Abstract
Characteristics of cancer cells include a more oxidized redox environment, metabolic reprogramming, and apoptosis resistance. Our studies with a lymphoma model have explored connections between the cellular redox environment and cancer cell phenotypes. Alterations seen in lymphoma cells made resistant to oxidative stress include: a more oxidized redox environment despite increased expression of antioxidant enzymes, enhanced net tumor growth, metabolic changes involving the mitochondria, and resistance to the mitochondrial pathway to apoptosis. Of particular importance, the cells show cross-resistance to multiple chemotherapeutic agents used to treat aggressive lymphomas. Analyses of clinical and tumor data reveal the worst prognosis when patients’ lymphomas have gene expression patterns consistent with the most oxidized redox environment. Lymphomas from patients with the worst survival outcomes express increased levels of proteins involved in oxidative phosphorylation, including cytochrome c. This is consistent with these cells functioning as metabolic opportunists. Using lymphoma cell models and primary lymphoma cultures, we observed enhanced killing using genetic and drug approaches which further oxidize the cellular redox environment. These approaches include increased expression of SOD2, treatment with a manganoporphyrin that oxidizes the glutathione redox couple, or treatment with a copper chelator that inhibits SOD1 and leads to peroxynitrite-dependent cell death. The latter approach effectively kills lymphoma cells that overexpress the anti-apoptotic protein BCL-2. Given the central role of mitochondria in redox homeostasis, metabolism and the intrinsic pathway to apoptosis, our studies support the development of new anti-cancer drugs to target this organelle.
Keywords: apoptosis, chemotherapy, lymphoma, metabolism, mitochondria, redox homeostasis
Redox Signaling in Cancer
Cancer cells frequently function with a more oxidized redox environment [5]. An expanded set of cancer hallmarks was proposed by Luo et al. in 2009 and includes oxidative stress [24]. Indications of altered cellular redox homeostasis in lymphomas compared to normal lymphoid tissue include differences in expression levels of antioxidant enzymes [2;41] and increased markers of oxidative damage [30]. These associations raise the important question of causality: do reactive oxygen species (ROS) drive lymphomagenesis or are they simply the consequence of oncogene activation and metabolic activity in highly proliferative cells? One argument in favor of ROS as drivers is that chronic inflammation increases the risk of cancer developing in the affected tissue. Examples of hematologic malignancies arising in this setting include aggressive lymphomas that develop in patients with rheumatoid arthritis or Sjögren syndrome [36]. The mechanism underlying the connection between chronic inflammation and cancer is an active area of investigation. It appears to be multi-faceted, involving redox signaling and oxidative damage to DNA. Inflammatory cells produce ROS and reactive nitrogen species (RNS) that can activate redox-sensitive signaling proteins [35]. These species can also cause DNA mutations and genomic instability [13].
Mitochondria have a central role in the cancer cell phenotypes of altered redox homeostasis, metabolic rewiring and apoptosis resistance (Fig. 1). Metabolism in cancer cells resembles proliferative tissues, which rely on aerobic glycolysis to a greater extent than do differentiated tissues, which are quiescent [43]. This was first reported in the 1920s by Otto Warburg, who noted that cancer cells take up glucose more avidly than their normal counterparts [44]. He postulated that they rely on aerobic glycolysis due to impaired oxidative phosphorylation. Metabolic profiling with modern tools has confirmed that aerobic glycolysis explains the increased demand for glucose seen in cancer cells [33;43]. Aerobic respiration is not impaired, but substrates for mitochondrial metabolism are provided through increased oxidation of fatty acids and glutaminolysis, in addition to the pyruvate supplied by glycolysis. Glutaminolysis converts glutamine to α-ketoglutarate that feeds into the tricarboxylic acid (TCA) cycle in mitochondria. This metabolic reprogramming provides the cancer cells with precursors needed for the synthesis of nucleic acids, lipids, and proteins, along with substrates for energy production [11]. An additional benefit of cancer metabolism is high levels of NADPH for redox homeostasis [47].
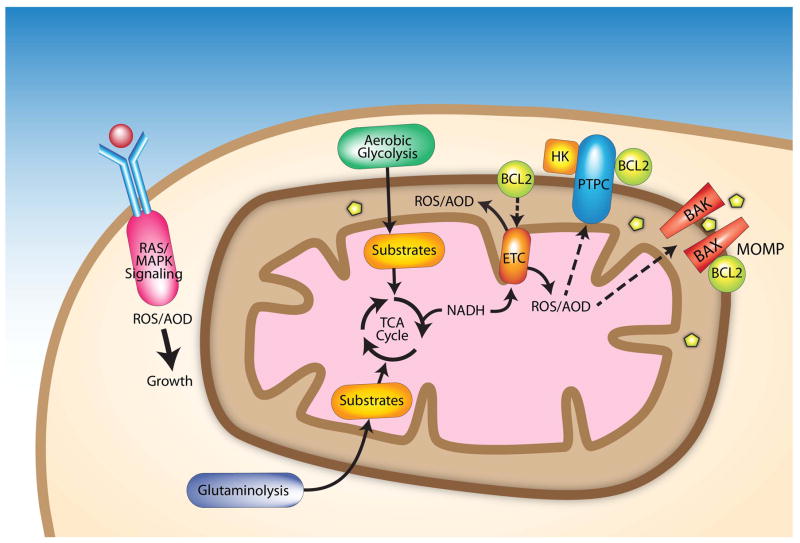
Figure 1.
Mitochondria are central to redox homeostasis, metabolism and apoptosis resistance in cancer cells. Factors contributing to a more oxidized redox environment in cancer cells include altered mitochondrial metabolism, changes in antioxidant defenses and increased oncogenic signaling. Signaling through growth factor receptor-mediated pathways leads to ROS generation by NADPH oxidases downstream of RAS; aberrant signaling due to RAS mutations or other oncogenes can elevate ROS levels. Aerobic glycolysis and glutaminolysis supply substrates for the TCA in mitochondria, which in turn generates substrates for the ETC, a major source of ROS. Mutations that alter ETC subunits can result in increased ROS. The anti-apoptotic protein BCL-2 appears to increase ROS levels by regulating ETC subunits. Mitochondrial-generated ROS regulate apoptosis by interacting with the permeability transition pore complex (PTPC) or pro-apoptotic proteins (e.g., BAX and BAK) that control mitochondrial outer membrane permeability (MOMP). Increased MOMP allows pro-apoptotic proteins including cytochrome c and apoptosis inducing factors to move from the intermembrane space to the cytosol. BCL-2 can inhibit apoptosis by regulating the PTPC and MOMP. Binding of hexokinase II (HK) to the PTPC inhibits apoptosis.
Resistance to chemotherapeutic drugs can often be traced to mitochondria as many of these agents work through the intrinsic pathway to apoptosis. A classic example is overexpression of BCL2, which was the first oncogene discovered to function by inhibiting cell death [20]. At the mitochondria, BCL-2 maintains the integrity of the outer mitochondrial membrane by interacting with pro-apoptotic BCL-2 family members [8]. This prevents the formation of pores through which several mitochondrial proteins, including cytochrome c, enter the cytoplasm. These proteins commit the cell to death. BCL-2 also functions in an indirect manner to prevent oxidative stress-induced apoptosis. While the molecular details have yet to be elucidated, the evidence points to BCL-2 regulating mitochondrial metabolism, with the result being an increase in ROS levels [21;23]. The cell responds by augmenting cellular defenses against ROS. This affords protection against further oxidative stress that would otherwise be lethal.
Manipulating Redox Homeostasis Results in More Aggressive Cancer Phenotypes
Our series of studies with a lymphoma model has investigated connections between the cellular redox environment and more aggressive cancer phenotypes. Oxidative stress-resistant variants of the WEHI7.2 murine lymphoma cell line were established through stable transfection with a catalase expression vector [38] or gradual selection for growth in the presence of 200 μM H2O2 [39]. The variants are designated as CAT2, CAT38, and 200R cells. All three have significantly higher catalase and superoxide dismutase activity than WEHI7.2 cells [40]. Despite this, they are in a more oxidized state, as shown by evaluation of the GSH/GSSG redox couple using the Nernst equation [40]. In contrast to measuring total GSH or the GSH/GSSG ratio, the Nernst equation gives a more accurate indication of the cellular redox environment by taking into account that two molecules of GSH are consumed when one molecule of GSSG is made [19;34]. As a further indication of the more oxidized redox environment in the variants, the expression of glutathione S-transferases and NAD(P)H:quinone oxidoreductase is increased compared to WEHI7.2 cells [40]. These phase II enzymes are up-regulated under conditions of increased oxidative stress.
Concurrent with a more oxidized redox environment, the WEHI7.2 variants acquire phenotypes of more malignant cells. When CAT38 cells are grown as xenografts in immunodeficient mice, they develop into significantly larger tumors compared to xenografts of control WEHI7.2 cells stably transfected with the neor vector [38]. The mitotic rate is not increased in the CAT38 cell xenografts, suggesting that the difference in net growth is caused by a lower rate of apoptosis. Increased resistance to cell death, as compared to WEHI7.2 cells, is evident when all three variants are exposed to agents used clinically to treat lymphoma: cyclophosphamide, doxorubicin, vincristine, and the synthetic glucocorticoid, dexamethasone [40]. Metabolic profiling of CAT38 cells indicates that these cells maintain mitochondrial production of ATP at a higher rate than WEHI7.2 cells following exposure to dexamethasone [42]. This likely contributes to their apoptosis resistance. Another potential contributor to this phenotype is increased hexokinase activity, along with a higher hexose/triose phosphate ratio, as seen in untreated CAT38 cells compared to WEHI7.2 cells [42]. Hexokinase regulates apoptosis through interactions with BCL-2 family members and the mitochondrial permeability transition pore complex [9;12;25]. A further metabolic difference between the CAT38 and WEHI7.2 cells is a pattern consistent with CAT38 cells using glutaminolysis to a greater extent than WEHI7.2 cells: a decrease in glutamine and glutamate coupled with an increase in aspartate when CAT38 cells are given fresh media, as compared to no or minimal change in these amino acids for WEHI7.2 cells under the same conditions [42].
Although the precise molecular mechanism for the increased resistance of the WEHI7.2 variants to chemotherapeutic agents is not clear, it involves mitochondria. A resistance mechanism that is intrinsic to mitochondria is revealed through cell-free assays testing for release of cytochrome c when mitochondria are isolated from the cells and incubated with the pro-apoptotic BCL-2 family member protein tBid [46]. Cytochrome c is released from WEHI7.2 mitochondria with 250 nM tBid, whereas higher concentrations are required for release from 200R or CAT38 (500 nM) and CAT2 (1,000 nM) mitochondria. This pattern mirrors the sensitivity of the intact cells to glucocorticoid-induced apoptosis; 200R or CAT38 cells show intermediate resistance, and CAT2 cells are the most resistant compared to WEHI7.2 cells [38;39]. Cell death induced by glucocorticoids involves the intrinsic pathway to apoptosis. Differences in levels of pro- or anti-apoptotic BCL-2 family members do not explain the resistance of the variant cells’ mitochondria to the intrinsic pathway to apoptosis [46]. Increased cytochrome c levels are present in mitochondria from the variants [45] and this may play a role.
Redox Homeostasis and Cancer Hallmarks in Lymphoma Patients
Lymphomas make up a heterogeneous group of neoplasms with subtypes arising from different stages of B and T cell development [15]. The majority of lymphomas are B cell-derived and the most common indolent and aggressive forms of these are follicular lymphoma and diffuse large B-cell lymphoma (DLBCL), respectively. DLBCL is noted for a variable response to treatment. The standard-of-care therapy cures approximately half of the patients; the remainder die relatively quickly of treatment-refractory disease present at the time of diagnosis or upon relapse. The variable outcomes seen for DLBCL patients suggest underlying biological differences between tumors that are not apparent from the morphological features. DLBCL was one of the first malignancies for which such differences were explored using gene expression profiling. The studies compared expression patterns in diagnostic tumor specimens to clinical characteristics (e.g., patient age and tumor stage) that are correlated with survival time following treatment. These studies reveal molecular signatures associated with survival outcomes that are based on the expression of genes involved in tumor proliferation, state of differentiation, and recognition by the immune system [1;32].
We have tested whether gene expression patterns in DLBCL support a connection between redox homeostasis and resistance to multi-agent therapy. From the set of genes included in the published DLBCL gene expression profiling data set [1;32], we chose genes encoding antioxidant defense enzymes, proteins involved in the thioredoxin system and genes that are upregulated in response to oxidative stress. Comparing the expression levels in the diagnostic tissue specimens to patients’ predicted survival time, the worst prognosis is associated with decreased expression of antioxidant enzymes (superoxide dismutases, SOD2, SOD3, and catalase) and increased activity of the thioredoxin system [41]. In the WEHI7.2 lymphoma cell model, the variants have increased catalase and SOD, but are resistant to multiple drugs used clinically to treat lymphoma [40]. The redox environment in those cells is more oxidized than in WEHI7.2 cells. Increased activity of the thioredoxin system in DLBCLs from patients with the worst prognosis is indicative of a more oxidized redox environment. Thus, chemoresistance appears to track with the overall balance of antioxidant defenses versus ROS, as indicated by the redox environment, rather than with the expression levels of antioxidant enzymes. In support of this, a study of 106 DLBCL patients used immunohistochemistry to show increased levels of thioredoxin, nitrotyrosine and 8-hydroxydeoxyguanosine in tumors from patients with worse survival outcomes [30]. Nitrotyrosine and 8-hydroxydeoxyguanosine are products of oxidative damage to protein and DNA, respectively.
To extend our analyses of the DLBCL gene expression data, we used a systems biology approach to evaluate the relationship between the redox environment and treatment outcome. The approach combines expression levels of the different proteins involved in redox homeostasis and takes into account that expression of some genes increases, while others decrease, in association with aggressive cancer phenotypes (Fig. 2). A mathematically-derived redox score is calculated for each patient; low redox scores reflect a more oxidized redox environment and increased thioredoxin system function [41]. The 5-year survival percentages for the quartiles of DLBCL patients with the lowest and highest redox scores are 37% and 57%, respectively (P < .001) [41]. When the patients are first separated into the subtypes previously shown to predict survival outcome based on the differentiation state of the tumor, patients with low redox scores still have significantly shorter 5-year survival than patients with higher scores. Thus, the redox score adds information to previously reported predictors of response to treatment in DLBCL.
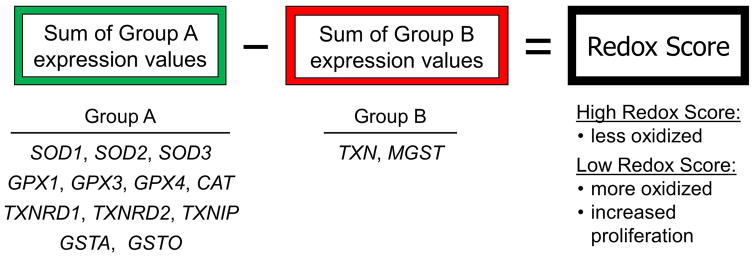
Figure 2.
A systems biology approach to assess the redox environment in tumors based on gene expression. A redox score is calculated for each tumor by summing the expression values of the genes in group A and subtracting the expression values of the genes in group B. Based on the known functions of these genes, a high redox score reflects a less oxidized tumor while a low redox score is indicative of a more oxidized tumor and higher proliferation rate. Gene names: SOD, superoxide dismutase; GPX, glutathione peroxidase; CAT, catalase; TXNRD, thioredoxin reductase; TXNIP, thioredoxin interacting protein; GSTA, glutathione S-transferase alpha; GSTO, glutathione S-transferase omega; TXN, thioredoxin; MGST, microsomal glutathione S-transferase.
Treatment-Refractory Lymphomas are Metabolic Opportunists
Alterations in mitochondrial metabolism may contribute to treatment-refractory disease in lymphoma patients. Monti et al. [27] have reported an “oxidative-phosphorylation (OxPhos)” gene expression signature in DLBCL that predicts response to therapy. The genes included in this signature are involved in oxidative phosphorylation and apoptosis. Cell lines derived from tumors with the OxPhos signature have enhanced mitochondrial energy transduction, greater flux through the TCA cycle and increased glutathione levels compared to non-OxPhos DLBCLs [7]. In light of these findings and the mitochondrial changes seen in the WEHI7.2 variants, we compared the expression of cytochrome c with response to therapy in different lymphoma subtypes [45]. No association is seen for patients with follicular lymphoma, an indolent form of the disease. Increased expression of cytochrome c does correlate with shorter survival in patients with the aggressive lymphoma subtypes: DLBCL and mantle cell lymphoma. Within the DLBCL patient set, significantly increased expression levels of genes needed for electron transport chain (ETC) function are seen in patients with the worst prognosis. These genes encode NADH dehydrogenase, cytochrome c reductase, and cytochrome c oxidase. These patients also have significantly decreased expression of pyruvate dehydrogenase kinase 3. This regulatory protein inhibits conversion of pyruvate to acetyl-Co-A, which is a substrate for the TCA cycle. Taken together, the expression patterns of mitochondrial proteins are consistent with increased use of the TCA cycle and oxidative phosphorylation in aggressive lymphoma. This reprogramming would allow the cells to function as metabolic opportunists, using aerobic glycolysis in conjunction with glutaminolysis to support biosynthesis needed for continual growth [43]. Our studies suggest that an additional advantage of this mitochondrial metabolism in aggressive lymphoma is enhanced chemoresistance.
Redox Homeostasis as a Chemotherapeutic Target
A number of chemotherapeutic agents cause increased oxidative stress in their target cells and this can contribute to the mechanism of cell killing. These agents include cyclophosphamide and glucocorticoids, used to treat aggressive lymphomas. A relatively new concept for cancer chemotherapy has been the development of agents to specifically target cellular components that maintain redox homeostasis [6;26;29;37]. The rationale is that the more oxidized redox environment in cancer cells places them closer to an apoptotic threshold, thus creating a therapeutic window for agents that deplete specific antioxidant defenses or increase levels of reactive species (ROS or RNS).
An attractive target for disrupting redox homeostasis in cancer cells is SOD2 (also known as manganese SOD). This mitochondrial enzyme converts the relatively short-lived superoxide anion radical to the more stable H2O2, which can traverse membranes and initiate redox signaling. SOD2 is overexpressed in some cancers but appears to function as a tumor suppressor in other types of cancer [14]. In DLBCL, a decreased level of SOD2 is associated with aggressive disease [41]. We used a genetic approach to examine the impact of increased SOD2 expression on lymphoma cells. Stable transfection with an SOD2 expression vector increases sensitivity of WEH7.2 cells to glucocorticoid-induced apoptosis [17]. As a pharmacologic approach, we treated WEHI7.2 cells with a manganoporphyrin, Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP5+). Based on its superoxide scavenging properties in cell-free systems [3], it was initially thought that MnTE-2-PyP5+ would act as an SOD2 mimetic. Studies with intact cells, however, indicate that manganoporphyrins can have either anti-oxidative or pro-oxidative properties depending on the cellular redox environment [4]. We found that treatment with MnTE-2-PyP5+ alone does not induce apoptosis but inhibits growth in a dose-dependent manner [17]. Co-treatment with MnTE-2-PyP5+ sensitizes WEHI7.2 cells to killing by glucocorticoids or cyclophosphamide [17]. Although H2O2 is required for enhanced killing in lymphoma cells treated with MnTE-2-PyP5+ and glucocorticoids, the mechanism of cell death does not simply involve elevated steady-state levels of this ROS, as one might expect for a drug acting simply as an SOD2 mimetic. Rather, MnTE-2-PyP5+ cycles with glutathione, oxidizes the 2GSH:GSSG redox couple and glutathionylates the p65 subunit of NF-κB [16]. NF-κB is critical for survival signaling in lymphoma cells [10] and glutathionylation inhibits its activity [31].
A second potential redox target in cancer cells is SOD1 (copper-zinc superoxide dismutase). This enzyme is primarily a cytoplasmic scavenger of superoxide, but is also found in the mitochondrial inter-membrane space of some cell types [28]. SOD1 is inhibited by the copper chelator ATN-224 (choline tetrathiomolybdate) [18]. This drug is FDA-approved for treatment of Wilson disease, a disorder of copper accumulation in the liver. Low nanomolar concentrations of ATN-224 effectively kill 200R cells and WEHI7.2 variants made resistant to oxidative stress and apoptosis through BCL-2 overexpression [22]. Treatment with ATN-224 leads to a rapid loss of SOD1 activity. This is accompanied by increases in superoxide and peroxynitrite levels in the cells. Peroxynitrite is a potent oxidant generated by the reaction of superoxide with nitric oxide. It is critical for ATN-224’s mechanism of action, as cell death is blocked in the presence of a peroxynitrite scavenger. ATN-224 induces death of tumor cells from patients with B-cell acute lymphoblastic leukemia [22]. This cancer commonly overexpresses BCL-2, indicating the potential clinical utility of ATN-224 for treatment-refractory lymphomas.
Conclusions
As we gain a better understanding of cancer, redox signaling has earned a rightful place next to kinase signaling. Conditions of chronic inflammation provide selective pressure for increased cellular resistance to oxidative stress. Results from studies with the WEHI7.2 lymphoma model indicate that altered redox homeostasis is accompanied by hallmarks of aggressive cancer. In patients with lymphoma, aggressive and treatment-refractory disease is predicted based on the expression of genes involved in redox homeostasis. Altered redox homeostasis is linked to metabolic reprogramming, as cancer cells optimize the synthesis of macromolecules needed for new cells with production of ATP and NADPH. Agents that target critical components of redox homeostasis can restore sensitivity to apoptosis. The central role of mitochondria in redox homeostasis, cancer cell metabolism, and the intrinsic pathway to apoptosis provides ample precedent for further development of anti-cancer drugs targeting this organelle.
Acknowledgments
The authors thank Debra Bowles in Biomedical Communications at the Arizona Health Sciences Center and Robert Hershoff in the Department of Pathology at the University of Arizona for the artwork in this article.
Funding
The authors and their research were supported by the funding from the National Cancer Institute [R01-CA71768, T32-CA009213, U54-CA143924, P30-CA023074 and P50-CA130805].
Abbreviations
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- TCA
tricarboxylic acid
- DLBCL
diffuse large B-cell lymphoma
- SOD1
copper-zinc superoxide dismutase
- SOD2
manganese superoxide dismutase
- MnTE-2-PyP5+
Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- ETC
electron transport chain
- MOMP
mitochondrial outer membrane permeability
- HK
hexokinase
References
- 1.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 2.Andreadis C, Gimotty PA, Wahl P, Hammond R, Houldsworth J, Schuster SJ, Rebbeck TR. Members of the glutathione and ABC-transporter families are associated with clinical outcome in patients with diffuse large B-cell lymphoma. Blood. 2007;109:3409–3416. doi: 10.1182/blood-2006-09-047621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 4.Batinic-Haberle I, Rajic Z, Tovmasyan A, Reboucas JS, Ye X, Leong KW, Dewhirst MW, Vujaskovic Z, Benov L, Spasojevic I. Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics. Free Radic Biol Med. 2011;51:1035–1053. doi: 10.1016/j.freeradbiomed.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 6.Cabello CM, Bair WB, III, Wondrak GT. Experimental therapeutics: targeting the redox Achilles heel of cancer. Curr Opin Investig Drugs. 2007;8:1022–1037. [PubMed] [Google Scholar]
- 7.Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, Zhou F, Green MR, Chen L, Monti S, Marto JA, Shipp MA, Danial NN. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 9.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 10.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 14.Holley AK, Dhar SK, St Clair DK. Curbing cancer's sweet tooth: is there a role for MnSOD in regulation of the Warburg effect? Mitochondrion. 2013;13:170–188. doi: 10.1016/j.mito.2012.07.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2001. World Health Organization Classification of Tumours. [Google Scholar]
- 16.Jaramillo MC, Briehl MM, Crapo JD, Batinic-Haberle I, Tome ME. Manganese porphyrin, MnTE-2-PyP5+, Acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radic Biol Med. 2012;52:1272–1284. doi: 10.1016/j.freeradbiomed.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaramillo MC, Frye JB, Crapo JD, Briehl MM, Tome ME. Increased manganese superoxide dismutase expression or treatment with manganese porphyrin potentiates dexamethasone-induced apoptosis in lymphoma cells. Cancer Res. 2009;69:5450–5457. doi: 10.1158/0008-5472.CAN-08-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juarez JC, Betancourt O, Jr, Pirie-Shepherd SR, Guan X, Price ML, Shaw DE, Mazar AP, Donate F. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin Cancer Res. 2006;12:4974–4982. doi: 10.1158/1078-0432.CCR-06-0171. [DOI] [PubMed] [Google Scholar]
- 19.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999;27:1208–1218. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 20.Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879–886. [PubMed] [Google Scholar]
- 21.Kowaltowski AJ, Fiskum G. Redox mechanisms of cytoprotection by Bcl-2. Antioxid Redox Signal. 2005;7:508–514. doi: 10.1089/ars.2005.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K, Briehl MM, Mazar AP, Batinic-Haberle I, Reboucas JS, Glinsmann-Gibson B, Rimsza LM, Tome ME. The copper chelator ATN-224 induces peroxynitrite-dependent cell death in hematological malignancies. Free Radic Biol Med. 2013;60:157–167. doi: 10.1016/j.freeradbiomed.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low IC, Kang J, Pervaiz S. Bcl-2: a prime regulator of mitochondrial redox metabolism in cancer cells. Antioxid Redox Signal. 2011;15:2975–2987. doi: 10.1089/ars.2010.3851. [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs. 2011;71:1385–1396. doi: 10.2165/11592590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RC, Dal CP, Ladd C, Pinkus GS, Salles G, Harris NL, la-Favera R, Habermann TM, Aster JC, Golub TR, Shipp MA. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 28.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 29.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Peroja P, Pasanen AK, Haapasaari KM, Jantunen E, Soini Y, Turpeenniemi-Hujanen T, Bloigu R, Lilja L, Kuittinen O, Karihtala P. Oxidative stress and redox state-regulating enzymes have prognostic relevance in diffuse large B-cell lymphoma. Exp Hematol Oncol. 2012;1:2. doi: 10.1186/2162-3619-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qanungo S, Starke DW, Pai HV, Mieyal JJ, Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 32.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, Lopez-Guillermo A, Grogan TM, Miller TP, LeBlanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T, Staudt LM. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 33.Samudio I, Fiegl M, Andreeff M. Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res. 2009;69:2163–2166. doi: 10.1158/0008-5472.CAN-08-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 35.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smedby KE, Baecklund E, Askling J. Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev. 2006;15:2069–2077. doi: 10.1158/1055-9965.EPI-06-0300. [DOI] [PubMed] [Google Scholar]
- 37.Tew KD, Townsend DM. Redox platforms in cancer drug discovery and development. Curr Opin Chem Biol. 2011;15:156–161. doi: 10.1016/j.cbpa.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tome ME, Baker AF, Powis G, Payne CM, Briehl MM. Catalase-overexpressing thymocytes are resistant to glucocorticoid-induced apoptosis and exhibit increased net tumor growth. Cancer Res. 2001;61:2766–2773. [PubMed] [Google Scholar]
- 39.Tome ME, Briehl MM. Thymocytes selected for resistance to hydrogen peroxide show altered antioxidant enzyme profiles and resistance to dexamethasone-induced apoptosis. Cell Death Differ. 2001;8:953–961. doi: 10.1038/sj.cdd.4400904. [DOI] [PubMed] [Google Scholar]
- 40.Tome ME, Frye JB, Coyle DL, Jacobson EL, Samulitis BK, Dvorak K, Dorr RT, Briehl MM. Lymphoma cells with increased anti-oxidant defenses acquire chemoresistance. Exp Ther Med. 2012;3:845–852. doi: 10.3892/etm.2012.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tome ME, Johnson DB, Rimsza LM, Roberts RA, Grogan TM, Miller TP, Oberley LW, Briehl MM. A redox signature score identifies diffuse large B-cell lymphoma patients with a poor prognosis. Blood. 2005;106:3594–3601. doi: 10.1182/blood-2005-02-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tome ME, Lutz NW, Briehl MM. Overexpression of catalase or Bcl-2 alters glucose and energy metabolism concomitant with dexamethasone resistance. Biochim Biophys Acta. 2004;1693:57–72. doi: 10.1016/j.bbamcr.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WARBURG O. In: Metabolism of Tumors. Dickens F, translator. Constable; London: 1930. [Google Scholar]
- 45.Wilkinson ST, Johnson DB, Tardif HL, Tome ME, Briehl MM. Increased cytochrome c correlates with poor survival in aggressive lymphoma. Oncol Lett. 2010;1:227–230. doi: 10.3892/ol_00000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson ST, Tome ME, Briehl MM. Mitochondrial adaptations to oxidative stress confer resistance to apoptosis in lymphoma cells. Int J Mol Sci. 2012;13:10212–10228. doi: 10.3390/ijms130810212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]