Abstract
Percutaneous kyphoplasty has a well-established role in the treatment of pathologic fractures in patients with multiple myeloma. Despite this, there is a scarcity of literature surrounding its use and efficacy in the sacrum. We present a case of successful symptom resolution in a patient with painful sacral fracture following sacroplasty, and review the existing literature.
An 81-year-man with multiple myeloma presented to the hematology/oncology clinic with a history of excruciating pain while seated. The impact of this pain on his quality of life subjectively was rated to be particularly high. Computed tomography of the sacrum confirmed the presence of pathologic fracture within the S1 and S2 vertebrae. Under fluoroscopic guidance, polymethyl methacrylate (PMMA) bone cement was injected via 11-gauge needles using an anterior-oblique approach. No immediate post-procedural complications occurred, such as foraminal extravasation or venous injection. The patient reported himself to be pain-free 1 day following the procedure, and this remains the case to date at 2 years of follow-up.
Sacroplasty is technically feasible and can provide durable relief of symptoms in patients with painful pathologic fractures of the sacrum. It is likely underused and can offer tremendous benefit to myeloma patients.
Keywords: Percutaneous, spine intervention, multiple myeloma, sacroplasty, pain management
Introduction
Pathological fractures identified on medical imaging aid in establishing the diagnosis of multiple myeloma. Currently, this diagnosis is often made late in disease progression and prognosis remains poor.1 The condition usually presents with bone pain that can lead to significant disability and impact on quality of life.2 Prolonged hospital stays and bed rest due to pain have resulted in increased medical care costs.3-6
Percutaneous kyphoplasty with the injection of polymethylmethacrylate (PMMA) has been shown to substantially and immediately alleviate pain associated with acute or subacute vertebral compression fractures.7-9 Excellent rates of improvement have been achieved in over 90% of patients using a variety of techniques in percutaneous verbroplasty.10 Likewise, intractable pain from osteoporotic sacral fractures has been successfully treated with percutaneous sacroplasty. The VAPOUR Trial recently examined vertebroplasty in the context of acute osteoporotic fractures with severe pain and demonstrated a statistically significant difference in pain relief versus a sham procedure in 120 patients.11 The application of percutaneous sacroplasty to control pain and facilitate rehabilitation in patients with severe and debilitating back pain secondary to sacral insufficiency fractures has been well described.12
The use of percutaneous kyphoplasty in the treatment of pathological fractures in patients with vertebral insufficiency fractures has also been well documented. However, vertebroplasty offers a safe intervention for both osteoporotic and neoplastic lesions in the spine, with a low incidence of side effects.13,14 However, there is a lack of literature on the use and efficacy of percutaneous sacroplasty in myeloma in particular. Here we report the successful application of percutaneous sacroplasty with symptom resolution in an elderly patient with severe pain from a sacral pathological fracture.
Case report
An 81-year-old man with multiple myeloma presented to the hematology/oncology clinic with a history of severe pain with sitting. Subjective pain assessment revealed a 5/10 pain (9/10 without analgesia) which was negatively impacting his quality of life. The pain had been preventing him from sitting normally, limiting him to 45 min at a time. For pain control, he had been taking hydromorphone extended release 3 mg twice a day. He felt constantly fatigued and would spend most of his day lying down in bed due to his pain. Plain radiographs of the sacrum confirmed the presence of pathologic fracture within the body of S2, later further characterized with computed tomography to reveal an S1 endplate fracture and severe osteopenia (Figure 1).
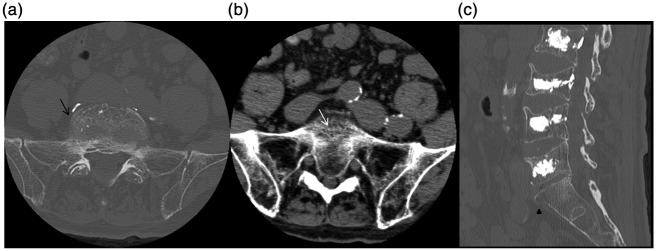
Figure 1.
(a) Axial bone-window algorithm CT demonstrates a concentric fracture pattern of the body of S1 (black arrows). (b) On the same presentation, the patient was found to have coexisting osteopenia of the bilateral sacral alae with multiple fine linear sacral body fractures (white arrow). (c) Sagittal reconstruction demonstrates an anterior endplate fracture.
The procedure was carried out under neuroleptic anesthesia. Intravenous antibiotics were administered prior to the procedure. The skin was cleaned and draped in a sterile manner. The injection was made at the level of the sacral body just below the L5/S1 facet joint. Local anesthetic solution consisting of 5 cc Xylocaine and Marcaine was used. Under fluoroscopic guidance, two 11-gauge M1M needles (Cook Medical, Bloomington, IN) were introduced into the S1 sacral alae bilaterally and advanced to the anterior third of the sacral body. PMMA cement, 8 ml per needle, was injected with excellent distribution across the alae without extravasation (Figures 2 and 3).
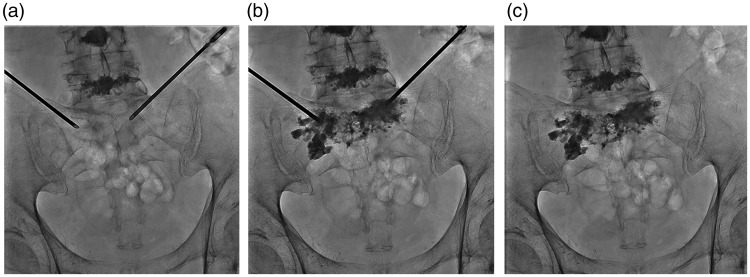
Figure 2.
(a) An anterior-oblique approach to the sacral body and alae simultaneously was selected, and (b) PMMA was injected into the anterior third of the sacral body for distribution within the body of S1 and S2. (c) post-injection fluoroscopy shows no extension towards the sacral canal, the neural foramina, or beyond the bone margins.
Figure 3.
(a) Left-lateral (LL) projection during the same procedure confirms the intended oblique approach anterior to the foramina, in line with the sacral alae. (b) Distribution of cement is confirmed within the areas of osteopenia seen on CT and (c) no venous or canal extravasation is seen on post-injection fluoroscopy.
There were no immediate complications. In follow-up 1 month post-sacroplasty, the patient was pain-free and able to sit comfortably. He has been able to resume weight-bearing, climbing stairs and reports he has comfortably returned to enjoying retired life at recent 2-year follow-up.
Discussion
It is occasionally underappreciated how crucial a role the sacral spine (consisting of the sacrum and coccyx) plays in sitting. Unlike the other sections of the vertebral column, the vertebrae found in those two regions are located between the hip bones. The body of a sacral vertebra differs from its lumbar, thoracic and cervical counterparts. The sacral vertebrae are generally fused in adults; and the upper half lateral surface forms a cartilaginous articulation with the ilium. Being a weight-bearing structure, sacral fractures cause pain that is exacerbated by sitting and may render such an essential resting position excruciating.15 The pain our patient experienced while sitting was debilitating. In the eyes of this patient, any palliation would have markedly improved his quality of life.
With its unique anatomical shape, the sacrum brings unique challenges to consideration of surgical intervention. Zhang et al.16 reported difficulties performing sacroplasty due to the challenge assessing needle depth and positioning as a result of the curved ventral cortical margin of the sacrum. This unique challenge potentially increases the risks of complications, such as cement migration and compression of sacral nerve roots. Other complications include bleeding, infection, transient radiculitis, nerve injury, dural puncture, local cement extravasation into the sacral canal or foramina, and cement embolus. Miller et al. recently investigated long and short-axis sacroplasty techniques in cadavers and found no difference in cement extravasation with either approach.17
Current standard of care for people with sacral fractures causing severe pain includes the administration of analgesics, prolonged bed rest, physical therapy and surgical interventions which often lengthen patients’ hospital stay, and often paints a grim prognosis due to higher mortality rate.18,19 Although not curative, sacroplasty has been shown to provide palliative pain relief. In a multicenter study the mean visual analog scale (VAS) score on a pain intensity score moved from 9.2 to 1.9 out of 10, in the pre-treatment and post-sacroplasty group, respectively.20 This subjective pain reduction, which represents a mean decrease of over 70% pain reduction on the VAS score, is seen in most patients post-intervention in studies. Frey et al.21 showed reduction from 8.1 to 0.8 in a 52-patient study, and Kamel et al.22 from 8 to 1.6 in a 19-patient study. In multiple myeloma patients, Basile et al. also demonstrated an improvement in Performance Status (PS) and reduced analgesic dose constant in follow-up.23
The literature on patients with history of multiple myeloma presenting with pathologic sacral fractures has been lacking. Recently, however, Kortman et al.20 sought to demonstrate the safety and efficacy of sacroplasty in multiple myeloma by performing a meta-analysis on 39 patients with focal mass or infiltrative sacral lesions, including 11 with multiple myeloma. Their findings included those of Basile et al.23 in a study of eight myeloma patients, two of which had only sacral lesions, those of Butler et al.24 with one patient presenting with bilateral myeloma involvement, and those of Wee and co-workers25 with one patient requiring large amount of opioids to manage his pain. The lasting pain relief in those patients is similar to our case, although our patient is relatively older: 81 years of age compared with an average of 68 years in the studies analyzed. This age difference may be significant, as our case provides further weight to the notion that advanced age should not be a contraindication to spinal intervention.26
Thirty years after the conception of percutaneous vertebroplasty in 1984, and 13 years after that of sacroplasty in 2002,27 the exact mechanism of pain relief remains unknown. The prevailing theory suggests that bone stabilization eases compression, while the exothermic reaction of the setting cement may damage penetrating nociceptors. Despite this unresolved question, sacroplasty has been successfully employed in treating pathologic sacral lesions. Recently, in their study of 57 patients with acute pain (less than 6 weeks) who underwent sacroplasty, Dougherty and co-workers28 found 34 patients had decreased opioid usage, 15 had unchanged usage and eight had increased usage in addition to their pain reduction. Although their follow-up time was relatively short—less than 3 weeks—their study further shows that sacroplasty represents a reasonable treatment option for patients suffering from pathologic sacral fractures.
Conclusion
Our case is a critical reminder that percutaneous sacroplasty can be used in elderly patients with sacral pathologic fractures in much the same way as vertebroplasty/kyphoplasty is employed in myeloma and sacroplasty in osteoporotic fracture. Relief was virtually instantaneous and allowed our elderly patient to sit down, thus improving his quality of life. The patient reported himself to be pain-free 1 day following the procedure, and this remains the case over 2 years post-procedure. A blinded trial akin to VAPOUR in elderly patients with painful pathologic fracture would help strengthen the quality of evidence supporting this valuable technique.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research did not receive any specific grant from a funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014; 28(5): 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78(1): 21–33. [DOI] [PubMed] [Google Scholar]
- 3.Cooper C, Atkinson EJ, O'Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: A population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 1992; 7(2): 221–227. [DOI] [PubMed] [Google Scholar]
- 4.Lyles KW, Gold DT, Shipp KM, et al. Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med 1993; 94(6): 595–601. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ., 3rd Epidemiology of spinal osteoporosis. Spine (Phila Pa 1976) 1997; 22(24 Suppl): 2s–11s. [DOI] [PubMed] [Google Scholar]
- 6.Ross PD, Davis JW, Epstein RS, et al. Pain and disability associated with new vertebral fractures and other spinal conditions. J Clin Epidemiol 1994; 47(3): 231–239. [DOI] [PubMed] [Google Scholar]
- 7.Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: Pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 2003; 226(2): 366–372. [DOI] [PubMed] [Google Scholar]
- 8.Grados F, Depriester C, Cayrolle G, et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000; 39(12): 1410–1414. [DOI] [PubMed] [Google Scholar]
- 9.Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: Technical aspects. AJNR Am J Neuroradiol 1997; 18(10): 1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BS, Hum B, Park JC, et al. Retrospective review of procedural parameters and outcomes of percutaneous vertebroplasty in 673 patients. Interv Neuroradiol 2014; 20(5): 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark W, Bird P, Diamond T, et al. Vertebroplasty for acute painful osteoporotic fractures (VAPOUR): Study protocol for a randomized controlled trial. Trials 2015; 16(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz AO, Brook AL. Sacroplasty. Techniques Vasc Interve Radiol 2009; 12(1): 51–63. [DOI] [PubMed] [Google Scholar]
- 13.Manfrè L. Vertebroplasty. Interv Neuroradiol 2003; 9(Suppl 2): 63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosanio G, Lavanga A, Vassallo P, et al. Vertebroplasty in the treatment of spine disease. Interv Neuroradiol 2005; 11(4): 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finiels H, Finiels PJ, Jacquot JM, et al. Fractures of the sacrum caused by bone insufficiency. Meta-analysis of 508 cases. Presse Med 1997; 26(33): 1568–1573. [PubMed] [Google Scholar]
- 16.Zhang J, Wu C-G, Gu Y-F, et al. Percutaneous sacroplasty for sacral metastatic tumors under fluoroscopic guidance only. Korean J Radiol 2008; 9(6): 572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JW, Diani A, Docsa S, et al. Sacroplasty procedural extravasation with high viscosity bone cement: Comparing the intraoperative long-axis versus short-axis techniques in osteoporotic cadavers. J Neurointerv Surg 2016; [epub ahead of print]. [DOI] [PubMed]
- 18.Pascal-Moussellard H, Broc G, Pointillart V, et al. Complications of vertebral metastasis surgery. Eur Spine J 1998; 7(6): 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol 1995; 13(9): 2330–2335. [DOI] [PubMed] [Google Scholar]
- 20.Kortman K, Ortiz O, Miller T, et al. Multicenter study to assess the efficacy and safety of sacroplasty in patients with osteoporotic sacral insufficiency fractures or pathologic sacral lesions. J Neurointerv Surg 2013; 5(5): 461–466. [DOI] [PubMed] [Google Scholar]
- 21.Frey ME, Depalma MJ, Cifu DX, et al. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: A prospective, multicenter, observational pilot study. Spine J 2008; 8(2): 367–373. [DOI] [PubMed] [Google Scholar]
- 22.Kamel EM, Binaghi S, Guntern D, et al. Outcome of long-axis percutaneous sacroplasty for the treatment of sacral insufficiency fractures. Eur Radiol 2009; 19(12): 3002–3007. [DOI] [PubMed] [Google Scholar]
- 23.Basile A, Tsetis D, Cavalli M, et al. Sacroplasty for local or massive localization of multiple myeloma. Cardiovasc Intervent Radiol 2010; 33(6): 1270–1277. [DOI] [PubMed] [Google Scholar]
- 24.Butler CL, Given CA, 2nd, Michel SJ, et al. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol 2005; 184(6): 1956–1959. [DOI] [PubMed] [Google Scholar]
- 25.Wee B, Shimal A, Stirling AJ, et al. CT-guided sacroplasty in advanced sacral destruction secondary to tumour infiltration. Clin Radiol 2008; 63(8): 906–912. [DOI] [PubMed] [Google Scholar]
- 26.Amelot A, Balabaud L, Choi D, et al. Surgery for metastatic spine tumors in the elderly. Advanced age is not a contraindication to surgery! Spine J 2015; [epub ahead of print]. [DOI] [PubMed]
- 27.Garant M. Sacroplasty: A new treatment for sacral insufficiency fracture. J Vasc Interv Radiol 2002; 13(12): 1265–1267. [DOI] [PubMed] [Google Scholar]
- 28.Dougherty RW, McDonald JS, Cho YW, et al. Percutaneous sacroplasty using CT guidance for pain palliation in sacral insufficiency fractures. J Neurointerv Surg 2014; 6(1): 57–60. [DOI] [PubMed] [Google Scholar]