Abstract
Introduction
Spinal cord injury is a cause of severe disability and mortality. The pharmacological and non-pharmacological methods used, are unable to improve the quality of life in spinal cord injury. Spinal disorders have been treated with human embryonic stem cells. Magnetic resonance imaging and tractography were used as imaging modality to document the changes in the damaged cord, but the magnetic resonance imaging tractography was seen to be more sensitive in detecting the changes in the spinal cord. The present study was conducted to evaluate the diagnostic modality of magnetic resonance imaging tractography to determine the efficacy of human embryonic stem cells in chronic spinal cord injury.
Materials and methods
The study included the patients with spinal cord injury for whom magnetic resonance imaging tractography was performed before and after the therapy. Omniscan (gadodiamide) magnetic resonance imaging tractography was analyzed to assess the spinal defects and the improvement by human embryonic stem cell treatment. The patients were also scored by American Spinal Injury Association scale.
Results
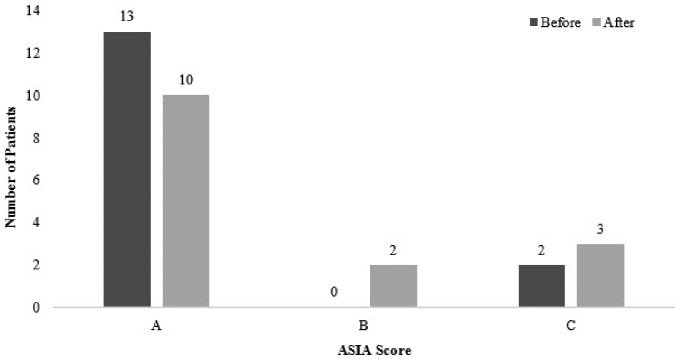
Overall, 15 patients aged 15–44 years with clinical manifestations of spinal cord injury had magnetic resonance imaging tractography performed. The average treatment period was nine months. The majority of subjects (n = 13) had American Spinal Injury Association score A, and two patients were at score C at the beginning of therapy. At the end of therapy, 10 patients were at score A, two patients were at score B and three patients were at score C. Improvements in patients were clearly understood through magnetic resonance imaging tractography as well as in clinical signs and symptoms.
Conclusion
Magnetic resonance imaging tractography can be a crucial diagnostic modality to assess the improvement in spinal cord injury patients.
Keywords: Spinal cord injury, magnetic resonance imaging tractography, human embryonic stem cell, American Spinal Injury Association
Introduction
Spinal cord injury (SCI) is a devastating disorder, impacting not only the physical, psychological, and social life of patients, but also making a significant impact financially.1 Approximately, 250,000–500,000 people suffer with SCI globally every year.2 In India, the prevalence of SCI is between 1.85–2.19%.3 According to the American Spinal Injury Association (ASIA), the severity of an injury is categorized as either complete or incomplete. A complete injury is defined as the absence of sensory and motor function below the level of injury. If there is some preserved motor or sensory function below the level of injury, the case is diagnosed as incomplete motor and sensory SCI.4,5
There are no specific diagnostic tests for SCI. Magnetic resonance imaging (MRI)6 and positron emission tomography7 are some of the imaging modalities for SCI, but amongst all methods tractography is more sensitive in detecting abnormalities in the spine.8,9
MRI scan is a commonly used imaging modality that can provide valuable information for the diagnosis of SCI.9 However, there are some limitations of MRI.10 MRI can identify different levels of SCI, but is unable to detect the functionality of SCI.
Tractography is a three-dimensional realm where the structure being determined extends beyond the plane of imaging. Tractography is an emerging MRI-based technique that can demonstrate spinal cord anatomy by measuring the fractional anisotropy. Tractography is the first non-invasive technique for measuring spinal cord fiber structure in vivo.11
In the present study, we have reported the MRI tractographic results of our observational longitudinal study of patients with SCI treated with human embryonic stem cells (hESCs).
Materials and methods
Cell culture and differentiation
The cells are cultured and maintained as per our patented technology (United States Granted Patent No. US 8592, 208, 52) in a good manufacturing practice (GMP), good laboratory practice (GLP), and good tissue practice (GTP) compliant laboratory. The cell lines are free of animal product and are chromosomally stable. The detailed procedure of cell culture and differentiation was elaborated previously.12 The safety and efficacy of these cells has been established in patients with incurable conditions.13
Study population
Patients (n = 15 (male: 13; female: 2); mean age 27 years) with clinical manifestations of SCI underwent MRI tractography of spine (paraplegic and quadriplegic) (Table 1). The study was approved by the independent Institutional Ethics Committee (IEC) and all procedures were performed according to ethical standards described in the Declaration of Helsinki. The study was conducted from August 2013–September 2015 in a good clinical practice (GCP) compliant center. Written and video consent from each patient was taken before initiation of the treatment. The whole treatment was supervised by the team of external consultants.
Table 1.
Demographic details of patients.
| Characteristics | n |
|---|---|
| Gender | |
| Male | 13 |
| Female | 2 |
| Origin | |
| India | 10 |
| Abroad | 5 |
Study design
The patients had to undergo a detailed examination by the doctors and the rehabilitation team before and after each treatment cycle. MRI tractography and biochemical investigations were done for all the patients before the start of the treatment and then at regular intervals. A separate team of doctors examined the observations documented by the various teams and further graded the patients. After diagnosis and procedural details, 0.05 ml of hESC was injected to the patients subcutaneously (s.c.) to test the hypersensitivity reactions. The study comprised of one treatment phase with gap phases in between. The patients entered the treatment phase (T, eight weeks for paraplegics and 12 weeks for quadriplegics) after the hypersensitivity testing.
During treatment, 0.25 ml (<4 million cells) of hESC was injected to patients through intramuscular (i.m.) route twice daily to “prime” the body and allow the patient’s immune system to accept the stem cells. About 1 ml of hESC (<16 million cells) was injected in patients through the intravenous (i.v.) route every 10 days to “home in” on the required area. For local action, 1–5 ml of hESC was injected in patients every 5–7 days through any of the supplemental routes (epidural infusion or injection/ caudal injection 5 ml; subarachnoid injection 2 ml and deep spinal injection 1 ml). As quadriplegic patients take more time to recover, the treatment and gap phase duration was varied for paraplegic and quadriplegic patients. The patients received no steroids and immunosuppressants during the treatment. The efficacy (clinical improvements observed in the patients and/or change in at least one grade of ASIA) of the therapy was assessed based on the change in ASIA scores and radiological examinations (axonal growth) of patients. ASIA has five grades which are A: complete impairment; B: sensory incomplete; C: motor incomplete (muscle grade less than 3); D: motor incomplete (muscle grade = 3) and E: normal.14
MRI scan
Preparation of Omniscan
Omniscan (gadodiamide) injection MRI scan was analyzed to assess the axonal growth in the spinal cord and the percentage of improvement by hESC treatment. An Omniscan kit was purchased from GE Healthcare Inc., Princeton, New Jersey, USA.
Fresh elutes of Omniscan were used to prepare the injection according to the manufacturer’s instructions and recommendations. In brief, 287 mg of Omniscan in 1 ml of sterile aqueous solution was added to produce Omniscan injection.
MRI acquisition
MRI was performed using a 1.5-Tesla scanner (Wipro GE, Milwaukee, Wisconsin, USA). T2-weighted imaging in the sagittal plane with slice thickness of 3 mm and slice gap of 1 mm was obtained. Diffusion tensor imaging (DTI) was performed in the sagittal plan using an eight-channel cervical thoracic lumbar (CTL) array spine coils with the following parameters: 25 direction echo-planar imaging tensor imaging (EPITI) (repetition time: 8700; time of echo (TE): min; b value: 1000; frequency: 128; phase: 128; number of excitations (NEs): 1; slice thickness: 3 mm with zero slice gap. Functool software provided by GE Healthcare was used for post-processing imaging. An experienced radiologist placed elliptical regions of interest (ROIs) (2 cm3) on the affected and implicated regions in the cord and the Functool software generated the fractional anisotropy (FA) value quantitatively and performed fiber tracking.
Image processing and interpretation
MRI tractography was interpreted by a certified nuclear medicine physician with extensive experience in spinal MRI scan interpretation. The evaluation of the patients was done using the ASIA scale.15
Results
Study patients
The data of 15 patients was analyzed, of which two subjects were females. The average treatment period was nine months. The demographic details of the patients are given in Table 1.
Outcomes
All the subjects showed an improvement in ASIA scores. Out of 15 patients, 13 patients were at score A, and two patients were at score C at the beginning of therapy. At the end of therapy, 10 patients were at score A, two patients were at score B and three patients were at score C. The change in ASIA score is shown in Figure 1.
Figure 1.
American Spinal Injury Association (ASIA) score before and after the treatment.
Radiological examinations
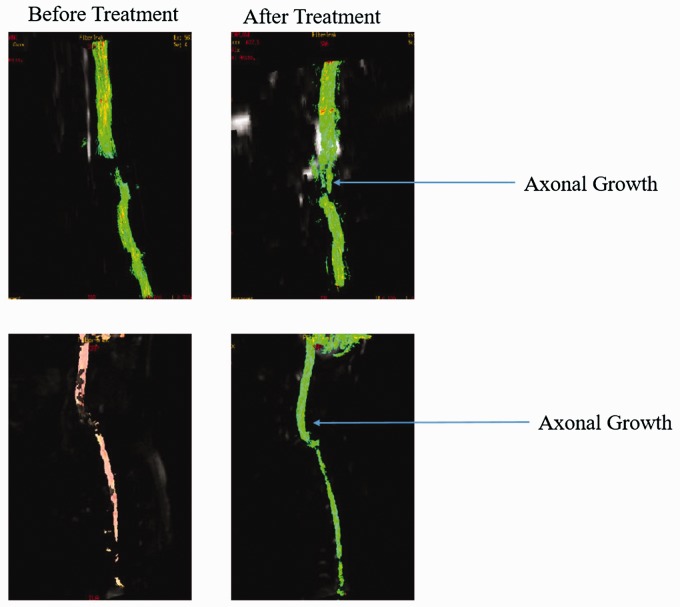
The radiological examinations for all the patients were done before and after the treatment (Figure 2, Table 2). Improvements in patients were clearly understood through the radiological examinations as well as from clinical signs and symptoms. Before hESC therapy, fracture dislocation of the spine at C6–C7 with stabilization of anterior plate was observed. There was anterior displacement of C6 vertebra with disruption of posterior osteoligamentous complex and partial tear of posterior longitudinal ligament. Also, the changes of cystic myelomalacia were noticeable at lower C5 to C7–D1 regions. After the therapy, significant bridging was observed in the previously non-visualized myelomalacic cord segment by the white matter tracts with only a small gap. The interpretation details of MRI tractography of patients is explained in Table 3.
Figure 2.
Magnetic resonance imaging (MRI) tractography images of patients before and after the therapy.
Table 2.
Radiological examinations of patients before and after the treatment.
| Patient no. | Radiological examinations (MRI tractography) |
|
|---|---|---|
| Before | After | |
| 80776 | There is significant vertebral body damage at T2–T8 with associated cord damage. Multiple areas of cord damage in the area with break in spinal tracts. | No significant degeneration of tracts. |
| 81027 | Multiple areas of vertebral body damage with associated cord damage and myelomalacia are seen in the cervical and dorsal regions. | Significant regeneration of tracts though there are areas of loss of communication. |
| 81078 | Significant vertebral body damage at C5–C8 with associated cord damage. Multiple areas of cord damage in the area with break in spinal tracts. | Significant regeneration of tracts with a minimal break in the tracts. |
| 81191 | There is vertebral body damage at C6–C8 with associated cord damage. Areas of cord neuromalacia in the area with break in spinal tracts. | Significant regeneration of tracts with almost complete regeneration of the tract with minimal separation of the proximal and distal segments of the tracts. |
| 81216 | There is anterior wedging of T11 with associated cord damage. | Significant regeneration of tracts with approximation of the segments though a minimal break in the tracts remains. |
| 80957 | There is significant vertebral body damage at T7–L1 with internal fixation and associated cord damage. | No significant degeneration of tracts. |
| 80438 | There is significant vertebral body damage at D12–L3 with internal fixation and associated cord damage. | Significant regeneration of tracts though there are areas of loss of communication. |
| 81107 | There is significant vertebral body damage at T4–T8 with internal fixation and associated cord damage. | Significant regeneration of tracts though there are areas of loss of communication. |
| 81263 | There is significant vertebral body damage at D1–D4 with internal fixation with associated cord damage. | Significant regeneration of tracts though there are areas of loss of communication. |
| 80836 | There is extensive vertebral body damage with internal fixation with associated cord damage. | Very minimal regeneration of tracts. |
| 81193 | There is significant vertebral body damage at C6 and C7 with associated cord damage. | Significant regeneration of tracts though there are areas of loss of communication. |
| 81160 | There is vertebral body damage at D2–D5 with associated cord damage. Areas of cord neuromalacia in the area with break in spinal tracts. | Significant regeneration of tracts with though there is residual area of separation of the proximal and distal segments of the tracts. |
| 80754 | There is significant vertebral body damage at C8–T5 with internal fixation and associated cord damage. | No significant deterioration of findings. |
| 81289 | Multiple areas of disruption in spinal tracts between D5 and D9. | Significant regeneration of tracts though there are areas of loss of communication. |
| 80985 | There is significant vertebral body damage at C4–C8 with internal fixation and associated cord damage. Multiple areas of cord damage in the area with break in spinal tracts. | No significant deterioration of tracts. |
MRI: magnetic resonance imaging.
Table 3.
Interpretation of magnetic resonance imaging (MRI) tractography before and after the treatment.
| Patient no. | MRI tractography interpretation |
|
|---|---|---|
| Before | After | |
| 80776 | Vertebral body fractures were found in D5 and D6 with anterior wedging of D6 with anterior, mildly displaced fragment. Myelomalacic change was seen at D5 and D6 vertebral levels. Stabilization of spine with pedicular screws was noted at D4–D5 and D8–D9. Non-visualization of fibers seen at myelomalacia level (D5–D6) and at caudally placed pedicular screws (D8–D9 level) with intervening cord showing some artefactual distortion at its cranial and caudal extent. | D5 and D6 vertebral body fractures were seen with anterior wedging of D6 with mildly displaced anterior fragment. Spine stabilization with pedicular screws at D4–D5 and D8–D9 were noted. Marked myelomalacic change at D5 and D6 vertebral levels was seen. Non-visualization of fibers seen at myelomalacia level (D5–D6) and at caudally placed pedicular screws (D8–D9 level) with intervening cord showing some artefactual distortion at its cranial and caudal extent. |
| 81027 | The evidence of pedicular screw spine stabilization at D11–L1 was found to cause distortion of anatomy including spinal canal from magnetic susceptibility artefacts. Mild anterior wedging of D12 vertebral body was noted. T1/T2 hyper-intense signal in anterior D12 vertebral body suggestive of lipid rest/hemangioma was noted. There was near complete transaction of the spinal cord at D12 level with very thin cord tissue seen adherent to the posterior thecal sac. An intradural anterior adhesion was also observed at upper D12 level. Cranial to this at D10 and D11 vertebral levels centromedullary T2 hypersensitivity in the cord suggested syrinx formation. MR tractography showed marked artefactual distortion and non-visualization of tracts in dorsal cords. | Sagittal T2-weighted MRI near complete transaction of the spinal cord at D12 level with very thin cord tissue seen adherent to the posterior thecal sac. An intradural anterior adhesion was also noted at upper D12 level. Cranial to this at D10 and D11 vertebral levels centromedullary T2 hyper-intensity in the cord suggest syrinx formation. MR tractography showed marked artefactual distortion with patchy interrupted and visualization of tracts in dorsal cords with complete non-visualization at D12 level (level of cord transaction) and at D11 vertebral level. There is an artefactual larger gap between the distal conus and the cauda equina compared to the previous tractography. |
| 81078 | Fracture dislocation of the spine at C6–C7 with anterior plate stabilization was seen. Minimal anterior displacement of C6 vertebra with disruption of posterior osteoligamentous complex and partial tear of posterior longitudinal ligament were noted. Changes of cystic myelomalacia were seen at lower C5 to C7–D1. No tracts were seen at C6 and C7 vertebral level. | Non-visualization of white matter tracts in the myelomalacic segment. Significant bridging of the previously non-visualized myelomalacic cord segment by the white matter tracts with only a small gap was found. |
| 81191 | There was fracture dislocation of spine at C7–D1 with mild anterior displacement of C7 vertebra with bilateral perched facets. At C7–D1, focal cystic myelomalacia was noted. Fracture of posterior spinous process of C7 and minimal C6 anterior subluxation were seen. Cord thinning at C6–C7 was observed along with T2 hyper-intense signal. Interruption of the cord nerve fibers at C6–C7 to C7–D1 level was suggested by MR tractography. | T2 hyper-intense myelomalacic change was seen in the spinal cord at C6–C7 vertebral levels. Focal cystic myelomalacia was present at C7–D1 level. Interruption of fiber tracts in the cord at the level of myelomalacic change with reduction of fractional anisotropy values to less than 0.150, in the MR tractography. Reduced values were seen extending up to mid dorsal level. |
| 81216 | D10–D12 laminectomy and old mild anterior wedge compression D11 vertebral body with mild kyphotic angulation of the spine was noted. Myelomalacic changes and focal thinning was observed at lower D10 level in the spinal cord. Cord contour expansion and linear/irregular gliotic scars on the sides along with D11–D12 disc level cystic myelomalacic changes. Ascending and descending white matter tracts were seen cephalic and caudal to the myelomalacic segments with patchy visualization of tracts along the length of the latter. | MRI tractography suggested no significant interval change in the patchy visualization of along the length of the myelomalacic segments with normal visualization of ascending and descending tracts cranial and caudal to it. Myelomalacic changes was observed at lower D10 level in the spinal cord focal thinning. |
| 80957 | On the sagittal T2-weighted images prominent magnetic susceptibility artefacts from fixation screws were noted at D8–D10 and D12–L1 level causing distortion of anatomy. Anterior wedging of D11 vertebral was noted with break in superior endplate. Minimal anterior displacement of D11 vertebral body was seen with marked height reduction of D11–D12 disc. There was mild thecal sac indentation from a mild posterior bulge of this disc. | On the sagittal T2-weighted images prominent magnetic susceptibility artefacts from fixation screws were noted at D8–D10 and D12–L1 level. Anterior wedging of D11 vertebral body with break in superior endplate was noted. Minimal anterior displacement of D11 vertebral body was seen with marked height reduction of D11–D12 disc. There was mild thecal sac indentation from a mild posterior bulge of this disc. Cystic myelomalacia was noted caudal to D9–D10 disc level with marked thinning of the cord caudal to lower D10 vertebral level. |
| 80438 | MRI tractography showed comminuted fracture of L1 vertebral body with significantly retro-pulsed poster superior fragment. The spinal canal was capacious at this level, post-laminectomy. Spine stabilization surgery with pedicular screws at D11, D12 and L2, L3 levels was noted with resultant magnetic susceptibility artefacts causing marked distortion of the spinal/canal cord at the level of the cranial screws. However, cystic myelomalacia was discerned at D11 and D12 level. Minimal to mild old anterior wedging of D11 and D12 vertebral bodies was noted. There was complete non-visualization of nerve fibers at D9–L3 at cranial normal cord fibers at D9 and D10 level not demonstrated due to presence of metal at adjoining caudal aspect. | MRI tractography showed comminuted fracture of L1 vertebral body with significantly retro-pulsed posterior superior fragment. The spinal canal was capacious at this level, post laminectomy. Spine stabilization surgery with pedicular screws at D11, D12 and L2, L3 levels was noted with resultant magnetic susceptibility artefacts causing marked distortion of the spinal/canal cord at the level of the cranial screws. However, cystic myelomalacia was discerned at D11 and D12 level. The spinal cord cranial to this level was seen normally. There was complete non-visualization of white matter tracts/nerve roots at D9–L3 levels with cranial normal cord at D9 and D10 level and upper cauda equina not seen due to presence of metal adjoining. There was no significant interval change on comparison with the last MRI tractography. |
| 81107 | There was distortion of the anatomy and fiber tracts consequent to pedicular fixation screws. On MRI tractography, ascending and descending white matter tracts were seen cephalic and caudal to the injured cord segment and showed thinning, loss of normal contour and myelomalacic changes (D3–D6). No tracts were seen at the level of cord changes. | There was distortion of the anatomy and fiber tracts consequent to pedicular fixation screws. There was no significant interval change on comparison with the last MRI tractography. The non-visualized upper dorsal segment distorted by metallic implants/screws was as before. |
| 81263 | Marked glottic scarring and cystic myelomalacic changes were seen in the cord at D1–D3 level. Caudal to this up to conus medullaris, syrinx formation was present with thin preserved peripheral rim of cord parenchyma. Changes of arachnoiditis were also noted in the dorsal and lumbar spine. Magnetic susceptibility artefacts from the metallic screws caused distortion of the anatomy/spinal canal in the upper dorsal spine. At MRI tractography, there was non-visualization of fiber tracts at the level of injury/surgery and susceptibility artefacts. Caudal to this up to the tip of the conus medullaris there was poor patchy interrupted fiber visualization. | Marked glottic scarring and cystic myelomalacic changes were seen in the cord at D1–D3/D4 level. Caudal to this up to conus medullaris, syrinx formation was present with thin preserved peripheral rim of cord parenchyma. Changes of arachnoiditis were also noted. At MRI tractography, there was non-visualization of fiber tracts at the level of injury/surgery and susceptibility artefacts. Compared to previous report there was no significant interval change. |
| 80836 | MRI tractography showed evidence of prior spine stabilization surgery with pedicular screws at D9–L3/L4 and multilevel laminectomies at dorsolumbar junction. There was artefactual distortion of the anatomy from the metallic screws. The cauda equina nerve roots were also not well visualized consequent to artefacts. | MRI tractography provided evidence of prior spine stabilization surgery with pedicular screws at D9–L3/L4 and multilevel laminectomies at dorsolumbar junction. There was marked artefactual distortion of the anatomy from the metallic screws. The cauda equina nerve roots were also not well visualized consequent to artefacts. |
| 81193 | There was mild thickening of the posterior longitudinal ligament from C4 to D1–D2 level. Mild broad-based posterocentral-right paracentral disc protrusion was seen at C7–D1 level causing thecal sac indentation with minimal impingement of ventral cord surface. Minimal posterior disc bulge was present at C4–C5 and C3–C4 level. Cystic myelomalacic changes were seen in the spinal cord at upper C6 to C7–D1 disc level. An oblique T2-hypointense linear soft tissue intensity was seen in the myelomalacic segment at C7 level, likely post traumatic glial scarring with hemosiderin deposition. Mild centro-medullary T2-hyperintensity was seen at D1–D2. MRI tractography showed artefactual distortion at C6, C7 level with interrupted visualization of nerve fibers/tracts in anterior third. Compared to the previous tractography, there was better visualization of fibers at C6–C7 levels. | There was mild thickening of the posterior longitudinal ligament from C4 to D1–D2 level. Mild broad-based posterocentral-right paracentral disc protrusion was seen at C7–D1 level causing indentation of the thecal sac. Minimal posterior disc bulge was present at C4–C5 and C3–C4 level. Cystic myelomalacic changes were seen in the spinal cord at upper C6 to C7–D1 disc level. An oblique T2-hypointense linear soft tissue intensity was seen in the myelomalacic segment at C7 level suggestive of post-traumatic hemosiderin stained glial scarring. MRI tractography shows artefactual distortion at C6, C7 level with interrupted visualization of nerve fibers/tracts. No continuous bridging of the cranial and caudal ends of the normal cord was seen. Compared to the previous tractography, artefactual distortion was more pronounced. No evident interval change was otherwise discerned. |
| 81160 | Mild kyphotic angulation was observed along with the partial collapse with anterior wedging and fusion of D5 and D6 vertebral bodies. Laminectomy was seen at D4, D5 and D6 with small pseudomeningocele at D4–D5 level. Occurrence of myelomalacia was showed by thinning at lower D2 to D6–D7 disc level with abnormal T2 hypersensitivity parenchymal signal. While at MRI tractography level, no white matter tracts/fibers at the level of myelomalacia. Milder cord thinning is present cranially up to C7 level while mild cord expansion was seen at D8–D9 vertebral level with diffused intramedullary T2 hyper intensity. | At MRI tractography, significantly smaller non-visualized cord segment was seen. Mild expansion at D8–D9 vertebral level with diffuse intramedullary T2-hyperintensity was present. |
| 80754 | D11–L1 vertebral body fractures with kyphotic angulation were seen. D9 and D11 had artefacts from pedicle screws on left side. Marked myelomalacia caudal to D9/D10 disc level were seen with abrupt cut-off of fibers in spinal cord caudal to their level up to distal conus level as shown by MRI tractography. Intervening segment had interrupted minimal visualization of cord fibers in caudal two-thirds. Focal posterior adhesion of spinal cord was noted proximal to the level of cut-off. | Marked anterior wedge compression of D12 vertebral body and mildly so of D11 body with normal marrow signal was observed. Significant kyphotic angulation of spine was seen. D12–L1 had bone formation at their anterior side along with their fusion. D11–D12 had irregularity of endplates with marked reduction of intervening disc height. Mild reduction of D12–L1 disc height was noted along with wide laminectomy at their level. Pedicular screw artefacts were seen at D9 and D11 levels. Gliotic scarring of the spinal cord at D11–D12 level with post-traumatic syrinx formation cranial to it up to lower D9 level. The spinal cord appears posteriorly adherent to the thecal sac. Non-visualization of cord fibers at D9–D10 and D11–D12 was shown in MRI tractography. FA values cranial to D8. |
| 81289 | Sagittal screening of the spine showed marked atrophy of the dorsal cord from D6–D7 disc to lower D11 vertebral body level with normal parenchymal signal. Rest of the spinal cord cranially up to cervicomedullary junction and caudally at D12 level was normal in size, outline and parenchymal signal. The nerve roots of cauda equine were unremarkable. Mild posterocentral-right paracentral disc protrusion was seen at L1–L2 level causing thecal sac indentation. At MRI tractography, there was non-visualization of fiber tracts in the atrophied cord segment with fraction anisotropy values cranial to this segment showing no significant interval change compared to the last tractography. In the atrophied segment values were inconsistent. | Sagittal T2 images of the spine showed marked atrophy of the dorsal cord from D6–D7 disc to lower D11 vertebral body level. The parenchymal signal was normal in the affected segment. Rest of the spinal cord cranially up to cervicomedullary junction and caudally at D12 level was normal in size, outline and parenchymal signal. At MRI tractography, there was non-visualization of fiber tracts in the atrophied cord segment with fraction anisotropy values cranial to this segment showed no significant interval change compared to the last tractography. In the atrophied segment the values were markedly reduced as before. |
| 80985 | It showed an evidence of anterior plate and screw fixation of the cervical spine from C3–C7. There was cervical straightening of cervical lordosis. Rightward rotation of the cervical vertebrae was noted with bony fusion of C4–C6 vertebral bodies on the right side. Right ventral thecal sac indentation was noted at these levels. There was marked thinning with deformity of the spinal cord at lower C4 to mid C6 vertebral level with T2-hyperintense myelomalacia cranially at mid-upper C4 level. MRI tractography showed interrupted visualization of the white matter fibers at lower C4–C6 levels with lower segments of discontinuity. | On the sagittal T2 weighted images anterior plate and screw fixation of the cervical spine was noted from C3–C7. There was straightening of cervical lordosis. Right ward rotation of the cervical vertebrae was noted with bony fusion of C4–C6 vertebral bodies on the right side with right ventral thecal sac indentation at these levels. There was marked thinning with deformity of the spinal cord at lower C4 to mid C6 level with T2-hyperintense myelomalacia cranially at mid-upper C4 level. MRI tractography shows interrupted visualization of the white matter fibers at lower C4–C6 levels. Compared to previously there is no significant interval change. |
MRI: magnetic resonance imaging.
Discussion
SCI is one of the most common causes of severe disability and mortality after trauma.16 Trauma can be associated with significant neurologic damages such as quadriplegia, paraplegia, and even death,17 and causes low quality of life, high cost of care for individual patients, and they will ultimately be short-lived.18 On the contrary, the importance of correct and timely diagnosis of patients with incomplete SCI to prevent progression to complete SCI has led to early detection and treatment of fractures, hematomas, and other compressive lesions on cord which is very important. Diagnostic imaging, particularly MRI, plays a vital role in the assessment and diagnosis of SCI. Demyelination of intact axons is a prominent feature of SCI19 and is an important contributor to functional loss in many CNS disorders, including trauma, multiple sclerosis, and stroke.20–22 In the present study, the efficacy of hESC therapy was evaluated based on the grade in MRI tractography and change in ASIA scores. An improvement in MRI tractography was observed in SCI patients after hESC therapy. The improvement was reflected by an axonal growth in the MRI scan of the spine. A remarkable change in MRI tractography was observed in majority of the subjects. This improvement was also reflected in ASIA scores, as the patients moved from A→C (bad→good) at the end of the treatment.
In the present study, MRI tractography was performed within one hour after injection of Omniscan. The contrast media, Omniscan is the most widely used contrast media for imaging SCI.23 This radiotracer is formed from the gadolinium complex of diethylenetriaminepenta acetic acid bismethylamide, and is an injectable, non-ionic extracellular enhancing agent for MRI tractography. By increasing the relaxation rate, Omniscan decreases both the T1 and T2 relaxation times in tissues where it is distributed. At clinical doses, the effect is primarily on the T1 relaxation time, and produces an increase in signal intensity. Omniscan does not cross the intact blood-brain barrier and, therefore, does not accumulate in the normal brain or in lesions that do not have an abnormal blood-brain barrier.24 A normal MRI tractography with Omniscan shows more intense axonal growth in the spinal cord.25 In our study, significant regeneration of tracts with a minimal break in the tracts was observed (Table 2, Figure 2).
In relation to clinical outcomes, some studies have compared the outcomes of MRI and MRI tractography. Chang et al.26 conducted a study to compare the MRI and tractography on 10 patients with chronic cervical SCI and on 10 controls. In patients with cervical cord injury, abnormal cervical levels detected on routine MRI were not correlated with clinical findings and tractography parameters. The author reported that tractography analyses were useful in the evaluation of patients with cervical SCI.26 Rajasekaran et al.27 conducted a study in demonstrating the partially severed spinal cord tracts with tractography and MRI scans on a 30-year-old male patient. MRI showed signal changes at the level of injury, but it was difficult to quantify the extent of neuronal damage in the spinal cord. Whereas, tractography of the spinal cord visualized the spinal cord tracts. Well-organized fiber tracts could be traced from the upper thoracic spinal cord till the site of hemi-section lesion. The fractional anisotropy and apparent diffusion coefficient values showed significant changes at the level of injury. The author suggests that tractography can clearly demonstrate the extent of injury to the spinal cord tracts.27
MRI tractography studies have increased our understanding of the spinal abnormalities in patients with SCI. MRI tractography is more valuable and sensitive than MRI, X-ray or computed tomography (CT) as it can detect more spinal defects compared to other morphologic modalities.17 X-ray and CT are less informative due to common problems associated with them such as inappropriate and inconsistent morphologic, radiologic, and pathologic findings and their uncertain relationship to the time estimation of SCI.17,28
Stem cells replace the damaged neurons involving both exogenous and endogenous neurons, glial cells, and endothelial cells. These cells act synergistically to repair spinal damage.29 Various studies demonstrated the differentiation of hESC-derived progenitor cells to the site of SCI30–32 that raised the hope that hESC therapy can be developed to treat SCI.33 In our previous study, we have observed remarkable clinical improvements in 11 SCI patients with hESC therapy.34
In our study, we have seen the spinal cord move towards joining and there are visible tracts. This is coupled with the patient’s clinical improvement. This could have been resulted due to the attachment of hESCs (“home in”) to the damaged area and its regeneration. The area of repair was first seen as whitish color that indicated regeneration of non-functional tracts and then, the color indicated functional tracts. The hESCs grow inside the body and the functioning of the cells usually follows. This might be due to the fact that all organs develop within 14–16 weeks of gestation during embryonic development in humans.35 On this basis, a gap phase of 4–6 months was included between our treatment periods to allow hESCs to grow, repair and regenerate the affected area. None of the patients have reported deterioration and their condition was stable. The results of the present study were derived from a small cohort of patients and need to be replicated in a larger sample population.
Conclusion
The extent of improvement in patients treated with hESCs was carefully monitored with MRI tractography and ASIA scores. Spinal MRI tractography may be an extremely useful tool in monitoring the treatment response to hESC therapy in patients with SCI. Further studies must be conducted to examine the diagnostic value of MRI tractography and to evaluate the possible effects of hESC therapy. MRI tractography helps in understanding the differential diagnosis and the disease progression. It may be used as a prognostic tool for the detection of the extent of improvement in SCI.
Acknowledgement
The author acknowledges the staff of Nutech Mediworld, all patients and their parents. The author also acknowledges Knowledge Isotopes Pvt. Ltd (http://www.knowledgeisotopes.com) for writing support.
Conflict of interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J Stem Cells 2014; 6: 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. International perspectives on spinal cord injury, http://www.who.int/disabilities/policies/spinal_cord_injury/report/en/ (accessed 23 November 2015).
- 3.Rehabilitation Council of India. Spinal cord injuries, http://www.rehabcouncil.nic.in/writereaddata/spinal.pdf (accessed 23 November 2015).
- 4.Grossman RG, Frankowski RF, Burau KD, et al. Incidence and severity of acute complications after spinal cord injury. J Neurosurg Spine 2012; 17: 119–128. [DOI] [PubMed] [Google Scholar]
- 5.Dalbayrak S, Yaman O, Yilmaz T. Current and future surgery strategies for spinal cord injuries. World J Orthop 2015; 6: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozzo A, Marcoux J, Radhakrishna M, et al. The role of magnetic resonance imaging in the management of acute spinal cord injury. J Neurotrauma 2011; 28: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nandoe Tewarie RD, Yu J, Seidel J, et al. Positron emission tomography for serial imaging of the contused adult rat spinal cord. Mol Imaging 2010; 9: 108–116. [PubMed] [Google Scholar]
- 8.Hayashi K, Yone K, Ito H, et al. MRI findings in patients with a cervical spinal cord injury who do not show radiographic evidence of a fracture or dislocation. Paraplegia 1995; 33: 212–215. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg AL, Kershah SM. Advances in imaging of vertebral and spinal cord injury. J Spinal Cord Med 2010; 33: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman D, Mills CM, Brant-Zawadzki M, et al. Magnetic resonance imaging of the spinal cord and canal: Potentials and limitations. AJR Am J Roentgenol 1983; 141: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 11.Stroman PW, Wheeler-Kingshott C, Bacon M, et al. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage 2014; 84: 1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shroff G. Establishment and characterization of a neuronal cell line derived from a 2-cell stage human embryo: Clinically tested cell-based therapy for neurological disorders. International Journal of Recent Scientific Research 2015; 6: 3730–3738. [Google Scholar]
- 13.Shroff G, Barthakur JK. Safety of human embryonic stem cells in patients with terminal/incurable conditions – a retrospective analysis. Ann Neurosci 2015; 22: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011; 34: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), http://www.physio-pedia.com/International_Standards_for_Neurological_Classification_of_Spinal_Cord_Injury_(ISNCSCI) (accessed 23 November 2015).
- 16.Yousefzadeh Chabok S, Safaee M, Alizadeh A, et al. Epidemiology of traumatic spinal injury: A descriptive study. Acta Med Iran 2010; 48: 308–311. [PubMed] [Google Scholar]
- 17.Parizel PM, van der Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imaging strategies. Eur Spine J 2010; 19: S8–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimi MV, Mohammadi M and Yazdi A. Comparison between nonoperative and operative care and timing of surgery in spinal cord injury. Hakim 2006; 9: 50–57.
- 19.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 2005; 486: 373–383. [DOI] [PubMed] [Google Scholar]
- 20.Gledhill RF, Harrison BM, McDonald WI. Demyelination and remyelination after acute spinal cord compression. Exp Neurol 1973; 38: 472–487. [DOI] [PubMed] [Google Scholar]
- 21.Blight AR. Delayed demyelination and macrophage invasion: A candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma 1985; 2: 299–315. [DOI] [PubMed] [Google Scholar]
- 22.Bunge RP, Puckett WR, Becerra JL, et al. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 1993; 59: 75–89. [PubMed] [Google Scholar]
- 23.Byrnes KR, Fricke ST, Faden AI. Neuropathological differences between rats and mice after spinal cord injury. J Magn Reson Imaging 2010; 32: 836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GE Healthcare. Omniscan™ (gadodiamide) injection, http://www3.gehealthcare.com/∼/media/documents/us-global/products/contrast-media_non-gatekeeper/clinical-product-information/omniscan/gehealthcare_omniscan-bulk-pack_prescribing-information.pdf?Parent=%7B21C1FA0B-7909-4C09-90C6-0FD83A4D7E61%7D (accessed 16 December 2015).
- 25.Newman TA, Woolley ST, Hughes PM, et al. T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: Involvement of metalloproteinases. Brain 2001; 124: 2203–2214. [DOI] [PubMed] [Google Scholar]
- 26.Chang Y, Jung TD, Yoo DS, et al. Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma 2010; 27: 2033–2040. [DOI] [PubMed] [Google Scholar]
- 27.Rajasekaran S, Kanna RM, Karunanithi R, et al. Diffusion tensor tractography demonstration of partially injured spinal cord tracts in a patient with posttraumatic Brown Sequard syndrome. J Magn Reson Imaging 2010; 32: 978–981. [DOI] [PubMed] [Google Scholar]
- 28.Chang AE, Matory YL, Dwyer AJ, et al. Magnetic resonance imaging versus computed tomography in the evaluation of soft tissue tumors of the extremities. Ann Surg 1987; 205: 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pabon MM, Borlongan CV. Advances in the cell-based treatment of neonatal hypoxic-ischemic brain injury. Future Neurol 2013; 8: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukovic D, Moreno Manzano V, Stojkovic M, et al. Concise review: Human pluripotent stem cells in the treatment of spinal cord injury. Stem Cells 2012; 30: 1787–1792. [DOI] [PubMed] [Google Scholar]
- 31.Hatami M, Mehrjardi NZ, Kiani S, et al. Human embryonic stem cell-derived neural precursor transplants in collagen scaffolds promote recovery in injured rat spinal cord. Cytotherapy 2009; 11: 618–630. [DOI] [PubMed] [Google Scholar]
- 32.Sharp J, Frame J, Siegenthaler M, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 2010; 28: 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005; 25: 4694–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shroff G, Agarwal P, Mishra A, et al. Human embryonic stem cells in treatment of spinal cord injury: A prospective study. J Neurol Res 2015; 5: 213–220. [Google Scholar]
- 35.Kota SK, Gayatri K, Jammula S, et al. Fetal endocrinology. Indian J Endocrinol Metab 2013; 17: 568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]