Abstract
Myotonic dystrophy type 1 (DM1) is a progressive multisystemic disease with common cognitive deficits and potential brain involvement in addition to the cardinal muscular and systemic symptoms. Impaired mental function associated with nonspecific pathological findings such as white-matter hyperintense lesions (WMHLs), ventricular enlargement and brain atrophy on brain MRI have been previously reported in DM1 patients. While some studies showed correlation of brain morphological changes with neuropsychological and clinical parameters including CTG repeat sizes and disease severity scales in DM1, others failed. The goal of this study was to retrospectively investigate cranial MR abnormalities, predominantly WMHLs, and their effects on clinical and cognitive deficits in a small, phenotypically or genotypically well-characterized cohort of DM1 patients.
Keywords: Cranial MRI, myotonic dystrophy, white-matter hyperintense lesions
Introduction
Myotonic dystrophy type 1 (DM1) is a progressive multisystemic disease caused by a trinucleotide (CTG) expansion in the 3′-untranslated region of the dystrophia myotonica protein kinase gene on chromosome 19.1 The range of CTG repeat numbers in myotonic dystrophy type 1 is 50–4000, in which repeat sizes of 50–80 may be associated with mild clinical phenotypes and large repeat expansions up to 4000 are often found in severe, mostly congenital forms of the disorder. It is characterized by common cognitive deficits and potential brain involvement in addition to the cardinal muscular and systemic symptoms. Daytime sleepiness, fatigue, executive and visuospatial dysfunctions and anxious personality traits (deteriorating with age) are among the common manifestations in patients with the classic adult (aDM1, age at onset ≥20 years) or juvenile (jDM1, age at onset <20 years) forms of DM1.2 The pathogenesis of central nervous system (CNS) symptoms is not entirely clear. Neurofibrillary degeneration with intraneuronal accumulation of abnormally modified microtubuli-associated tau protein has been demonstrated in DM1 brains connecting this disorder to a subset of neurodegenerative diseases.3–5
Brain involvement in DM1 has been demonstrated in vivo using different neuroimaging techniques. In these patients, routine brain magnetic resonance imaging (MRI) often shows nonspecific pathological findings such as white-matter hyperintensities (WMHs), ventricular enlargement and brain atrophy.6–8 The effect on WM was quantified either by morphometric techniques (brain volumetry or voxel-based morphometry (VBM)) or assessed nerve fiber integrity using diffusion-tensor imaging (DTI) and tractography.9,10 The correlations of brain morphological changes with clinical and cognitive features in DM1 have been assessed by several reports with conflicting results. Disconnection of cortical regions by changes of the interconnecting WM is a potential mechanism for cognitive dysfunction in various neurological disorders and may also be responsible for CNS symptoms in myotonic dystrophies.11–13
The goal of this study was to investigate WM abnormalities and their effects on clinical and cognitive deficits in a small, phenotypically and genotypically well-characterized cohort of DM1 patients.
Materials and methods
A total of 13 DM1 patients attending our Neuromuscular Outpatient Clinics were enrolled in the study and their data were evaluated retrospectively. The diagnosis of DM1 was based on clinical examination, electromyography (EMG) findings and Southern blot analysis showing the CTG repeat expansion on chromosome 19. Patients with history of congenital DM, traumatic brain injury history, intracranial mass, other major medical, neurological or psychiatric illnesses, and/or any contraindication to MRI were excluded from the study. MRI scans from three patients were excluded because of movement artifacts and lack of medical records. Therefore, 10 DM1 patients were included in the analysis. Neurological evaluation consisted of history collection and assessment of muscle involvement using the Muscular Impairment Rating Scale (MIRS).14
Genetic assessment performed during outpatient clinic follow-up consisted of molecular analysis of CTG triplet repeat expansions including small-pool polymerase chain reaction (PCR) analysis using serial dilutions of EcoR1 digested genomic DNA (50–500 pg). EMG examination of all patients demonstrated myotonic discharges and myopathic changes. MRI scans were acquired using a 3 T system. All MRI post-processing was performed by observers blinded to subject’s identity. A comprehensive analysis of WMHLs using a visual scale (Wahlund and age-related white-matter changes (ARWMC) scale)15 and brain atrophy was performed. WM lesion (≥5 mm) quantifications on T2-images consisted of grading within five regions, separately for each hemisphere, ranging from 0 (no lesions) to 3 (diffuse involvement of the entire region). Cerebral atrophy was evaluated relative to age. Brain atrophy was rated as follows: no, mild, moderate and severe.
Cognitive functions were explored by an experienced neuropsychologist blinded to MRI results. Tests included a screening test for cognitive impairment (Mini-Mental State Examination, MMSE), global cognitive status with the Montreal Cognitive Assessment (MoCA) and handedness survey based on the Edinburgh Handedness Inventory (EHI). Memory with Wechsler-Adult Intelligence Scale (WAIS) and Buschke Selective Reminding test, attention, visuospatial abilities, phonemic and semantic fluency tests were also performed. Frontal Assessment Battery included five-word test, Stroop, and clock-drawing test. The full battery is shown in Table 1. The full battery was applied to nine of the patients. Results were considered abnormal if participants performed at least 1.0 standard deviation below the normative mean score of the healthy population according to the appropriate references. The Number Cruncher Statistical System (NCSS) 2007 Statistical Software (NCSS LLC, Kaysville, UT, USA) program was used for statistical evaluation. Data were analyzed using descriptive statistical methods (mean, standard deviation, median, frequency and percentage). The comparison between groups of quantitative data not showing normal distribution was carried out using Mann–Whitney U test and for the evaluation of their relationship Spearman’s correlation analysis was used. Fisher’s exact test was used for the comparison of qualitative data. Results were evaluated at 95% confidence intervals and a p < 0.05 level was significant.
Table 1.
Full cognitive functions assessment battery.
| COGNITIVE FUNCTION | SCREENING TEST |
|---|---|
| Handedness survey | Edinburgh Handedness Inventory |
| Global cognitive impairment | • MMSE • MoCA |
| Verbal attention and capacity to proceed attention and working memory | Digit Span Test |
| Visual attention and capacity to proceed attention and working memory | Corsi Block Test |
| Verbal memory | Buschke Selective Reminding test |
| Nonverbal memory | WMS visual memory subtest |
| Visuospatial and construction abilities | Cube drawing test |
| Language functions | • Spontaneous talking • Modified BNT • Writing • Reading |
| Frontal assessment battery | • Clock-drawing test • Stroop Test • Verbal fluency tests • (Animal-COWAT test) |
| High cortical functions | • KMDT Subtest • WAIS Perceptual Reasoning Subtest • WAIS Processing Subtest |
WMS: Wechsler Memory Scale; Boston Naming Test; COWAT: Controlled Oral Word Association Test; KMDT: Key Math Diagnostic Test; WAIS: Wechsler Adult Intelligence Scale.
Results
The age range of 10 (seven male/three female) patients was 19–57 years (37.30 ± 14.47). Patients’ clinical data are shown in Table 2. EMG showed myotonic and myopathic motor unit potentials (MUPs) in all patients. Mild cognitive impairment was detected in 72% of the patients. Minimal impairment in the learning and recall phases of verbal memory was also detected. Nonverbal memory impairment was mild especially in recording and recall phases. Visual-spatial/visual-constructive abilities were mildly impaired in three of the patients. With respect to evaluation of language functions, mild dysarthric speech was detected in three patients and impairment in writing skills in one patient. The Frontal Assessment Battery showed impairment in the ability to maintain attention and working memory, difficulty suppressing inappropriate responses, and reduction in categorical and lexical fluency (lexical fluency damage was more pronounced than categorical fluency). MoCA scores ranged between 24 and 30 and the average was 28.10 ± 1.85. Table 3 shows the detailed score of the neuropsychological tests. WMHLs were observed in 40% (n: 4) of the patients (Figure 1). Wahlund score of all patients with WMHLs and the subscore according to the anatomical distribution is shown in Table 4. Wahlund score ranged between 0 to 3 and the average score was 0.70 ± 1.05. A total of five patients did not show brain atrophy (50%). Mild brain atrophy was detected in three (n: 4; 40%) and moderate brain atrophy in one (n: 1; 10%) patient, respectively. When evaluating the relationship between the presence of WMHLs to age, gender and MoCA scores, a statistically significant difference was not found (p = 0.285; p = 0.324; p = 1.000) (Table 5). Mild cognitive impairment was prominent particularly in patients with temporal involvement. A negative correlation (19.4%) that was not statistically significant (r = –0.194; p = 0.592) was found between Wahlund and MoCA score. Also, a positive correlation that was not statistically significant (r = 0.216; p = 0.549) was detected between Wahlund score and brain atrophy (Table 6).
Table 2.
Main demographic and clinical data.
| PATIENTS | |
|---|---|
| Age at MRI RI | 43 ± 8.1 |
| CTG repeat length (range) | 800–1000 |
| Sex (M/F) | 11/2 |
| Duration of disease (years) | 15.6 ± 5.3 |
| Severity of disease (Muscular Impairment Rating Scale) | 3.4 ± 0.2 |
MRI: magnetic resonance imaging; CTG: cytosine-thymine-guanine; M: male; F: female.
Table 3.
The total scores of the detailed neuropsychological tests battery.
| SCREENING TEST | DM1 patients Avg ± SD |
|---|---|
| • MMSE | 28 ± 2.9 |
| • MoCA | 28.1 ± 1.8 |
| Digit Span Test | 15.5 ± 0.4 |
| Buschke Selective Reminding test | 57.2 ± 10.1 |
| WMS visual memory subtest | 11.3 ± 3.4 |
| Cube drawing test | 4.1 ± 1.3 |
| • Spontaneous talking | Normal: 7 Mild dysarthria: 3 |
| • Modified BNT | 7 |
| • Writing | Writing:9/1 |
| • Reading | Reading: 10 |
| • Clock-drawing test | 11.8 ± 1.7 |
| • Stroop Test | 2.2 ± 2.4 |
| • Verbal fluency tests | 17.3 ± 5.9 |
| • (Animal-COWAT test) | 19.1 ± 2.9 |
DM1: myotonic dystrophy type 1; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; WMS: Wechsler Memory Scale; Boston Naming Test; COWAT: Controlled Oral Word Association Test.
Figure 1.
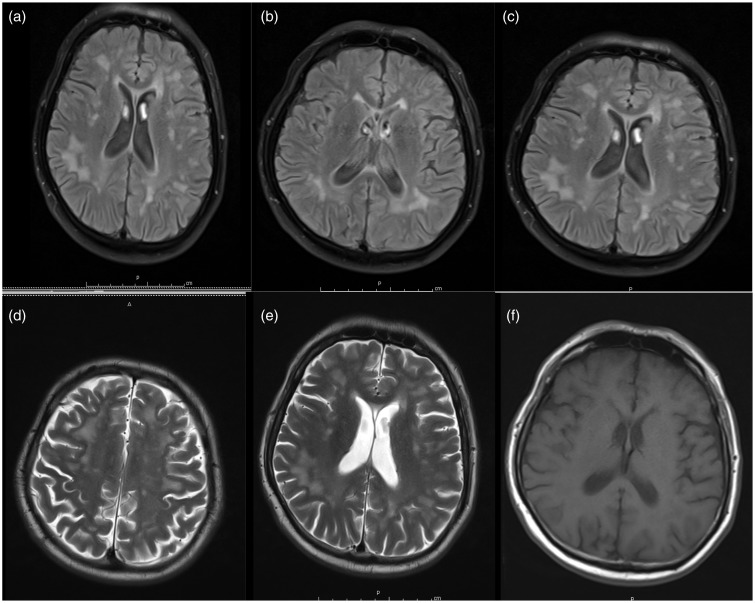
Axial brain magnetic resonance images of a 50-year-old myotonic dystrophy type 1 (DM1) patient. (a)–(c) White-matter hyperintense lesions (WMHLs) were bilaterally detected in both subcortical and periventricular areas, single or confluent on fluid-attenuated inversion recovery. (d) and (e) Areas of high signal in the subcortical white matter of the anterior temporal lobes (anterior temporal WMHLs) in T2-weighted sequences and (f) T1-weighted sequences.
Table 4.
WMHLs rated according the Wahlund scale.
| PATIENTS | |
|---|---|
| Frontal subscore | 1.16 ± 1.89 |
| Parietal subscore | 1.2 ± 1.5 |
| Temporal subscore | 0.8 ± 0.7 |
| Infratentorial subscore | 0 |
WMHLs: white-matter hyperintense lesions.
Table 5.
The relationship between the presence of WMHLs to age and MoCA.
| MR lesion (+) | MR lesion (–) | a p | ||
|---|---|---|---|---|
| Age | Min–Max (median) | 27–57 (47) | 19–51 (40) | 0.285 |
| Avg ± SD | 44.50 ± 12.61 | 32.50 ± 14.58 | ||
| MoCA Score | Min–Max (median) | 24–30 (27.50) | 27–30 (28.50) | 0.324 |
| Avg ± SD | 27.25 ± 2.50 | 28.67 ± 1.21 | ||
|
n (%) |
n (%) | b p | ||
| Gender | Female | 1 (25.0) | 1 (16.7) | 1.000 |
| Male | 5 (75.0) | 3 (83.3) | ||
Mann–Whitney U test. bFisher’s exact test.
WMHLs: white-matter hyperintense lesions; MoCA: Montreal Cognitive Assessment; Min: minimum; Max: maximum.
Table 6.
The relationship between Wahlund score to MoCA score and brain atrophy.
| Wahlund score | ||
|---|---|---|
| MoCA score | r | −0.194 |
| p | 0.592 | |
|
n (%) |
||
| Brain atrophy | r | 0.216 |
| p | 0.549 | |
r: Spearman’s correlation. MoCA: Montreal Cognitive Assessment.
Discussion
The progressive decline in working capacity and physical activity in patients with DM1 has in general been attributed to the progression of muscular symptoms and respiratory insufficiency. So far there has been an attempt to demonstrate the clinical relevance of the abnormal neuropsychological test scores and neuroimaging findings in patients with DM1.
We present clinical, cognitive and cranial MRI data in a relatively small series of patients with noncongenital DM1. Our DM1 patients showed generally a moderate muscular impairment, impaired cognitive performance over a broad range of functions, including frontal, visuospatial, naming and memory abilities and WM abnormalities. The degree of cognitive impairment in classical aDM1 patients is greatly variable, and significant cognitive impairment is observed only in some patients. Symptoms such as mild depression and anxiety as well as deficits of visuospatial abilities, executive functions, reasoning and naming have been frequently reported in DM1 patients.16,17 We demonstrated WMHLs in DM1 patients mainly located in the anterior temporal, frontal, parieto-occipital and periventricular WM regions (Figure 1). Several MRI studies have shown that DM1 patients have WMHLs, which are generally more prominent in the frontal, parietal and temporal regions and increase with the progression of the disease.2,5–7,18,19 Qualitative scales were mostly used to evaluate the spatial distribution of WMHLs. In this study we provide a quantitative assessment of the anatomic location of WMHs using the Wahlund scale. These radiological changes may be explained by myelin alterations, changes in interaxonal water content and decreased magnetization transfer ratio of the normal-appearing WM observed in DM1 patients.20
Although based on a small number of patients, our data confirm selective progressive frontal lobe function (attentional) involvement. Several previous studies suggested a relationship between the extent of WMHLs and cognitive deficits in DM1 patients although other studies did not confirm these findings.3–7,21 In our DM1 patients, the severity of cognitive deficits was more prominent in patients with WM damage and in particular, WMHL burden was associated with memory, executive, reasoning, and visuospatial impairments. In aDM1 patients MRI findings have been mostly related to disease duration, CTG repeat expansion size, MIRS score, manual motor performance and facial muscle volumes but rarely with cognitive impairment.8,22 A correlation between WM damage and intelligence tests was reported in congenital and jDM1.23 These conflicting results concerning the correlation of structural brain abnormalities, clinical features and cognitive/behavioral findings can be attributed mainly to the high variability of the DM1 clinical presentation based on the somatic mosaicism of CTG repeats, but also to the small and heterogeneous cohorts of patients involved in previous reports including ours. The nature of CNS abnormalities revealed by MRI is still unclear: The ones located at the temporal poles seem to be characteristics of the disease, while others, small, diffuse WMHLs suggest findings similar to the age-related alterations.
The retrospective design of our study does not allow concluding on the neurodegenerative and/or developmental origin of the brain abnormalities observed in DM1. Still, the exploratory nature of the performed neuropsychological tests and WM lesions analyses provide data that support the hypothesis of a degenerative process present in DM1 patients. The lack of a control group and the relatively small number of the included patients constitute limitations in this study.
Conclusion
Overall, these results suggest that DM1 is characterized by brain involvement, and WMHLs may be responsible for the global intellectual dysfunctions in aDM1 patients. Studies including larger number of patients may shed light on the different mechanisms underlying the brain involvement and their relationship to the cognitive impairment in aDM1.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Turner C, Hilton-Jones D. The myotonic dystrophies: Diagnosis and management. J Neurol Neurosurg Psychiatry 2010; 81: 358–367. [DOI] [PubMed] [Google Scholar]
- 2.Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve 2007; 36: 294–306. [DOI] [PubMed] [Google Scholar]
- 3.Vermersch P, Sergeant N, Ruchoux MM, et al. Specific tau variants in the brains of patients with myotonic dystrophy. Neurology 1996; 47: 711–717. [DOI] [PubMed] [Google Scholar]
- 4.Sergeant N, Sablonniere B, Schraen-Maschke S, et al. Dysregulation of human brain microtubule-associated tau mRNA maturation in myotonic dystrophy type 1. Hum Mol Genet 2001; 10: 2143–2155. [DOI] [PubMed] [Google Scholar]
- 5.Itoh K, Mitani M, Kawamoto K, et al. Neuropathology does not correlate with regional differences in the extent of expansion of CTG repeats in the brain with myotonic dystrophy type 1. Acta Histochem Cytochem 2010; 43: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber SJ, Kissel JT, Shuttleworth EC, et al. Magnetic resonance imaging and clinical correlates of intellectual impairment in myotonic dystrophy. Arch Neurol 1989; 46: 536–540. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann G, Damian MS, Koch M, et al. The clinical and genetic correlates of MRI findings in myotonic dystrophy. Neuroradiology 1996; 38: 629–635. [DOI] [PubMed] [Google Scholar]
- 8.Romeo V, Pegoraro E, Ferrati C, et al. Brain involvement in myotonic dystrophies: Neuroimaging and neuropsychological comparative study in DM1 and DM2. J Neurol 2010; 257: 1246–1255. [DOI] [PubMed] [Google Scholar]
- 9.Ota M, Sato N, Ohya Y, et al. Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neurosci Lett 2006; 407: 234–239. [DOI] [PubMed] [Google Scholar]
- 10.Wozniak JR, Mueller BA, Lim KO, et al. Tractography reveals diffuse white matter abnormalities in myotonic dystrophy type 1. J Neurol Sci 2014; 341: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009; 132: 239–249. [DOI] [PubMed] [Google Scholar]
- 12.Minnerop M, Weber B, Schoene-Bake JC, et al. The brain in myotonic dystrophy 1 and 2: Evidence for a predominant white matter disease. Brain 2011; 134(Pt 12): 3530–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caso F, Agosta F, Peric S, et al. Cognitive impairment in myotonic dystrophy type 1 is associated with white matter damage. PloS One 2014; 9: e104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathieu J, Boivin H, Meunier D, et al. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology 2001; 56: 336–340. [DOI] [PubMed] [Google Scholar]
- 15.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 16.Abe K, Fujimura H, Toyooka K, et al. Involvement of the central nervous system in myotonic dystrophy. J Neurol Sci 1994; 127: 179–185. [DOI] [PubMed] [Google Scholar]
- 17.Meola G, Sansone V, Perani D, et al. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2). Neuromuscul Disord 2003; 13: 813–821. [DOI] [PubMed] [Google Scholar]
- 18.Ogata A, Terae S, Fujita M, et al. Anterior temporal white matter lesions in myotonic dystrophy with intellectual impairment: An MRI and neuropathological study. Neuroradiology 1998; 40: 411–415. [DOI] [PubMed] [Google Scholar]
- 19.Di Costanzo A, Di Salle F, Santoro L, et al. Pattern and significance of white matter abnormalities in myotonic dystrophy type 1: An MRI study. J Neurol 2002; 249: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 20.Naka H, Imon Y, Ohshita T, et al. Magnetization transfer measurements of cerebral white matter in patients with myotonic dystrophy. J Neurol Sci 2002; 193: 111–116. [DOI] [PubMed] [Google Scholar]
- 21.Romeo V, Pegoraro E, Ferrati C, et al. Brain involvement in myotonic dystrophies: Neuroimaging and neuropsychological comparative study in DM1 and DM2. J Neurol 2010; 257: 1246–1255. [DOI] [PubMed] [Google Scholar]
- 22.Franc DT, Muetzel RL, Robinson PR, et al. Cerebral and muscle MRI abnormalities in myotonic dystrophy. Neuromuscul Disord 2012; 22: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wozniak JR, Mueller BA, Ward EE, et al. White matter abnormalities and neurocognitive correlates in children and adolescents with myotonic dystrophy type 1: A diffusion tensor imaging study. Neuromuscul Disord 2011; 21: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]