12-color FACS (Gunderson et al., 2015; Ruffell et al., 2012), we developed qualitative gating strategies for the panels (Figures 3C, 3D, and S4A–C). For comparative analyses between image cytometry and FACS, the same pieces of human surgical specimens were divided into two pieces, and evaluated by single-cell suspension-based FACS analysis and FFPE section-based image cytometry, and observed positive correlations in percentages of T and B cells measured by both methodologies (N = 9, Figures S4D, S4E), thus validating the image cytometric approach.
In situ leukocyte analysis enables tumor subclassification and prognosis in HNSCC
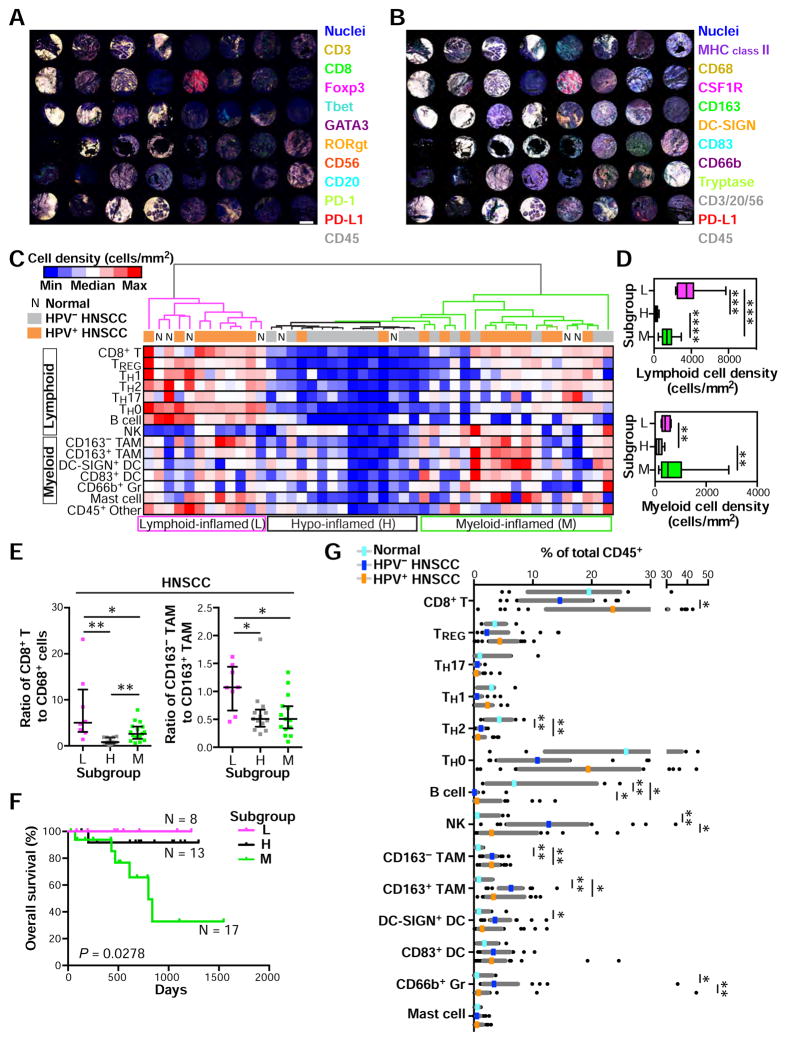
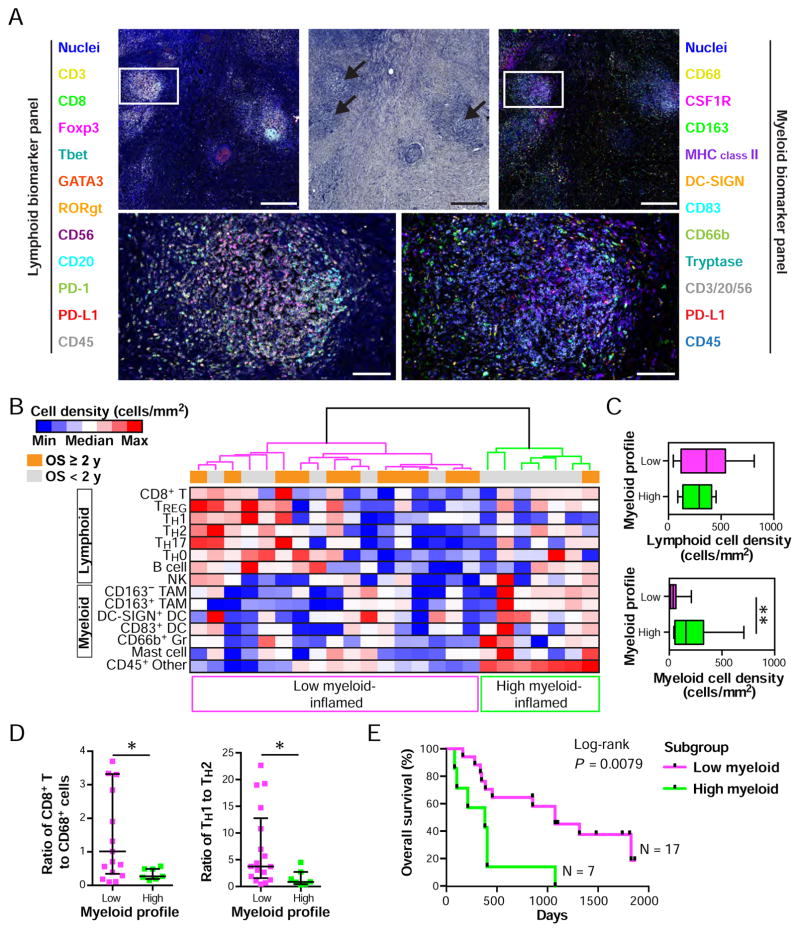
To verify the capability of the multiplex imaging and quantitation platform, we evaluated a tissue microarray (TMA) of oropharyngeal head and neck squamous cell carcinomas (HNSCC), wherein presence of oncogenic human papilloma virus (HPV) is associated with immunogenic gene signatures (Keck et al., 2014; Thurlow et al., 2010), thus serving as a validation control for the platform. A HNSCC TMA was assembled from 2 mm cores reflecting pathologist-selected representative intratumoral areas including 21 HPV-positive and 17 HPV-negative tissues, with 8 non-malignant pharyngeal tissues (Table S2). IHC evaluation with lymphoid and myeloid panels was performed on two adjacent FFPE sections, wherein antibodies detecting p16 were included to assess HPV positivity (Figures 4A and 4B). Following quantification of cell densities and ratios of 14-different immune cell lineages identified by image cytometry gating strategies (Figures 3C, 3D, S4B, and S4C), an unsupervised hierarchical clustering analysis was performed to identify distinct tumor subgroups based on immune complexity profiles (Figure 4C). This analysis revealed presence of lymphoid-inflamed, hypo-inflamed and myeloid-inflamed subgroups, wherein lymphoid and myeloid lineage cells were differentially present in stroma (Figures 4C and S5A). This observation was supported by cell density analyses among the three groups (Figure 4D). Utilizing transversal quantification of multiple immune cell lineages, we evaluated ratios of CD8+ T cell to CD68 as IHC-based favorable predictors of clinical outcomes as reported in other malignancies (DeNardo et al., 2011; Ruffell and Coussens, 2015) as well as CD163 expression on TAMs which is associated with anti-inflammatory TH2 phenotype and tumor initiation and progression (DeNardo et al., 2011; Mantovani et al., 2002). While the hypo-inflamed group unsurprisingly revealed low scores reflecting a “cold” inflammatory status, both ratios in the lymphoid-inflamed subgroup appeared significantly higher than those in the myeloid-inflamed subgroup, (Figure 4E). In comparison, the myeloid-inflamed subgroup exhibited the shortest overall survival among three subgroups regardless of HPV-status (Figures 4F and S5B). A leukocyte composition analysis of total CD45+ revealed high CD163− and CD163+ TAMs, and low TH2 and B cells in HNSCC tissues in comparison to normal pharynx (Figure 4G). HPV-positive status was associated with high CD8+ T cells, while HPV-negative correlated with high NK and DC-SIGN+ DC, and CD66b+ Gr (Figure 4G), together indicating presence of differential immune profiles between benign and malignant tissues as well as HPV-status. Distinct immune profiles depending on HPV-status were also confirmed by cell density-based analysis (Figure S5C). Results comparing HPV-status were similarly observed by analysis of The Cancer Genome Atlas (TCGA) (Figure S5D), supporting validation of the method for evaluating HNSCC subtypes. To exclude potential bias from effects of differential tumor-stroma ratios on immune cell densities, we also evaluated tumor area percentage as a function of total tissue in each core, and observed no significant differences between subgroups (Figures S5E and S5F). Interestingly, although the lymphoid- and hypo-inflamed subgroups were clearly segregated between HPV-positive and -negative status respectively, the myeloid-inflamed subgroup with poor prognosis exhibited heterogeneity in HPV-status (Figure 4C), indicating the possibility that further stratification of patients with HNSCC based on immune profiles beyond HPV-status may be warranted. These data demonstrate that this quantitative imaging approach depicts distinct immune cell characteristics corresponding to tumor subtypes according to immune cell densities and HPV-status associating with prognosis.
Figure 4. Quantitation of immune cell density-based subgrouping, enables stratification for prognosis and human papilloma virus (HPV) status in HNSCC.
(A, B) Two FFPE sections from a HNSCC-assembled TMA including HPV-negative (N = 17), HPV-positive oropharyngeal tumor (N = 21) and normal oropharynx (N = 8) were stained using the lymphoid (A) and myeloid biomarker antibody panels (B). Scale bar = 1.0 mm. (C) Cell densities (cells/mm2) of 15 immune cell lineages in each single core were quantified using image cytometry. Data sets from the two panels reflecting lymphoid and myeloid biomarkers were normalized based on CD45+ cell number. A heat map according to color scale (upper left) is shown with a dendrogram of unsupervised hierarchical clustering, depicting lymphoid-, hypo-, and myeloid-inflamed subgroups (L, H and M in bottom, respectively). See also Figure S5C and Table S2. (D) Immune cell densities of lymphoid and myeloid cell lineages comparing subgroups in Figure 4C. Bars, boxes and whiskers represent median, interquartile range and range, respectively. (E) Ratios of cell percentages comparing subgroups are shown. Bars show median with interquartile range. (F) Kaplan-Meier analysis of overall survival of HNSCC patients stratified by subgroups. Statistical significance was determined via log-rank test. (G) Immune cell percentages were quantified as a percentage of total CD45+ cells. Statistical differences in (D), (E) and (G) were determined via Kruskal-Wallis tests with false discovery rate (FDR) adjustments, with * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
Differential intratumoral immune complexity stratifies therapeutic response to neoadjuvant GVAX in patients with PDAC
Based on the differential success of immune therapies utilizing vaccines or therapeutic antibodies targeting co-stimulatory or co-inhibitory molecules, we predicted that effective auditing of tumor leukocyte biomarkers might provide stratification metrics with which to improve success of these and other therapies. Thus, following validation of the multiplex imaging and quantitation approach using archival HNSCC, we sought to evaluate similar immune metrics to determine if the approach would stratify patients based on therapeutic response to an immune therapy. To accomplish this, we utilized archival FFPE specimens from previously reported pancreatic ductal adenocarcinoma (PDAC) surgical specimens reflecting patients who received neoadjuvant GVAX therapy, a granulocyte-macrophage colony-stimulating factor (GM-CSF)–secreting PDA cell-based vaccine (Lutz et al., 2014) (ClinicalTrials.gov Identifier: NCT00727441) where intratumoral lymphoid aggregates develop in some patients as a post-vaccination response (Table S3). Adjacent FFPE tissues sections were stained using the lymphoid and myeloid-selective antibody panels (Figures 5A, S6A, and S6B), and quantitatively evaluated by image cytometry (Figures S6C and S6D) in three areas per FFPE tissue (Figures S6E and S6F) that typically included lymphoid aggregate regions (Figure 5A). The three 25 mega pixel each images (approximately 2.5 × 2.5 mm square) were selected as regions of interest (ROI) based on geometrical mapping analyses of CD45+ leukocyte cell densities (see Experimental Procedures. Figure S9). Following an unsupervised hierarchical clustering analysis similar to Figure 4C, we observed differential immune complexity profiles showing low and high myeloid-inflamed status (Figure 5B). Interestingly, the two clusters showed differential myeloid cell densities, but not lymphoid cell densities (Figure 5C), compatible with robust induction of lymphoid aggregate regions post-GVAX. However, despite relatively high lymphoid cell densities in both groups, the existence of immunosuppressive profiles dominated the high myeloid-inflamed group, based on ratios of CD8+ T cells to CD68+ cells and TH2 polarization of CD8− T cells (Figures 5D and S6G). Comparison of these results associated with immunosuppressive status, the high myeloid-inflamed group was associated with short overall survival based on Kaplan-Meier analysis (Figure 5E). Together these results support the hypothesis that intratumoral leukocyte analysis, and specifically a myeloid-inflamed stroma may limit efficacy of GVAX therapeutic responses despite successful induction of lymphoid infiltration.
Figure 5. Immune complexity correlates with therapeutic response to neoadjvant GVAX therapy in pancreatic ductal adenocarcinoma (PDAC).
(A) Two adjacent FFPE sections from human PDAC tissues derived from neoadjuvant GVAX-treated (N = 24, see Table S3) individuals were analyzed by multiplex IHC. Representative 12-color composite images of myeloid and lymphoid biomarker panels are shown with a corresponding hematoxylin image. Biomarkers and colors are shown. The boxes represent the magnified area below. Scale bars = 500 μm (low) and 100 μm (high magnification). Corresponding single marker images are shown in Figures S6A and S6B. (B) Immune cell densities (cells/mm2) of three leukocyte hotspots in intratumoral regions (see Figure S6E) were assessed by multiplex IHC/image cytometry, in analogues to Figure 4C. A heat map according to color scale (upper left) is shown with a dendrogram of unsupervised hierarchical clustering, depicting low and high myeloid-inflamed subgroups. (C) Immune cell densities of lymphoid and myeloid cell lineages comparing subgroups in (B). Bars, boxes and whiskers represent median, interquartile range and range, respectively. (D) Ratios of cell percentages comparing subgroups are shown. Bars show median with interquartile range. Statistical differences in (C) and (D) were determined via Kruskal-Wallis tests, with * P < 0.05, and ** P < 0.01. (E) Kaplan-Meier analysis of neoadjuvant GVAX-treated PDAC cohort (N = 24) stratified by subgroups. Statistical significance was determined via log-rank test.
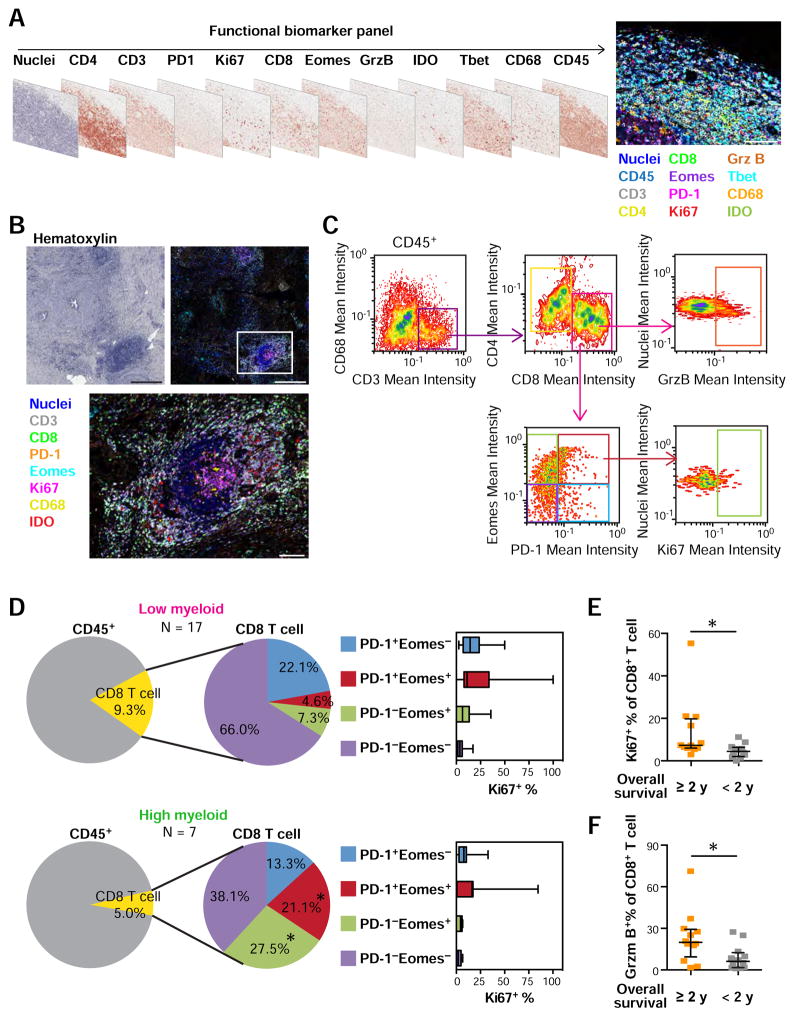
CD8+ T cell status correlates with myeloid-inflammation and reflects outcome in response to GVAX therapy
Since tumor-associated myeloid populations contribute to cancer progression based in part on their immunosuppressive capabilities regulating T cell dysfunction (Paley et al., 2012; Twyman-Saint Victor et al., 2015; Wherry and Kurachi, 2015), e.g., proliferation (DeNardo et al., 2011; Ruffell et al., 2014), recruitment (Affara et al., 2014), differentiation and effector function (Gunderson et al., 2015; Mantovani et al., 2002; Palucka and Coussens, 2016), we sought to integrate in situ assessment of T cell functional status as related to myeloid versus lymphoid status of tumors. To achieve this, we developed a third antibody panel (Figures 6A and S7A) for multiplex IHC (Figures 6B and S7B) in combination with quantitative image cytometry (Figure 6C), to examine T cell activation or exhaustion status in the same three tissue areas analyzed for lymphoid and myeloid complexity (Figure 5A). Images were quantitatively evaluated by image cytometry, followed by further dissection based on CD45+CD3+CD8+ T cells expressing the co-inhibitory receptor, programmed cell death-1 (PD-1), effector T cell development-associated Eomesodermin (Eomes), followed by assessment of proliferation (Ki67) and cytotoxic (granzyme B) activity (Figures 6C–F, S7C and S7D). Comparative analysis between the low and high myeloid-inflamed profiles observed in Figure 5B revealed that high myeloid-profiles correlated with PD-1+Eomes+ CD8+ T cells, linked to an exhausted phenotype (Twyman-Saint Victor et al., 2015; Wherry and Kurachi, 2015), together with expansion of PD-1−Eomes+ late effector T cells (Figure 6D). Notably, the expanded PD-1+Eomes+ CD8+ T cell component in the myeloid-inflamed group exhibited relatively low Ki67 expression, further indicating presence of exhausted T cells in relation to myeloid lineage enrichment (Figures 6D and S7C). Further analysis of the prognostic impact of in situ T cell functional status revealed that short overall survival was associated with low proliferation status of CD8+ T cells (Figures 6E, and S7D), as well as low activation status assessed by granzyme B (Figure 6F). Together these data indicate that myeloid-dominated immune environments associate with restricted T cell functionality regardless of the degree of lymphoid-inflammation, and adversely correlates with clinical outcome following neoadjuvant GVAX therapy.
Figure 6. In situ T cell functional biomarker assessment elucidates CD8+ T cell status in non-responders to neoadjuvant GVAX treatment.
(A) T cell functional biomarker panel is shown as digital scans of bright field sequential IHC derived from a single FFPE section of human tonsil tissue. Scale bar = 100 μm. Corresponding single marker images are shown in Figure S7A. (B) Representative images from PDAC tissue including lymphoid aggregates. Biomarkers and colors are shown. Boxes represent the magnified area below. Scale bars = 500 μm (low) and 100 μm (high magnification). See also Figure S7B. (C) Gating strategy for image cytometry of the T cell functional biomarker. (D) CD8+ T cells in neoadjuvant GVAX-treated PDAC tissues (N = 24) were assessed by T cell functional biomarker panel in three regions per tissue matched to analyzed regions in Figure 5C. Left pie charts represent average of CD8+ T cell percentages of total CD45+ cells, comparing low and high myeloid-inflamed profiles defined in Figure 5C. Middle pie charts show average percentages showing a composition of CD8+ T cells stratified by PD-1 and Eomes expression. Box whisker plots in right show Ki67+ percentages evaluated in each CD8+ T cell subpopulation. Bars, boxes and whiskers represent median, interquartile range and range, respectively. Statistical significances between the two groups were determined via Kruskal-Wallis tests with FDR adjustments, with * P < 0.05. (E, F) Percentages of Ki67 (E) and Granzyme B (F) in CD8+ T cells in neoadjuvant GVAX-treated PDAC tissues were shown, comparing overall survival ≥ 2 y (N = 12) and < 2 y (N = 12). Statistical significances were determined via Kruskal-Wallis tests, with * P < 0.05.
Myeloid PD-L1 correlates with activated CD8+ T cell status, associated with favorable prognosis following GAVX
Considering these data revealed the significance of understanding T cell activation/exhaustion status in the context of myeloid-mediated inflammation, and that expression of PD-1 ligand, PD-L1 on myeloid cells may provide prognostic information for therapeutic response to immune therapy (Parsa et al., 2007; Patel and Kurzrock, 2015; Topalian et al., 2012), and that blockade of the PD-1/PD-L1 axis reflects a therapeutic strategy for HNSCC and PDAC (Paley et al., 2012; Topalian et al., 2012), we sought to examine PD-L1 expression in relation to T cell functional status to assess its biomarker potential for patient stratification using both the HNSCC and PDAC samples evaluated above.
In the HNSCC TMA (Figure 4), six of 38 cases exhibited diffuse PD-L1 expression in HNSCCs (Figure S8A), where PD-L1 expression was observed in a spectrum of leukocyte lineages including CD163+ and CD163− TAMs, CD83+ and DC-SIGN+ DCs, NK, CD66b+ Gr, mast cells, T cells, and B cells (Figure S8B), in agreement with previous reports (Lyford-Pike et al., 2013; Pardoll, 2012; Soares et al., 2015). To quantitatively verify these observations, positive percentages of PD-L1 expression in each cell lineage was then quantified by image cytometry, and transversely analyzed across cell lineages together with subclassification of tumor/normal tissue types. Among cell lineages including CD45− neoplastic cells, the highest frequency of PD-L1 expression was observed on myeloid cells (Figure S8C). High PD-L1 expression on myeloid cells was observed particularly in HPV-associated tumors (Figure S8C), associated with lymphoid-inflamed profiles (Figure 4C).
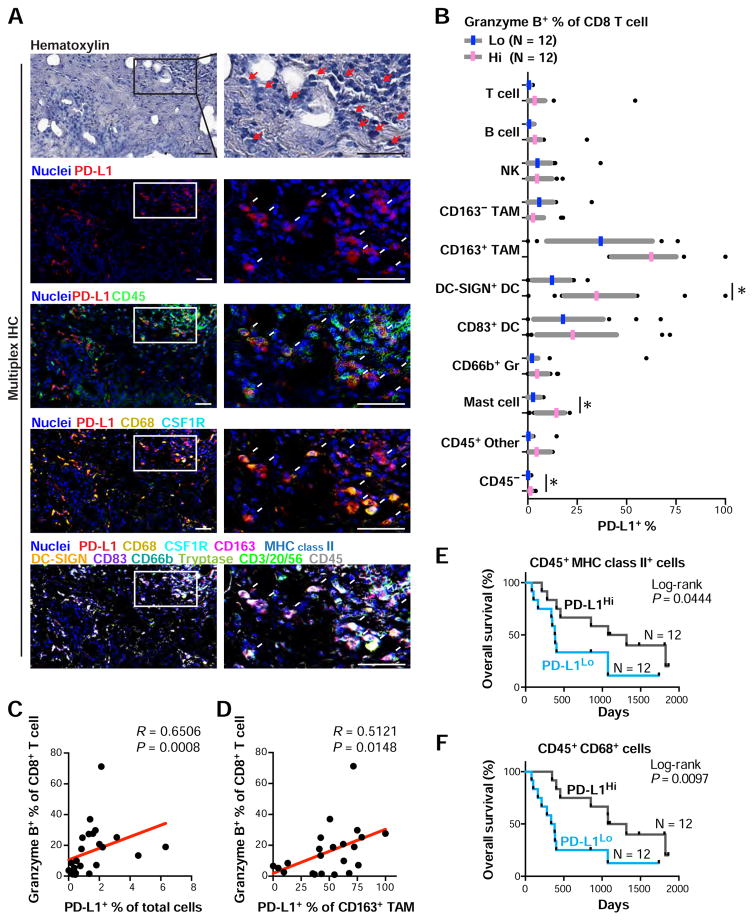
In the GVAX-treated PDAC samples (Figure 5), PD-L1 expression was identified predominantly in CD45+ cells, and particularly in CD68+ and MHC class II+ cells and DCs, rather than CD45− populations (Figures 7A and 7B). To investigate a potential association between T cell functional status and PD-L1, we examined the T cell activation marker granzyme B, and observed a significant correlation with PD-L1+ cells (Figures 7C and 7D), but independent of CD8+ T cell frequency (Figure S8D). This observation was further supported by analysis of granzyme B+CD8+ T cell density (Figure S8E). Furthermore, as was observed in Figure 7A, PD-L1 expression on CD163+ TAMs and DC-SIGN+ immature DCs also correlated with granzyme B positivity of CD8+ T cells (Figures 7B and S8F). As expected, high PD-L1 expression on myeloid cells was associated with high granzyme B expression in CD8+ T cells, indicating a correlation between PD-L1 upregulation and activated T cell status. Since activated T cell status was linked to favorable prognosis (Figure 6F) and high PD-L1 expression was observed on myeloid lineages (Figure 7B), we then evaluated prognostic significance of myeloid PD-L1 based on Kaplan-Meier analyses, revealing that high PD-L1 expression on myeloid lineages represented by MHC class II+ cells and CD68+ cells indicated significantly longer overall survival (Figures 7E and 7F). Given that PD-L1 plays a physiological function for auto-regulatory immune mechanisms, these results together imply that upregulated PD-L1 on myeloid cells could be a result of phenotypic changes in response to abundant inflammatory cytokines following successful induction of lymphoid-inflamed profiles. Simultaneously, these observations also indicate that PD-L1 expression on myeloid cells potentially serves as a biomarker for vaccination therapy as well as therapeutic targets via immune checkpoint blockade.
Figure 7. Myeloid PD-L1 expression correlates with favorable prognosis following neoadjuvant GVAX treatment, and associates with CD8+ T cell activation status.
(A) Multiplex IHC images showing PD-L1 expression in neoadjuvant GVAX-treated PDAC tissues. Arrows depict PD-L1+ cells, demonstrating colocalization with CD45+ CD68+ CSF1R+ macrophages. Scale bars = 100 μm. (B) PD-L1+ percentages were assessed in cell lineages shown, and comparing low (N = 12) and high (N = 12) groups in granzyme B percentages of CD8+ T cells. Median (11.7%) was used for the cutoff line of granzyme B-status. Three regions per tissue matched to analyzed regions in Figure 5C were evaluated. Statistical significances were determined via Kruskal-Wallis tests with FDR adjustments, with * P < 0.05. (C, D) Spearman correlations of granzyme B+ percentages of CD8+ T cells versus PD-L1+ percentages of total cells (C) or CD163+ tumor associated macrophages (TAMs) (D) are shown with estimated regression lines (red) in the neoadjuvant GVAX-treated PDAC cohort (N = 24). (E, F) Kaplan-Meier analyses of neoadjuvant GVAX-treated PDAC stratified by PD-L1+ percentages in CD45+ CD68+ cells (E) and CD45+ MHC class II+ cells (F). Median (15.7% and 18.7%) was used for the cutoff line of PD-L1-status for E and F, respectively. Statistical significance was determined via log-rank test.
DISCUSSION
In this study, we investigated tumor immune characteristics of archival HNSCC tumors, and in PDAC specimens from patients who had received neoadjuvant GVAX therapy, utilizing a multiplex IHC methodology, optimized for immune complexity and phenotype analyses, accompanied by quantitative studies utilizing a computational image processing workflow. The imaging approach and analysis pipeline enables quantitative assessment of immune-infiltrates based on sequential IHC using FFPE specimens, to identify clinical correlations for patient stratification.
The three antibody panels described herein enable simultaneous evaluation of leukocyte presence, complexity and functional status in a variety of FFPE tissue contexts. In addition to HNSCC and PDAC evaluated herein, this platform has been utilized for quantitative assessment of papillary thyroid carcinomas, and longitudinal core biopsy samples of mesothelioma, breast and pancreas tumors from patients receiving various regimens of immune therapy (data not shown), as well as murine tissues/tumors (Gunderson et al., 2015; Liu et al., 2016), to thus reveal the broad applicability of the approach utilizing minimal FFPE tissue sections. Considering that FFPE sections are widely utilized for routine preparation of diagnostic pathology, this method accelerates retrospective biomarker studies in archival tissue sections as well as prospective and longitudinal assessment using core biopsy specimens to monitor response.
While current gene expression-based profiling is not capable of assessing single cell-based phenotypes or retention of tissue context information, our approach described herein circumvents these issues. One possible limitation however is a lack of direct correlation between protein expression level and signal intensity; chromogenic amplification provides nonlinear correlation with protein expression levels. In order to compensate for this issue, we adopted thresholding approaches to identify positive and negative cell populations based on qualitative gating, in which thresholds were determined by basic signal intensities in negative controls (Figures S4A–C). Based on this qualitative gating strategy, we observed that image cytometry and flow cytometry data performed similarly in quantification of lymphocytes (Figure S4E); thus, this imaging approach serves as a platform to perform multiparametric assessment of various cell lineages enabling tumor localization information. Although this method enables lineage identification based on multiple lineage-selective markers (Figure 2), as specific cell types have a great diversity with regards to lineage-restricted biomarkers, an ultimate classification of leukocyte subsets based on markers utilized herein remains limited and awaits further technologic and bioinformatic innovations.
Analysis of the archival HNSCC cohort described herein supports the notion that immune complexity of HNSCC reflects clinical outcome and tumor-molecular phenotype, including presence of viral antigens. In comparison with previous reports revealing that malignancies associated with oncogenic viruses typically induce viral antigen-specific CD8+ T cells infiltration (Gentles et al., 2015), the multiplex platform affirmed that HPV-associated HNSCCs contain significantly higher CD8+ T cell densities, and together with other TH1-associated immune infiltrates such as Tbet+ TH1 cells and CD163− TAMs (Figure 4G), indicates presence of anti-tumor immunoreactivity possibly against HPV viral antigens. On the other hand, immune cell complexity profiles of HNSCC revealed lymphoid, myeloid, and hypo-inflamed signature-based subtypes not previously identified by gene expression analyses, where myeloid-enriched TH2-biased tumors were associated with decreased overall survival (Figure 4C–4F). Notably, although there were clear tendencies of lymphoid-inflamed tumors correlating with HPV-positive tumors, a portion of HPV-positive tumors also correlated with myeloid-inflamed profiles, and these associated with poor prognosis (Figure 4C), potentially indicating association between myeloid-driven tumor characteristics and disease aggressiveness. Taken together, these observations indicate that tumor characteristics impact infiltration and phenotypes of tumor-infiltrating immune cells, simultaneously confirming the capability of this intratumoral in situ imaging approach.
Similar to findings in HNSCC, regardless of lymphoid lineage quantity in PDAC specimens, myeloid-enriched immune profiles associated with TH2-driven phenotypes and poor prognosis in response to neoadjuvant GVAX vaccination therapy (Figures 5B–5E), again indicating that myeloid enrichment negatively impacts anti-tumor immune responses as predicted by numerous murine modeling studies (DeNardo et al., 2011; Mantovani et al., 2002; Ruffell et al., 2014; Ruffell and Coussens, 2015; Ruffell et al., 2010). Quantitative evaluation of functional indicators of T cell differentiation, proliferative and effector status further supported these observations in revealing that myeloid-inflamed tumors correlated with shortened survival and were linked to CD8+ T cell exhaustion status, e.g., low Ki67 and granzyme B expression (Figures 6D–6F). Conversely, activated CD8+ T cell status correlated instead with high PD-L1 expression on myeloid cells, as well as favorable prognosis (Figures 7E and 7F), indicating importance of understanding total immune complexity and phenotype originating from both lymphoid and myeloid lineages. Together, data revealed from PDAC and HNSCC specimens through multiplex IHC and computational image processing analysis supports the tenant that immune contexture can be effectively utilized as a metric for predicting clinical outcomes and responses to therapy. Importantly, results described herein also reveals characteristics of myeloid lineages whose presence in tumors restricts induction of anti-tumor immunity, and thus highlights the therapeutic potential for select myeloid antagonists in combination with vaccine and/or checkpoint-targeted immune therapy.
EXPERIMENTAL PROCEDURES
Clinical samples and TMA construction
FFPE surgical specimens from a total of 38 patients with previously untreated oropharyngeal squamous cell carcinoma were obtained from the Oregon Health and Science University (OHSU) Knight Biolibrary, and the OHSU Department of Dermatology research repository, and were used to create a TMA. Cohort characteristics of HNSCC are shown in Table S2. A total of 24 human PDAC tumor specimens with presence of intratumoral lymphoid aggregates were obtained from our previous study wherein allogeneic GM-CSF-secreting pancreatic tumor vaccine (GVAX) was administered intradermally either alone or in combination with immune modulatory doses of cyclophophamide as neoadjuvant treatment for patients with resectable PDAC (NCT00727441, Table S3) (Lutz et al., 2014). Further details can be found in Supplemental Experimental Procedures.
Sequential IHC and image acquisition
Chromogenic sequential IHC was conducted with 5 μm of FFPE tissue sections. Following de-paraffinization, slides were stained by hematoxylin (S3301, Dako) for 1 min, followed by whole tissue scanning using Aperio ImageScope AT (Leica Biosystems). Slides were subjected to endogenous peroxidase blocking followed by heat-mediated antigen retrieval. Then, sequential IHC consisting of iterative cycles of staining, scanning, and antibody/chromogen stripping was performed according to a modified protocol based on previous reports (Glass et al., 2009; Lan et al., 1995; Tramu et al., 1978). Primary antibodies, HRP-conjugated polymer and chromogenic detection were serially added in the indicated order and condition shown in Table S1. Two forms of negative controls were used during analyses; slides for conventional negative controls were treated with 2.5% goat serum in PBS without primary antibodies; slides for sequential IHC negative controls were used for confirmation of complete antibody/signal stripping (Figure S2A). Further details can be found in Supplemental Experimental Procedures.
Image processing and analysis
The digital image workflow encompasses three steps: image preprocessing, visualization, and quantitative image analysis as shown in Supplemental Experimental Procedures. In image preprocessing, iteratively digitized images were co-registered so that cell features overlap down to a single pixel level, using a CellProfiler Version 2.1.1 pipeline, “Alignment_Batch.cppipe” available under GPLv2 at https://github.com/multiplexIHC/cppipe. Pseudocodes for algorithms used are available in Supplemental Experimental Procedures. In the PDAC surgical specimen analysis, a heat map of CD45+ cell density was utilized for selection of three rectangle ROIs within intratumoral high CD45-density area (approximately 6.25 mm2, or less if analyzable cancerous area is smaller than 3.0 × 6.25 mm2) (Figure S6F). Visualization was performed via conversion of co-registered images to pseudo-colored single-marker images in ImageJ Version 1.48 (Schneider et al., 2012) and ImageScope (Leica Biosystems). In quantitative image assessment, single cell segmentation and quantification of staining intensity was performed using a CellProfiler Version 2.1.1 pipeline, “CellID_FlowCyt - 6.9.15.cpproj” (available under GPLv2 at https://github.com/multiplexIHC/cppipe). Pseudocodes for algorithms used are available in Supplemental Experimental Procedures. All pixel intensity and shape-size measurements were saved to a file format compatible with flow and image cytometry data analysis software, FCS Express 5 Image Cytometry Version 5.01.0029 (De Novo Software). Further details can be found in Supplemental Experimental Procedures.
Flow cytometry
Flow cytometry studies using freshly resected human tissue was performed as described previously (Gunderson et al., 2015; Ruffell et al., 2012).
Statistics
Kruskal-Wallis tests were used to determine statistically significant differences in un-paired and paired data. Spearman correlation coefficient was used to assess correlations of cell percentages and densities among cell lineages. Overall survival was estimated using Kaplan-Meier methods, and differences were assessed with log-rank tests. An unsupervised hierarchical clustering was performed with Ward’s minimum variance method (“hclust” from “R”). P values were adjusted for multiple comparisons using Benjamini-Hochberg false discovery rate (FDR) adjustments. All statistical calculations were performed by R software, version 3.2.3 (www.r-project.org) and SAS software version 9.4. P < 0.05 was considered statistically significant.
Study approval
All studies involving human tissue were approved by institutional IRB (protocol #809 and #3609), and written informed consent was obtained.
Supplementary Material
Acknowledgments
The authors thank Justin Tibbitts, Teresa Beechwood, and Chase Smith for regulatory and technical assistance; and Dr. Gunderson for FACS analysis. This project was supported by Oregon Clinical and Translational Research Institute (OCTRI, #UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH), Stand Up To Cancer – Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant, and P30 CA069533-17 OHSU Knight Cancer Institute. MKM acknowledges support from the NCI (CA192405). LMC also acknowledges support from the NIH/NCI, DOD BCRP Era of Hope Scholar Expansion Award, Breast Cancer Research Foundation, Susan B. Komen Foundation, and the Brenden-Colson Center for Pancreatic Health. A patent application related to the methodology described in the present work has been filed by T.T., S.K., R.N.B., V.A., and L.M.C.
Footnotes
AUTHOR CONTRIBUTIONS
T.T., S.K. and L.M.C. conceived and designed the experiments; T.T. wrote the manuscript that was edited by L.M.C; J.W.G. reviewed the manuscript; T.T., R.K. and G.C. performed the experiments; T.T., R.N.B., V.A., G.T., Y.H.C., A.B., P.L. and A.M. developed computational algorithms and software; E.R.L., L.Z. and E.M.J. contributed to design of IHC panels and provided tissues for PDAC studies; M.M. supported statistical data analysis; D.S., M.K.M., E.E., D.R.C. and P.W.F. provided tissues for human HNSCC analyses; L.M.C. supervised the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass G, Papin JA, Mandell JW. SIMPLE: a sequential immunoperoxidase labeling and erasing method. J Histochem Cytochem. 2009;57:899–905. doi: 10.1369/jhc.2009.953612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, et al. Bruton’s Tyrosine Kinase (BTK)-dependent immune cell crosstalk drives pancreas cancer. Cancer Discov. 2015;6:270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck MK, Zuo Z, Khattri A, Stricker TP, Brown C, Imanguli M, Rieke D, Endhardt K, Fang P, Bragelmann J. Integrative analysis of Head and Neck Cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-2481. clincanres. 2481.2014. [DOI] [PubMed] [Google Scholar]
- Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem. 1995;43:97–102. doi: 10.1177/43.1.7822770. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Z, De La Torre R, Barling A, Tsujikawa T, Hornick N, Hanifin J, Simpson E, Wang Y, Swanzey E, et al. Trim32 deficiency enhances Th2 immunity and predisposes to features of atopic dermatitis. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.09.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A. Immunotherapy Converts Nonimmunogenic Pancreatic Tumors into Immunogenic Foci of Immune Regulation. Cancer Immunology Research. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K, Eddy WF, Crowley JC. A novel algorithm for optimal image thresholding of biological data. J Neurosci Methods. 2010;193:380–384. doi: 10.1016/j.jneumeth.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Coussens LM. Macrophages and Therapeutic Resistance in Cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruifrok AC, Katz RL, Johnston DA. Comparison of quantification of histochemical staining by hue-saturation-intensity (HSI) transformation and color-deconvolution. Appl Immunohistochem Mol Morphol. 2003;11:85–91. doi: 10.1097/00129039-200303000-00014. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T cell infiltration into pancreatic tumors. Journal of immunotherapy (Hagerstown, Md: 1997) 2015;38:1. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow JK, Murillo CLP, Hunter KD, Buffa FM, Patiar S, Betts G, West CM, Harris AL, Parkinson EK, Harrison PR. Spectral clustering of microarray data elucidates the roles of microenvironment remodeling and immune responses in survival of head and neck squamous cell carcinoma. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.24.8724. JCO. 2009.2024. 8724. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramu G, Pillez A, Leonardelli J. An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem. 1978;26:322–324. doi: 10.1177/26.4.207771. [DOI] [PubMed] [Google Scholar]
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wählby C, SINTORN IM, Erlandsson F, Borgefors G, Bengtsson E. Combining intensity, edge and shape information for 2D and 3D segmentation of cell nuclei in tissue sections. J Microsc. 2004;215:67–76. doi: 10.1111/j.0022-2720.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature Reviews Immunology. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.