Abstract
Purpose
Obesity is a risk factor for incident prostate cancer (PC) as well as risk of disease progression and mortality. We hypothesized that men diagnosed with lower-risk PC and who elected active surveillance (AS) for their cancer management would likely initiate lifestyle changes that lead to weight loss.
Methods and Patients
Patients were enrolled in the Prostate Active Surveillance Study (PASS), a multicenter prospective biomarker discovery and validation study of men who have chosen AS for their prostate cancer. Data from 442 men diagnosed with PC within 1 year of study entry who completed a standard of care 12-month follow-up visit were analyzed. We examined the change in weight and body mass index (BMI) over the first year of study participation.
Results
After one year on AS, 7.5% (33/442) of patients had lost 5% or more of their on-study weight. The proportion of men who lost 5% or more weight was similar across categories of baseline BMI: normal/underweight (8%), overweight (6%) and obese (10%, Chi-Square test p=0.44). The results were similar for patients enrolled in the study one year or six months after diagnosis. By contrast, after one year, 7.7% (34/442) of patients had gained more than 5% of their weight.
Conclusion
Only 7.5% of men with low-risk PC enrolled in AS lost a modest (≥5%) amount of weight after diagnosis. Given that obesity is related to PC progression and mortality, targeted lifestyle interventions may be effective at this ‘teachable moment’, as men begin AS for low-risk PC.
Introduction
Obesity has been associated with increased risk of prostate cancer (PC) progression among patients managed with active surveillance (AS).1–6 Evidence from animal models suggests that weight loss may improve PC outcomes; however, this has not yet been demonstrated in human clinical trials.7,8 In addition to a potential reduction in PC progression as well as other morbidities with weight loss, weight reduction may reduce the risk of treatment-related complications (e.g., urinary incontinence) in those men who ultimately require treatment in subsequent years due to disease progression.3,9,10
Many patients demonstrate remarkable lifestyle modifications after a diagnosis of low-risk prostate cancer.11,12 These lifestyle changes include improved eating habits, increased daily physical activity, and weight loss. Few data exist on the effectiveness of these self-imposed lifestyle modifications that are done outside of formal programs. Population-based data suggest limited lifestyle modifications after diagnosis and potentially a reduction in exercise.13,14
A diagnosis of cancer is thought to represent a teachable moment for patients, at which time patients would be more likely to adopt healthy lifestyle changes resulting in weight loss. If these types of lifestyle changes are implemented, there may be an impact on cancer progression as well as a reduced risk of cardiovascular complications in the future.15 We sought to investigate whether a new cancer diagnosis without specific discussion of lifestyle changes led changes in body mass index utilizing the Canary Prostate Active Surveillance Study (PASS), a large, multi-institutional, prospective study of men with localized prostate cancer on active surveillance.
Methods
PASS Study Population
Data are from the Canary Prostate Cancer Active Surveillance Study (PASS), a multicenter, prospective study (clinicaltrials.gov NCT00756665) of men who elected AS for PC management.16,17 All men provided written informed consent prior to participation, and study procedures were approved by the local institutional review board for each study site. Men being seen in urology clinics were eligible for PASS if they had clinical stage T1-2 localized prostate cancer, no previous cancer treatment, no history of other malignancies (except non-melanoma skin cancer), an Eastern Cooperative Oncology Group (ECOG) status of 0 or 1, and were willing to participate in a structured AS protocol including quarterly PSA measures, semi-annual digital rectal examinations, and periodic surveillance prostate biopsies. There were no lifestyle or behavioral components as part of the protocol. The primary outcomes of PASS are the discovery and validation of biomarkers that predict outcomes to improve management of individual patients with prostate cancer.
Data Collection, Definitions, and Outcomes
At baseline, participants reported information on race/ethnicity, smoking status (never/former/current), alcohol use in the past year (one or more drinks per month), and family history of PC; clinic staff measured height and weight. Body mass index was calculated as weight (kg) divided by height (m2) and was categorized as normal (BMI < 25), overweight (25 ≤ BMI < 30), and obese (BMI ≥ 30).18 The primary outcome of this analysis was percentage weight change, calculated as the difference in weight between baseline and year 1 visits divided by weight at baseline visit.
Statistical Analyses
Weight loss of 5% or more, a level endorsed by the U.S. Centers for Disease Control and Prevention for improving cardiovascular health (http://www.cdc.gov/healthyweight/losing_weight/index.html), was of particular interest.18 Weight maintenance was defined as change in weight of ≤+/− 3% relative to baseline. Minimal weight change was defined as +/−3.1%–4.9%, and modest weight change was defined as ≥+/− 5%, an amount considered clinically meaningful. Weight change from baseline was compared across BMI categories using Fisher’s exact tests. Multiple linear regression models were used to evaluate the association of percentage weight change with demographic characteristics, including age (<55 (reference), 55–64 and 65+ years), race (Caucasian (reference), African-American, Other), BMI (normal (reference), overweight, obese), smoking status (current/former vs. never (reference), alcohol intake (<1 drink/month (reference) vs. 1+ drink/month) and family history of PC (no (reference) vs. yes). Study site was considered as a random effect in linear mixed models, but was found to neither improve model fit nor markedly change the fixed effect estimates and therefore was not included in final models. Since the potential for weight change might be greatest among men with newly diagnosed PC, data and analyses are presented separately for the subset of men who enrolled in PASS within 12 months or within 6 months of their PC diagnosis date. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina). All statistical hypotheses were 2 sided, and p < 0.05 was considered statistically significant.
Results
Between September, 2008 and February, 2015, a total of 745 patients enrolled in PASS and had completed a 12-month follow-up visit. Of these men, 466 enrolled in PASS within one year, and 258 within 6 months, of PC diagnosis. Men enrolled later than 12-months from diagnosis or had missing data missing for any of the examined covariates were excluded, leaving 442 men for these analyses, 242 of whom were enrolled within 6 months of diagnosis. Demographic and health-related characteristics of study patients are displayed in Table 1. For the sample of patients enrolled within one year of cancer diagnosis, the mean age was 63 (SD=7), mean weight was 194 pounds (SD=32), and mean height was 70 inches (SD=3). Twenty-three percent (n=100) were normal, 52% (n=231) were overweight, and 25% (n=111) were obese. Overall, 7.5% (n=33) lost more 5% or more of their body weight after 1 year. The distribution of demographic characteristics among the subset of patients enrolled within 6 months of their diagnosis was similar to the overall study sample. While men with a BMI < 25 lost on average 0.1 pounds (SD=4.0), overweight and obese men gained 0.4 (SD=3.7) and 0.1 (SD=4.3) pounds, respectively, over the year after diagnosis.
Table 1. Demographics of the study cohort.
Results are shown for all participants diagnosed with prostate cancer within 1 year before study enrollment and a subset of those diagnosed within 6 months before enrollment.
| Demographics | Diagnosed within 1 year of enrollment N(%) or mean (SD) |
Diagnosed within 6 months of enrollment N(%) or mean (SD) |
|---|---|---|
| N=442 | N=242 | |
|
| ||
| Age (years) | 63 (7) | 634 (7) |
|
| ||
| < 55 | 57 (13) | 22 (9) |
| 55 ≤ Age < 65 | 182 (41) | 106 (44) |
| ≥ 65 | 203 (46) | 114 (47) |
|
| ||
| Race | ||
|
| ||
| Caucasian American | 384 (87) | 206 (85) |
| Hispanic/Latino | 20 (5) | 12 (5) |
| African American | 19 (4) | 10 (4) |
| Asian | 12 (3) | 9 (4) |
| Other | 7 (1) | 5 (2) |
|
| ||
| Weight, baseline (pounds) | 194 (32) | 195 (32) |
|
| ||
| Height, baseline (inches) | 70 (3) | 70 (3) |
|
| ||
| Body Mass Index (kg/m2) | ||
|
| ||
| <25 | 100 (23) | 53 (22) |
| 25 ≤ BMI < 30 | 231 (52) | 127 (52) |
| ≥ 30 | 111 (25) | 62 (26) |
|
| ||
| Lost 5% Body Weight | 33 (7) | 22 (9) |
|
| ||
| Cigarette Use | ||
|
| ||
| Current | 17 (4) | 7 (3) |
| Former | 172 (39) | 101 (42) |
| Never | 253 (57) | 134 (55) |
|
| ||
| Alcohol Use (in past 12 mo.) | ||
|
| ||
| Yes | 370 (84) | 198 (82) |
| No | 72 (16) | 44 (18) |
|
| ||
| Family History of PCa | ||
|
| ||
| Yes | 111 (25) | 65 (27) |
| No | 331 (75) | 177 (73) |
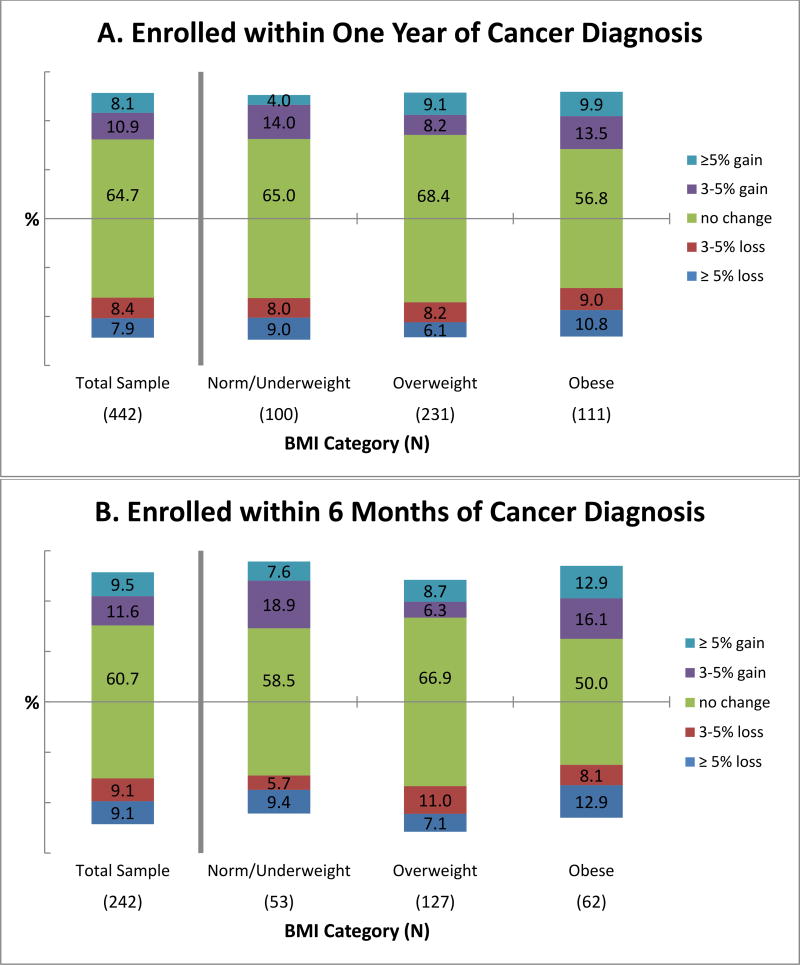
Figure 1 shows the proportion of patients, for both the overall study sample and by BMI group, who lost, maintained, or gained weight during the first year of study enrollment after PC diagnosis. In the overall sample of patients enrolled within one year of cancer diagnosis (Figure 1A) and the subset of patients enrolled within 6 months of cancer diagnosis (Figure 1B), the majority of men maintained their enrollment weight (65% and 61%changed weight by ±3% or less, respectively). Among the overall sample of patients enrolled within 12 months of PC diagnosis, similar proportions lost or gained more than 5% of their enrollment weight during the first year of study (7.5% and 7.7%, respectively; Figure 1A). A greater proportion of obese men had a weight loss of ≥5% compared to overweight or normal/underweight men (9.9% versus 6.1% and 8.0%, respectively), and a similar pattern was seen for weight gain of ≥5% (9.9% versus 8.2% and 4.0%, respectively), although the differences in weight gain ≥5% across baseline BMI categories was not statistically significant (Fisher’s exact test p-value=0.24).
Figure 1.
Change in weight during first year of enrolment in an active surveillance protocol. Results are shown for the total cohort and by BMI category, both for all participants enrolled in the study within one year of cancer diagnosis (A) and for those enrolled within 6 months of cancer diagnosis (B).
Among the subset of 242 patients who enrolled within 6 months of PC diagnosis, a slightly greater proportion of men lost or gained ≥5% of enrollment weight, compared to men who enrolled within 12 months of PC diagnosis (9.1% vs. 7.5% with modest loss and 9.1% vs. 7.7% with modest gain, respectively). In addition, the proportions of obese, overweight and normal/underweight men who lost ≥5% weight were slightly greater among the subset of patients enrolled within 6 vs. 12 months of diagnosis (12.9% vs. 9.9% for obese, 7.1% vs. 6.1% for overweight and 9.4% vs. 8.0% for normal weight, respectively).
Among the 442 patients who enrolled within 1 year of diagnosis, none of the baseline variables significantly predicted weight change (Table 2). In the subset of patients who enrolled within 6 months of diagnosis, men who had a family history of PC or did not regularly consume alcohol tended to lose more weight than men who did not have a family history of PC or were regular drinkers although these differences were neither clinically nor statistically significant (Table 2).
Table 2. Association between weight change and patient characteristics.
Weight change is recorded as Least Square (LS) means and standard errors (SE) from linear regression model.
| Baseline Demographics |
Diagnosed within 1 Year of enrollment (N = 442) |
Diagnosed within 6 Months of enrollment (N = 242) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Baseline Weight (lb) |
Weight change (lb) from baseline to year 1 |
P-value* | Mean Baseline Weight (lb) |
Weight change (lb) from baseline to year 1 |
P-value* | |
| Age | ||||||
|
| ||||||
| < 55 | 194 | 0.26 (0.63) | 190 | −0.19 (1.02) | ||
| 55 ≤ Age < 65 | 200 | 0.26 (0.48) | 0.59 | 200 | −0.53 (0.72) | 0.94 |
| ≥ 65 | 189 | −0.13 (0.46) | 191 | −0.53 (0.67) | ||
|
| ||||||
| Race | ||||||
|
| ||||||
| African American | 197 | 0.62 (0.91) | 202 | −0.37 (1.39) | ||
| Caucasian | 194 | −0.19 (0.31) | 0.67 | 195 | −0.32 (0.47) | 0.97 |
| Other | 192 | −0.04 (0.67) | 194 | −0.55 (0.92) | ||
|
| ||||||
| Body Mass Index | ||||||
|
| ||||||
| Under / Normal | 162 | −0.40 (0.61) | 162 | −0.90 (0.90) | ||
| Overweight | 190 | 0.33 (0.49) | 0.40 | 191 | −0.75 (0.74) | 0.41 |
| Obese | 232 | 0.46 (0.61) | 232 | 0.41 (0.90) | ||
|
| ||||||
| Smoking Status | ||||||
|
| ||||||
| Current / Former | 197 | 0.29 (0.48) | 0.40 | 199 | −0.08 (0.73) | 0.24 |
| Never | 192 | −0.04 (0.45) | 192 | −0.74 (0.65) | ||
|
| ||||||
| Alcohol intake (prior year) | ||||||
|
| ||||||
| 1+ drink per month | 196 | 0.47 (0.40) | 0.18 | 197 | 0.21 (0.60) | 0.09 |
| <1 per month | 188 | −0.21 (0.57) | 186 | −1.03 (0.84) | ||
|
| ||||||
| Family history of PCa | ||||||
|
| ||||||
| Yes | 198 | −0.07 (0.50) | 0.36 | 198 | −0.88 (0.76) | 0.13 |
| No | 193 | 0.33 (0.45) | 194 | 0.05 (0.65) | ||
P-value from Type 3 Test, which tests the significance of each variable in the model, given all other variables in the model. Regression models are adjusted for baseline weight.
Discussion
In a sample of men with newly diagnosed, early stage PC enrolled in an AS study, of whom three-quarters were overweight or obese, we found little evidence to suggest that men made lifestyle changes that resulted in weight loss during the one-year period following enrollment in an AS study. The majority of men (286/442, 65%) maintained their enrollment weight (≤±3%), and only 7.5% of men lost 5% weight or more during the first year of AS. As troublesome, but perhaps not surprising, was the observation that a nearly equivalent number of men gained 5% or more from their enrollment weight (34/442, 7.7%) over the ensuing year.
These findings are consistent with those recently noted in a subset (n=511) of the Prostate testing for cancer and Treatment (PROTECT) trial in the United Kingdom in which no change in BMI after a diagnosis of PC was found.19 No change in BMI was noted in this cohort despite approximately one-third of men modifying their behavior after their cancer diagnosis by reducing alcohol intake, quitting smoking, or increasing physical activity without deliberate intervention.12,19 Men in the Prostate Cancer Prevention Trial (PCPT) overall only gained 1–2 1bs on average over the 7 years; however, the most at risk group for weight gain was men with low-grade cancer (71% higher annual weight gain than men with no cancer, p=0.002).20 This indicates men with a low grade prostate cancer diagnosis may actually be at risk for weight gain. Preliminary evidence suggests that small lifestyle modifications may make a difference in PC outcomes. For instance, in the Health Professionals Follow-up Study, a modest amount of vigorous activity for ≥ 3 hours a week was associated with lower overall and PC-specific mortality.21
A diagnosis of PC, even when ‘low-risk’, can be a psychologically-traumatizing event.22 We sought to determine whether men make lifestyle changes that would result in weight loss. With the growing evidence that cancer-specific survival for men with low-risk PC on AS is remarkably high (98% or more), we further wondered if behavioral change and weight loss could contribute to these outcomes.3,23
The majority of men in this large prospective PC AS study had either not changed, or increased, their weight within one year after diagnosis. This striking observation supports the opportunity for a ‘teachable moment’ at the time of PC. Certainly, the simplest opportunity occurs if the patient decides to pursue a strategy of AS in which no other elements of his life change. However, weight loss and dietary changes surrounding other prostate cancer therapies may also be beneficial.24
There is no single best method to achieve weight loss; however, elements that are important for clinical success mentioned by the National Heart, Lung, and Blood Institute (NIH) include setting goals that are specific, doable, and forgiving.25 They give an example of a goal that meets these requirements, such as “walk 30 minutes, 5 days each week” (https://www.nhlbi.nih.gov/health/educational/lose_wt/behavior.htm). This pointed information at the time of low-grade cancer diagnosis could be helpful to evoke behavioral change.
Men on AS for PC have a very low disease-specific mortality rate, and instead have a higher risk of death from competing causes. As a result, this moment is when the clinician can explain to the patient that lifestyle changes that reduce the risk of other causes of death (e.g., myocardial infarction, stroke, diabetes) would have a far-greater impact on health than interventions related to PC.26,27, 25, 13 This study and the PROTECT trial show that overweight or obese men on AS are unlikely to modify their lifestyle and lose weight. Recently, the American Society of Clinical Oncology has established an initiative, which includes providing tools and resources for providers and patients regarding obesity including access to weight loss programs for cancer survivors.28 Formal programs to provide support should be a component of follow-up plans for men on AS.
Only 7.5% of men embarking in a program of active surveillance for low-risk prostate cancer had a modest amount of weight loss of at least 5% reduction from baseline weight. Physician facilitated multi-disciplinary weight loss programs for all patients on surveillance for prostate cancer should be further investigated.
Acknowledgments
Funding: PASS is funded by Canary Foundation and coordinated by National Cancer Institute’s Early Detection Research Network (EDRN) (grant number U01 CA086402). Additionally, Dr. Liss is supported through the DOD Prostate Cancer Research Program (PCRP) Physician Research Training Award. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under Award No. W81XWH-15-1-0441. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Footnotes
Previously Presented: None
Conflict of interest
The authors declare no competing financial interests in relation to the work described in this manuscript.
References
- 1.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. The New England journal of medicine. 2011;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 2.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. The New England journal of medicine. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 4.Bhindi B, Kulkarni GS, Finelli A, Alibhai SM, Hamilton RJ, Toi A, et al. Obesity Is Associated with Risk of Progression for Low-risk Prostate Cancers Managed Expectantly. European urology. 2014 doi: 10.1016/j.eururo.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. European urology. 2013;63(5):800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grambsch PL, Grande JP, et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutrition and cancer. 2009;61(2):265–275. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 8.Blando J, Moore T, Hursting S, Jiang G, Saha A, Beltran L, et al. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer prevention research. 2011;4(12):2002–2014. doi: 10.1158/1940-6207.CAPR-11-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 10.Moyer VA Force USPST. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;157(5):373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 11.Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(8):1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery KN, Donovan JL, Gilbert R, Davis M, Emmett P, Down L, et al. Men with prostate cancer make positive dietary changes following diagnosis and treatment. Cancer causes & control : CCC. 2013;24(6):1119–1128. doi: 10.1007/s10552-013-0189-x. [DOI] [PubMed] [Google Scholar]
- 13.Williams K, Steptoe A, Wardle J. Is a cancer diagnosis a trigger for health behaviour change? Findings from a prospective, population-based study. British journal of cancer. 2013;108(11):2407–2412. doi: 10.1038/bjc.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newsom JT, Huguet N, McCarthy MJ, Ramage-Morin P, Kaplan MS, Bernier J, et al. Health behavior change following chronic illness in middle and later life. The journals of gerontology Series B, Psychological sciences and social sciences. 2012;67(3):279–288. doi: 10.1093/geronb/gbr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA: the journal of the American Medical Association. 1998;280(23):2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 16.Newcomb LF, Brooks JD, Carroll PR, Feng Z, Gleave ME, Nelson PS, et al. Canary Prostate Active Surveillance Study: design of a multi-institutional active surveillance cohort and biorepository. Urology. 2010;75(2):407–413. doi: 10.1016/j.urology.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newcomb LF, Thompson IM, Jr, Boyer HD, Brooks JD, Carroll PR, Cooperberg MR, et al. Outcomes of active surveillance for the management of clinically localized prostate cancer in the prospective, multi-institutional Canary PASS cohort. The Journal of urology. 2015 doi: 10.1016/j.juro.2015.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn G. Effect of degree of weight loss on health benefits. Obesity research. 1995;3(Suppl 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 19.Hackshaw-McGeagh LE, Penfold CM, Walsh E, Donovan JL, Hamdy FC, Neal DE, et al. Physical activity, alcohol consumption, BMI and smoking status before and after prostate cancer diagnosis in the ProtecT trial: Opportunities for lifestyle modification. International journal of cancer Journal international du cancer. 2015 doi: 10.1002/ijc.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Tangen C, Goodman P, Parnes HL, Lucia MS, Thompson IM, et al. Finasteride, prostate cancer, and weight gain: evidence for genetic or environmental factors that affect cancer outcomes during finasteride treatment. The Prostate. 2008;68(3):281–286. doi: 10.1002/pros.20637. [DOI] [PubMed] [Google Scholar]
- 21.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(6):726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell K. Remaking the self: trauma, teachable moments, and the biopolitics of cancer survivorship. Culture, medicine and psychiatry. 2012;36(4):584–600. doi: 10.1007/s11013-012-9276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(16):2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 24.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer prevention research. 2011;4(12):2062–2071. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and science in sports and exercise. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 26.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA : the journal of the American Medical Association. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 27.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA : the journal of the American Medical Association. 2004;291(22):2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 28.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(31):3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]