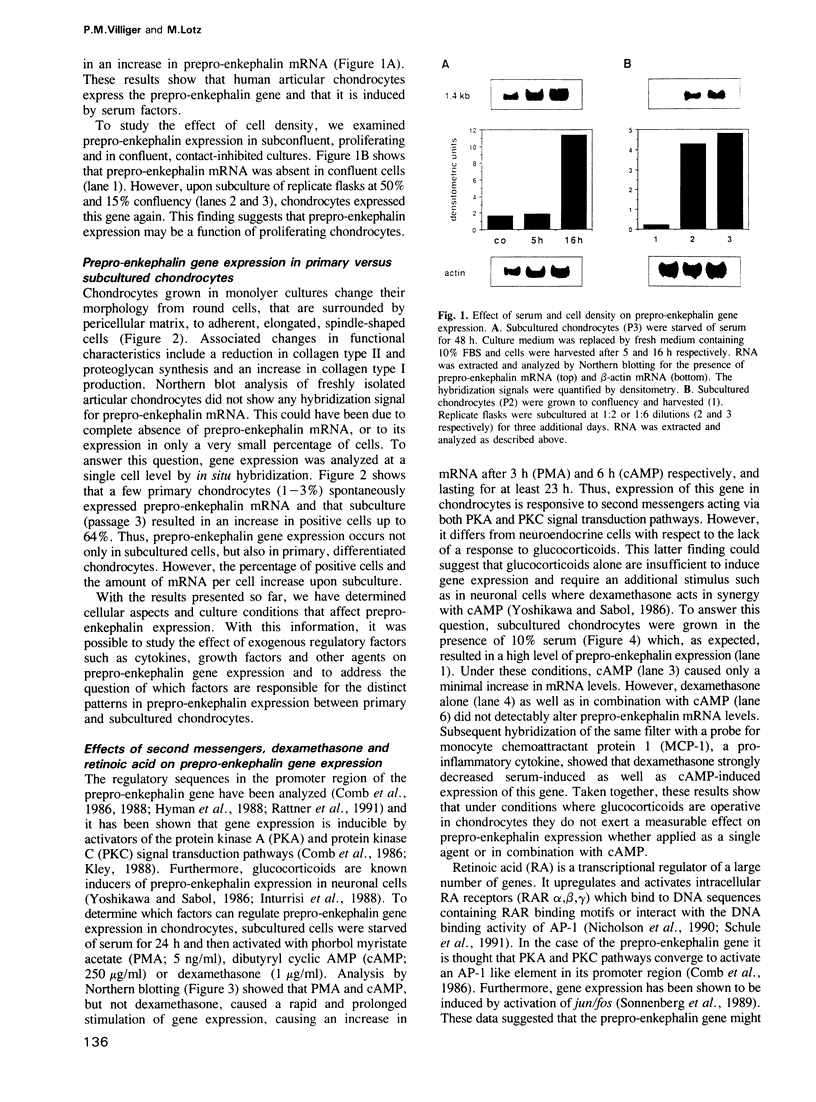
Abstract
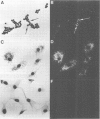
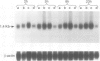
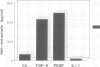
This study shows that cultured human articular chondrocytes express high levels of 1.4 kb prepro-enkephalin mRNA. Chondrocytes store met-enkephalin intracellularly and secrete this neuropeptide in mature as well as in precursor form. Gene expression is inducible by serum factors. High levels of prepro-enkephalin mRNA are detected in proliferating chondrocytes but not in confluent, contact-inhibited cells. Phorbol myristate acetate and dibutyryl cyclic AMP, but not dexamethasone, increase levels of prepro-enkephalin mRNA. Furthermore, transforming growth factor beta (TGF beta) and platelet derived growth factor (PDGF) upregulate gene expression, whereas retinoic acid, which inhibits chondrocyte proliferation, suppresses both basal and induced gene expression. Using in situ hybridization it is shown that only 1-3% of primary chondrocytes express prepro-enkephalin mRNA, whereas 52 +/- 12% of subcultured cells are strongly positive. Analysis of DNA synthesis, by autoradiography of incorporated [3H]thymidine, shows that these numbers correspond to the percentage of cells in S-phase of the cell cycle. In cultures of primary chondrocytes TGF beta promotes the formation of cartilage nodules and stimulates proliferation of adherent cells. This is associated with high levels of prepro-enkephalin mRNA in proliferating cells but not in contact-inhibited cells in cartilage nodules. In contrast, formation of cartilage nodules, proliferation and the expression of enkephalin are suppressed by interleukin-1 beta. In summary, expression of prepro-enkephalin in human articular chondrocytes is differentially controlled by cartilage regulatory factors and closely associated with cell proliferation.
Full text
PDF
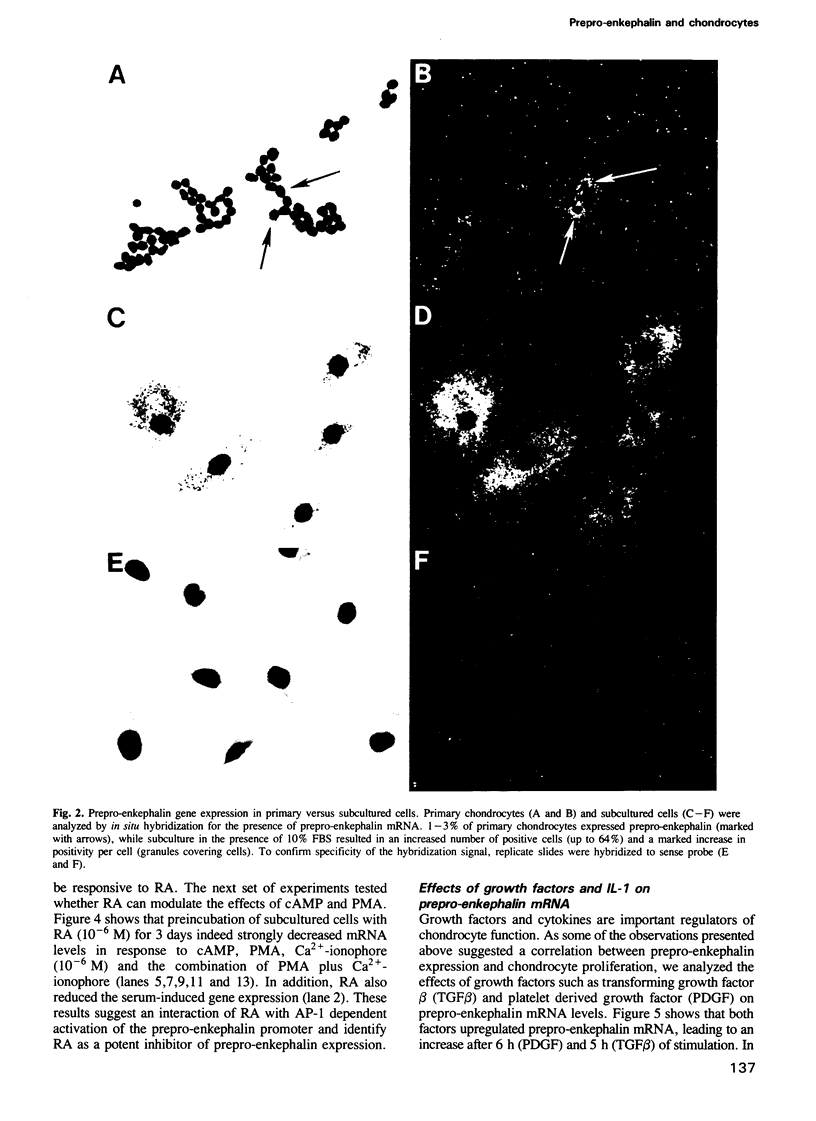
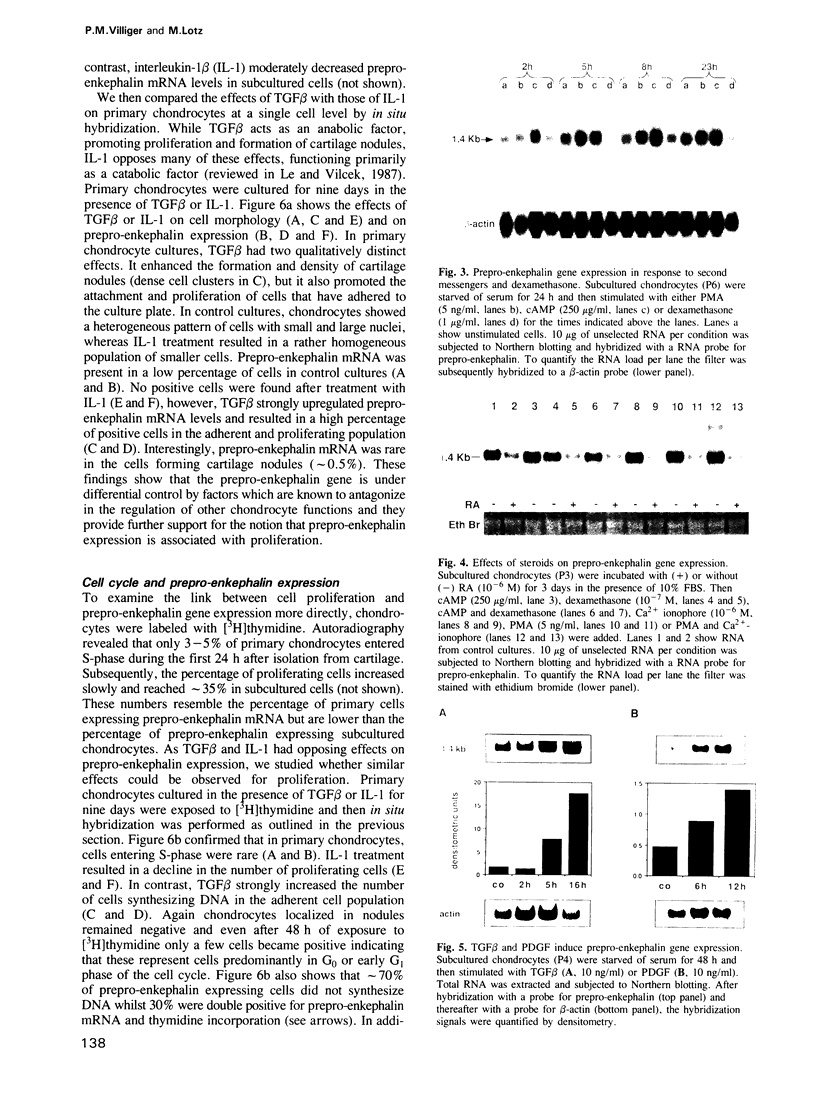

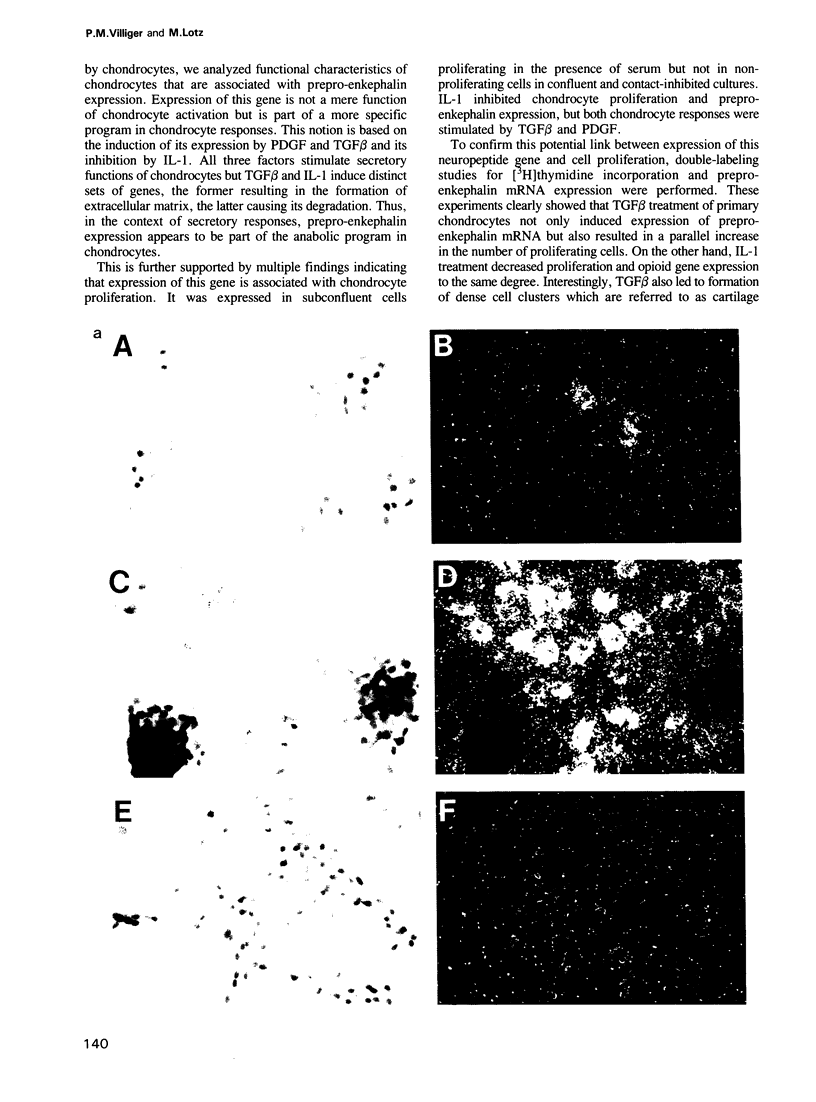
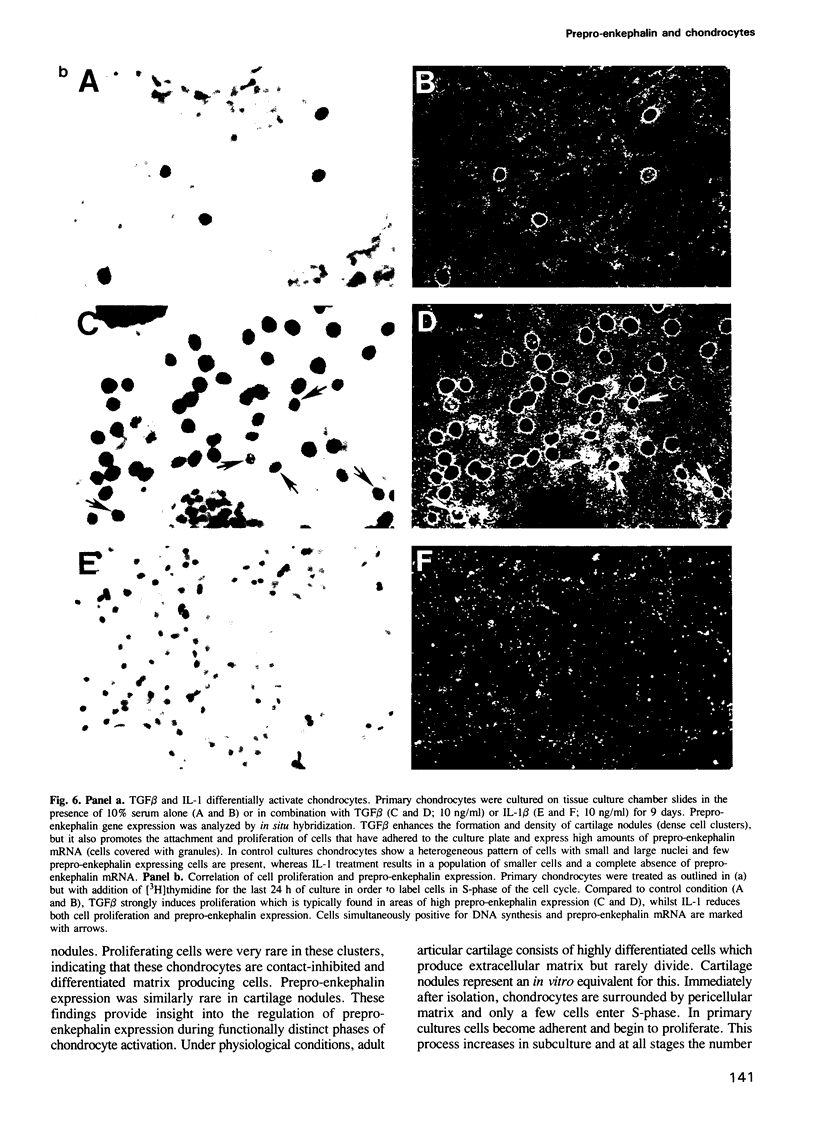


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos B., Lotan R. Retinoid-sensitive cells and cell lines. Methods Enzymol. 1990;190:217–225. doi: 10.1016/0076-6879(90)90026-w. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Comb M., Mermod N., Hyman S. E., Pearlberg J., Ross M. E., Goodman H. M. Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. EMBO J. 1988 Dec 1;7(12):3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas P., Burgos J., Baird A. Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem Biophys Res Commun. 1988 Oct 31;156(2):611–618. doi: 10.1016/s0006-291x(88)80887-8. [DOI] [PubMed] [Google Scholar]
- Eiden L. E. The enkephalin-containing cell: strategies for polypeptide synthesis and secretion throughout the neuroendocrine system. Cell Mol Neurobiol. 1987 Dec;7(4):339–352. doi: 10.1007/BF00733787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fóris G., Medgyesi G. A., Gyimesi E., Hauck M. Met-enkephalin induced alterations of macrophage functions. Mol Immunol. 1984 Aug;21(8):747–750. doi: 10.1016/0161-5890(84)90029-4. [DOI] [PubMed] [Google Scholar]
- Giguère V., Ong E. S., Evans R. M., Tabin C. J. Spatial and temporal expression of the retinoic acid receptor in the regenerating amphibian limb. Nature. 1989 Feb 9;337(6207):566–569. doi: 10.1038/337566a0. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Carson D. A., Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- Hauser K. F., Osborne J. G., Stiene-Martin A., Melner M. H. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990 Jul 9;522(2):347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagy W., Laurance M., Cohen E., Finberg R. Neurohormones regulate T cell function. J Exp Med. 1990 May 1;171(5):1625–1633. doi: 10.1084/jem.171.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucklebridge F. H., Hudspith B. N., Lydyard P. M., Brostoff J. Stimulation of human peripheral lymphocytes by methionine enkephalin and delta-selective opioid analogues. Immunopharmacology. 1990 Mar-Apr;19(2):87–91. doi: 10.1016/0162-3109(90)90043-e. [DOI] [PubMed] [Google Scholar]
- Hyman S. E., Comb M., Lin Y. S., Pearlberg J., Green M. R., Goodman H. M. A common trans-acting factor is involved in transcriptional regulation of neurotransmitter genes by cyclic AMP. Mol Cell Biol. 1988 Oct;8(10):4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi C. E., Branch A. D., Robertson H. D., Howells R. D., Franklin S. O., Shapiro J. R., Calvano S. E., Yoburn B. C. Glucocorticoid regulation of enkephalins in cultured rat adrenal medulla. Mol Endocrinol. 1988 Jul;2(7):633–640. doi: 10.1210/mend-2-7-633. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Smith E. M., Torres B. A., Blalock J. E. Regulation of the in vitro antibody response by neuroendocrine hormones. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4171–4174. doi: 10.1073/pnas.79.13.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Polakiewicz R. D., Itin A., Ornoy A., Rosen H. Proenkephalin A is expressed in mesodermal lineages during organogenesis. EMBO J. 1989 Oct;8(10):2917–2923. doi: 10.1002/j.1460-2075.1989.tb08441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kley N. Multiple regulation of proenkephalin gene expression by protein kinase C. J Biol Chem. 1988 Feb 5;263(4):2003–2008. [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Lynch D. R., Snyder S. H. Neuropeptides: multiple molecular forms, metabolic pathways, and receptors. Annu Rev Biochem. 1986;55:773–799. doi: 10.1146/annurev.bi.55.070186.004013. [DOI] [PubMed] [Google Scholar]
- Martin J., Prystowsky M. B., Angeletti R. H. Preproenkephalin mRNA in T-cells, macrophages, and mast cells. J Neurosci Res. 1987;18(1):82–87. doi: 10.1002/jnr.490180114. [DOI] [PubMed] [Google Scholar]
- Melner M. H., Low K. G., Allen R. G., Nielsen C. P., Young S. L., Saneto R. P. The regulation of proenkephalin expression in a distinct population of glial cells. EMBO J. 1990 Mar;9(3):791–796. doi: 10.1002/j.1460-2075.1990.tb08175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn N. A., Lum L. G. Immunoregulatory effects of alpha-endorphin, beta-endorphin, methionine-enkephalin, and adrenocorticotropic hormone on anti-tetanus toxoid antibody synthesis by human lymphocytes. Clin Immunol Immunopathol. 1989 Sep;52(3):376–385. doi: 10.1016/0090-1229(89)90152-9. [DOI] [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakiewicz R. D., Rosen H. Regulated expression of proenkephalin A during ontogenic development of mesenchymal derivative tissues. Mol Cell Biol. 1990 Feb;10(2):736–742. doi: 10.1128/mcb.10.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A., Korner M., Rosen H., Baeuerle P. A., Citri Y. Nuclear factor kappa B activates proenkephalin transcription in T lymphocytes. Mol Cell Biol. 1991 Feb;11(2):1017–1022. doi: 10.1128/mcb.11.2.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Itin A., Schiff R., Keshet E. Local regulation within the female reproductive system and upon embryonic implantation: identification of cells expressing proenkephalin A. Mol Endocrinol. 1990 Jan;4(1):146–154. doi: 10.1210/mend-4-1-146. [DOI] [PubMed] [Google Scholar]
- Rosen H., Polakiewicz R. D., Simantov R. Expression of proenkephalin A mRNA and enkephalin-containing peptides in cultured fibroblasts. Biochem Biophys Res Commun. 1990 Sep 14;171(2):722–728. doi: 10.1016/0006-291x(90)91206-8. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Umesono K., Mangelsdorf D. J., Bolado J., Pike J. W., Evans R. M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990 May 4;61(3):497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- Shinoda H., Marini A. M., Cosi C., Schwartz J. P. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science. 1989 Jul 28;245(4916):415–417. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Springhorn J. P., Claycomb W. C. Preproenkephalin mRNA expression in developing rat heart and in cultured ventricular cardiac muscle cells. Biochem J. 1989 Feb 15;258(1):73–78. doi: 10.1042/bj2580073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce B. A., Curtis R., Wilkin G. P., Glover D. M. A neuropeptide precursor in cerebellum: proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990 Jun;9(6):1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Hassan A. H., Przewłocki R., Gramsch C., Peter K., Herz A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5935–5939. doi: 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle C. A., Trice M. E., Ollivierre F., Awbrey B. J., Treadwell B. V. Regulation of cartilage remodeling by IL-1: evidence for autocrine synthesis of IL-1 by chondrocytes. J Rheumatol. 1987 May;14(Spec No):11–13. [PubMed] [Google Scholar]
- Verbeeck M. A., Draaijer M., Burbach J. P. Selective down-regulation of the pro-enkephalin gene during differentiation of a multiple neuropeptide-co-expressing cell line. J Biol Chem. 1990 Oct 25;265(30):18087–18090. [PubMed] [Google Scholar]
- Villiger P. M., Cronin M. T., Amenomori T., Wachsman W., Lotz M. IL-6 production by human T lymphocytes. Expression in HTLV-1-infected but not in normal T cells. J Immunol. 1991 Jan 15;146(2):550–559. [PubMed] [Google Scholar]
- Vindrola O., Padrós M. R., Sterin-Prync A., Ase A., Finkielman S., Nahmod V. Proenkephalin system in human polymorphonuclear cells. Production and release of a novel 1.0-kD peptide derived from synenkephalin. J Clin Invest. 1990 Aug;86(2):531–537. doi: 10.1172/JCI114740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Sabol S. L. Glucocorticoids and cyclic AMP synergistically regulate the abundance of preproenkephalin messenger RNA in neuroblastoma-glioma hybrid cells. Biochem Biophys Res Commun. 1986 Aug 29;139(1):1–10. doi: 10.1016/s0006-291x(86)80071-7. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987 May 26;412(1):68–72. doi: 10.1016/0006-8993(87)91440-5. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]
- van Epps D. E., Saland L. Beta-endorphin and met-enkephalin stimulate human peripheral blood mononuclear cell chemotaxis. J Immunol. 1984 Jun;132(6):3046–3053. [PubMed] [Google Scholar]