Abstract
Patients with connective tissue diseases are thought to be at a higher risk for a number of cerebrovascular diseases such as intracranial aneurysms, dissections, and acute ischemic strokes. In this report, we aim to understand the prevalence and occurrences of such neurovascular manifestations in four heritable connective tissue disorders: Marfan syndrome, Ehlers-Danlos syndrome, Neurofibromatosis Type 1, and Loeys-Dietz syndrome. We discuss the fact that although there are various case studies reporting neurovascular findings in these connective tissue diseases, there is a general lack of case-control and prospective studies investigating the true prevalence of these findings in these patient populations. Furthermore, the differences observed in the manifestations and histology of such disease pathologies encourages future multi-center registries and studies in better characterizing the pathophysiology, prevalence, and ideal treatment options of neurovascular lesions in patents with connective tissue diseases.
Keywords: Ehlers–Danlos syndrome, Marfan syndrome, neurofibromatosis type 1 (NF1), Loeys–Dietz syndrome, intracranial aneurysm, dissection
Introduction
Patients with connective tissue diseases such as Marfan syndrome, Ehlers–Danlos syndrome (EDS), neurofibromatosis type 1 (NF1) and Loeys–Dietz syndrome (LDS) are thought to be at a higher risk for a number of cerebrovascular diseases such as intracranial aneurysms, dissections and acute ischemic strokes. The most commonly accepted causal explanation of this association is that genetic mutations involved in such connective-tissue diseases affect the collagen and proteoglycans that construct the extracellular matrix, thus resulting in the weakening of the vessel wall. Because of the purported higher prevalence of these neurovascular complications in patients with connective-tissue diseases, it is important for neurovascular specialists including neurologists, neurosurgeons, and neuroradiologists to be aware of the various cerebrovascular manifestations of these diseases. We present a comprehensive review of the neurovascular manifestations occurring in the most common inheritable connective-tissue diseases.
Description of diseases
Marfan syndrome
Marfan syndrome is the most commonly inherited connective-tissue disorder, with a reported prevalence of two or three per 10,000 individuals. An autosomal dominant mutation of the fibrillin 1 (FBN1) gene is the most frequent cause of Marfan syndrome. Sporadic de-novo mutations account for 25% of Marfan cases. Marfan syndrome has no racial or gender predisposition.1 Aortic root disease, mitral valve prolapse, long bones and joint laxity, arachnodactyly, and ectopia lentis are some of the more well-known classical presentations of Marfan syndrome.2 Currently, the revised Ghent nosology published in 2010 is used as the diagnostic criteria of Marfan syndrome, and places heavy emphasis on aortic root dilation/dissection, ectopia lentis, and genetic information.3
Once the diagnosis is made, the current management recommendations center around the use of beta blockers, restriction of vigorous physical exercise, routine and noninvasive monitoring of aortic diameter, and elective surgical repair of the aorta. There are currently no specific recommendations for screening of intracranial aneurysms and/or dissections.
The FBN1 mutation causes an alteration in the structural role of microfibrils in coordinating tissue morphogenesis, homeostasis, and response to hemodynamic stress.4 Histologically, fragmentation of lamellae, cystic medial necrosis (lacunar appearance of medial degeneration), fibrosis, and loss of smooth muscle cells are observed in the medial layer of the aortic root in patients with Marfan syndrome.5–9 The wide variation in histological appearance among Marfan patients warrants further investigation of the direct cause of their vascular abnormalities.8
EDS
EDS describes a group of disorders with shared primary characteristics of skin hyperextensibility, joint hypermobility, and tissue fragility. This disease is seen in approximately one out of 5000 individuals, with some types being rarer than others.10 Each of the six major types of EDS has unique genetic defects and inheritance patterns.10–12 The most commonly seen of these types are classic (EDS type I and II), hypermobility (EDS type III), and vascular (EDS type IV), with vascular EDS having the most prominent neurovascular manifestations.13 The diagnosis of EDS and its various types should be suspected when some combination of their shared features are present—joint hypermobility, multiple joint dislocations, translucent skin, poor wound healing, easy bruising, unusual scars, spontaneous ruptures of organs, and dissections of blood vessels. In addition, genetic testing for specific genes is available for all EDS types except for the hypermobility type. With the primary interest of our review being in the neurovascular manifestations of connective-tissue diseases, manifestations of vascular EDS (type IV) in the current literature will be emphasized.
Histological analysis of the aortic walls of patients with EDS IV show a broadened intima with fibrosis, abundant cholesterol crystals, and derangement of elastic fibers.14 Skin biopsies of EDS patients indicate collagen bundles that are thin and rare in the dermis and hypodermal septae. Under polarized light, such collagen bundles are less refringent than in normal skin.15 Type III collagen, mutated in EDS IV, is essential in providing structure and strength to connective tissue (skin, blood vessels, and internal organs) as a major component of the extracellular matrix. It is hypothesized that the poor assembly of these type III collagen fibers in EDS IV results in the neurovascular manifestations of EDS IV.
NF1
NF1, also known as von Recklinghausen disease, is the most common form of neurofibromatosis. It is an autosomal dominant mutation of the neurofibromin 1 (NF1) gene on chromosome 17 that results in the malformation of neurofibromin protein. NF1 affects one in every 2600 to 3000 individuals, with half of its presentation being familial, and the other half being de novo in nature.16,17 It is suggested that advanced paternal age increases the likelihood of de novo NF1.18 There is a typical order of appearance of its key clinical manifestations: café-au-lait macules, axillary/inguinal freckling, Lisch nodules, and neurofibroma tumors.19,20 Some other complications seen include osseous lesions, optic pathway glioma in children, childhood hypertension, and malignant transformations of tumors in adolescence and adulthood.21–23 The diagnosis for NF1 is clinical, and follows the diagnostic criteria set by the United States (US) National Institutes of Health (NIH) Consensus Conference. These diagnostic criteria are highly specific and sensitive, with 97% of patients meeting the diagnostic criteria by age 8.19 Because of this, genetic testing is not required, but can be helpful in the diagnosis of individuals who do not fully meet the diagnostic criteria.
NF1 vasculopathy is a significant but under-recognized complication of systemic NF1, and its pathogenesis is hypothesized to be due to the mutant neurofibromin protein expressed in the endothelial and smooth muscle cell layers of blood vessels.24,25 One study in rats determined the contribution of NF1 expression to NF1 vasculopathy by locating neurofibromin in blood-vessel structures.26 Neurofibromin was detected in the endothelial cell layer of rat cerebral vessels, renal arteries, and aorta. Neurofibromin was also detected in the smooth muscle layer specifically of the aorta, but not of the cerebral or renal vessels. Histologic features of affected NF1 patients include concentric intimal proliferation of spindle cells, marked proliferation of the intima with fibrous thickening, aneurysmal formation with irregular smooth muscle loss, and nodular proliferation of epithelioid and spindle cells. It is hypothesized that all of the above features are contributing factors to the neurovascular malformations seen in patients with NF1.
LDS
LDS is an autosomal-dominant connective-tissue disorder that stems from a variety of genetic alterations that all result in aortic aneurysms, generalized arterial tortuosity, hypertelorism, and bifid/broad uvular or cleft palate.27 The most widely understood mutations that are associated with LDS are transforming growth factor B receptor 1 (TGFBR1), transforming growth factor B receptor II (TGFBR2), decapentaplegic homolog 3 (SMAD2), and transforming growth factor B 2 ligand (TGFB2). All mutations show similar defective changes in the transforming growth factor-B (TGF-B) signaling cascade, and affected individuals show widespread arterial involvement with a proposed increase in risk of aneurysms and dissections.28–30 The diagnosis of LDS is clinical in nature, with the finding of aortic aneurysm and characteristic facial features being the most suggestive. However, the definitive diagnosis of LDS can be accomplished only through genetic testing.31
In the first description of LDS by Van Laer et al., an examination of aortic tissue demonstrated the following histologic features: fragmentation of elastic fibers, loss of elastin content, and accumulation of amorphous matrix components in the aortic media.30 Structural analysis showed a loss of intimate spatial association between elastin deposits and vascular smooth muscles and a marked excess of aortic wall collagen.32 Thus, although not specific, LDS aortic samples have significantly more diffuse medial degeneration than those of patients with Marfan syndrome.33 Genetically, an interesting phenomenon of increased TGF-B signaling is observed, despite the mutations in LDS being loss of function in nature. As there is no specific understanding of the pathogenesis of vascular malformations and aneurysms in patients with LDS, it is hypothesized that similar changes to the aorta are also seen in the neurovasculature.
Intracranial aneurysms in inheritable connective-tissue diseases
In review, the current hypotheses that exist for the neurovascular manifestations of these connective-tissue diseases differ slightly from one another. In Marfan syndrome, the medial smooth muscle cell layer of the vascular wall is histopathologically affected, with suggested causes being alterations of microfibrils and/or bioavailability of TGF-beta. In EDS, fibrosis of the tunica intima is observed, with the suggested cause being the lack of collagen type III, a fundamental feature of reticular fibers. In NF1, the deposition of neurofibromin in the endothelial cell layer of cerebral vessels, concentric intimal proliferation, and irregular smooth muscle loss of the medial vascular wall layer are attributed to vessel weakening. Finally, in LDS, the fragmentation of elastic fibers and diffuse medial degeneration of vasculature is hypothesized to be the cause of neurovascular malformation. Examples of cerebral aneurysms seen in connective-tissue disease patients are provided in Figure 1.
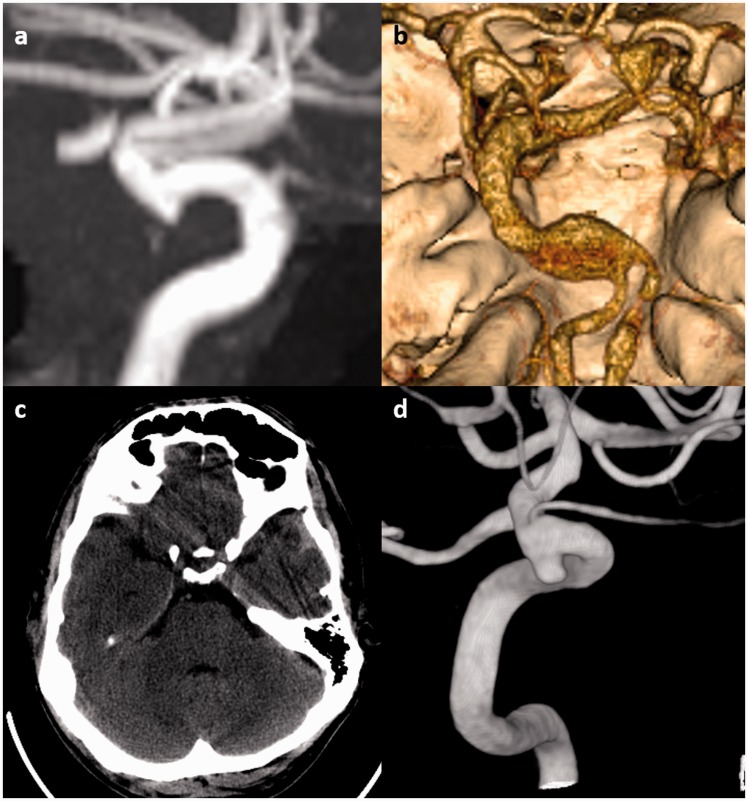
Figure 1.
Examples of intracranial aneurysms in connective-tissue diseases. (a) Incidental 3 mm supraclinoid internal carotid artery (ICA) aneurysm in a 64-year-old male Marfan syndrome patient. The aneurysm was managed conservatively. (b) An 8 mm dolichoectatic and fusiform aneurysm of the basilar artery in a 46-year-old female with Ehlers–Danlos syndrome. This aneurysm was managed conservatively because of the lack of good treatment options for this type of aneurysm in the setting of connective-tissue disease. (c) Non-contrast head computed tomography (CT) of a 50-year-old male with neurofibromatosis type 1 (NF1) shows a small amount of subarachnoid and subdural hemorrhage adjacent to the left temporal lobe. (d) Cerebral angiography demonstrates a 3 mm ruptured superior hypophyseal aneurysm that was effectively treated with stent-assisted coiling with no complications.
Marfan syndrome
A number of studies have been performed to examine the association between intracranial aneurysms and Marfan syndrome.34,35 Aneurysms in Marfan syndrome can be saccular, fusiform or dissecting. There is a propensity for aneurysms in Marfan syndrome to be located in the proximal intracranial carotid artery.36 A series of 10 clinical reports, one pathology case, and an autopsy series of seven patients suggested that individuals with Marfan syndrome may have an increased prevalence of intracranial aneurysms. However, there are many speculations that these reports did not clearly confirm clinical manifestations of Marfan, thus leading to the questioning of a true diagnosis of Marfan syndrome in these patients.37 One recently published study by Kim et al found that the prevalence of intracranial aneurysms among patients with Marfan was 14% (8/59).33 To date, no prospective intracranial aneurysm screening study has been performed among Marfan syndrome patients to determine the true incidence of intracranial aneurysms.
EDS
The potential relationship between collagen deficiencies in EDS and cerebral aneurysms has long been speculated, but not tested in the form of a prospective screening study.38,39 Intracranial aneurysms in EDS patients can be saccular or fusiform and are most commonly located in the cavernous sinus.36 Studies examining the prevalence of aneurysm and subarachnoid hemorrhage in EDS patients have yielded varying results. In a study of 419 patients with EDS or a family history of the disease, Pepin et al. found six patients who had intracranial aneurysms.40 In a study of 202 patients, North et al. noted that six patients had cavernous carotid fistulae (CCF), four had ruptured intracranial aneurysms and four had intracranial hemorrhage of uncertain etiology.41 However, not all patients in these studies received screening for intracranial aneurysms with imaging. The largest study to date on the prevalence of unruptured intracranial aneurysms among EDS patients with intracranial vascular imaging found a prevalence of 11%.33
NF1
There are varying reports regarding the prevalence of intracranial aneurysms in NF1 patients. One large study found that only one of 316 NF1 patients undergoing brain magnetic resonance imaging (MRI) had an intracranial aneurysm. It is important to note only eight of these 316 patients had magnetic resonance angiography (MRA) performed. Since MRI without MRA is not sensitive for the detection of intracranial aneurysms, it is likely that the prevalence of aneurysms in this population was higher than reported.24 A small autopsy study by Conway et al. found that none of the 25 patients diagnosed with NF1 had intracranial aneurysms on autopsy.42 On the contrary, a study conducted by Schievink et al. compared a sex- and age-matched control population with an NF1 population for the incidental detection of aneurysms with MRI brain examination. In this comparison of 39 NF1 patients to 526 control patients, the authors demonstrated a prevalence rate of 9% in NF1 patients versus 0% in the control group.43 Lastly, in a study of 47 patients with intracranial neurovascular imaging, Kim et al found an aneurysm prevalence of 11%.33 Ultimately, further studies are needed to confirm these findings.
LDS
There are only a few reports on the prevalence of intracranial aneurysms in LDS patients.44–48 In one neuroradiologic study conducted in 25 patients with positive genetic testing for LDS, eight (32%) had intracranial aneurysms, suggesting the importance of serial noninvasive imaging monitoring in younger patients.49 In another study of 25 patients with clinical or genetic diagnosis of LDS, Kim et al found that 28% of patients had an intracranial aneurysm.33 In the original study describing LDS, approximately 2% of the patients died of cerebral bleeding and 10% of patients had head or neck aneurysms.
Treatment and screening recommendations
In general, screening of intracranial aneurysms in these patient populations is not advised owing to the high risk of surgical or endovascular treatment of intracranial aneurysms in these patients. Incidentally detected unruptured aneurysms are generally managed conservatively because of the extremely friable nature of blood vessels in patients with connective-tissue diseases.
There are few series and case reports that describe surgical or endovascular treatment of intracranial aneurysms in patients with connective-tissue diseases. Among patients with Marfan syndrome, connective-tissue fragility is not a major problem in the surgical treatment of intracranial lesions; however, the extreme ectasia and tortuosity of the carotid and vertebral arteries can make endovascular treatment of these lesions difficult.36 Surgery in the setting of EDS is particularly challenging. In a small series of four EDS patients undergoing intracranial aneurysm surgery, Schievink et al. reported one patient who died as a direct result of the surgery and a high rate of postoperative complications including spontaneous pneumothorax and vertebral artery dissections.50 Surgical repair of aneurysms in NF1 can be complicated by the presence of intracranial arterial occlusive disease, excessive vascular fragility and anatomic distortion from sphenoid wing dysplasia.36 Some small studies and case reports suggest that despite the vessel fragility seen in LDS patients, surgical and endovascular management are safe, effective, and durable treatment modalities.46,48,51 To our knowledge, there are no systematic studies reporting outcomes of treatment of aneurysms in connective-tissue disease.
Dissections, pseudoaneurysms, and inheritable connective-tissue disease
Cervical carotid dissections
Marfan syndrome
The annual incidence rate for spontaneous internal carotid artery dissections in the general population is 1.72 per 100,000 individuals. However, owing to its asymptomatic nature, the true incidence may be higher.52,53 While no study has demonstrated an association between Marfan syndrome and cervical carotid dissection to date, Marfan syndrome patients are known to sometimes suffer from these dissections, both isolated to the cervical and intracranial vasculature as well as a result of extension of Stanford type A thoracic aortic dissection (Figures 2–4).54,55 The pathophysiology of cervical and intracranial dissections is similar to that of intracranial aneurysms. Pathological studies have found that a combination of intimal proliferation, medial degeneration, and fragmentation of the internal elastic lamina is responsible for dissections in these patients.56,57 Mucopolysaccharide deposition in the tunica media, fibromuscular dysplasia and cystic medial necrosis and focal fragmentation of the internal elastic lamina have also been suggested as potential causes.57,58 Pathology findings of dissections in EDS, NF1, and LDS are currently not reported.
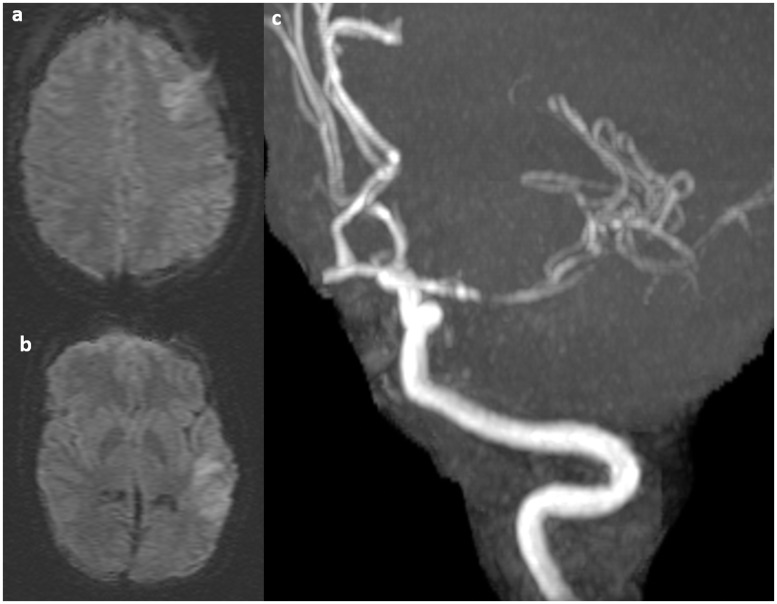
Figure 2.
A 43-year-old male with Marfan syndrome presented with infarctions in the left frontal and temporal lobes ((a) and (b)). Time of flight magnetic resonance angiography (MRA) shows a focal dissection of the left M1.
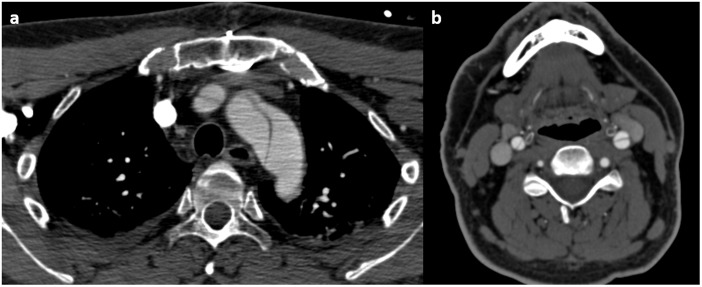
Figure 3.
A 52-year-old male with Marfan syndrome with Stanford type A dissection (a) extending into both common carotid arteries (b).
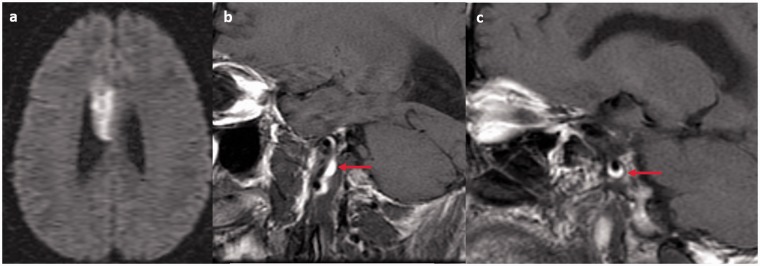
Figure 4.
A 36-year-old male with neurofibromatosis type 1 (NF1). (a) Diffusion-weighted imaging (DWI) images show an acute infarct in the right corpus callosum. ((b) and (c)) Sagittal T1-weighted images show a dissection of the distal cervical and petrous internal carotid artery with high T1 signal in the vessel wall consistent with intramural hematoma (red arrows).
EDS
While many case reports of cervical carotid and vertebral artery dissections have been reported in EDS patients, only a few studies have examined the incidence of dissection in this population.59–64 Brandt et al. conducted a study of skin biopsies on 65 patients with proven non-traumatic spontaneous cervical artery dissection (sCAD). Although only three patients had clinical manifestations of EDS, 36 patients had ultrastructural aberrations of the connective tissue commonly found in EDS II or EDS III, suggesting that CADs are associated with ultrastructural connective tissue abnormalities such as EDS.65 This study further supported the same association suggested in a smaller cohort of 25 cervical arterial dissection patients in which 17 (68%) patients had ultrastructural abnormalities associated with EDS II or III.66 Ulbricht et al. conducted a study of skin biopsies on seven consecutive patients with sCADs. In five of the seven patients (71%), histologic, immunohistochemical, and ultrastructural changes similar to EDS were found, again suggesting that sCADs are associated with dermal alterations seen in EDS.67
NF1
While no definite association between NF1 and extracranial dissections has been established, a number of case reports have been published on this topic.68–70 It is reasonable to assume that the vasculopathy resulting from NF1 could predispose these patients to cervical dissections.
LDS
In the neuroradiologic study by Rodrigues et al. of 25 patients with a positive genetic testing for LDS, three had dissections of the carotid and vertebrobasilar arteries. However, the exact number of an intracranial or extracranial nature is not described.49 Because LDS patients are prone to formation of aortic aneurysms and dissections, some cervical dissection cases can result from extension of a Stanford type A dissection, similar to the pattern seen in Marfan syndrome.71
Management of cervical dissections
Guidelines for management of patients with extracranial carotid and vertebral artery dissections were published by the American Heart Association in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography.72 The guideline first recommends that a confirmatory diagnosis of cervical dissection be made using contrast-enhanced computed tomography angiography (CTA), MRA, or catheter-based angiography. Once confirmed, the first-line treatment for cervical dissections associated with ischemic stroke or transient ischemic attack (TIA) centers around medical antithrombotic treatment with anticoagulation or antiplatelet therapy for at least three to six months. However, it is of interest to note a recently published randomized control study of 250 patients with carotid and vertebral artery dissections found no differences in efficacy of antiplatelet and anticoagulation drugs at preventing stroke and death in patients with symptomatic carotid and vertebral artery dissections.73 In general, invasive procedures such as carotid artery stenting are not advocated in the treatment of cervical dissections in the general population and would likely be even less warranted in the connective-tissue disease population because of the high risk of iatrogenic injury from friable vasculature. The effectiveness and safety of therapy with a-adrenergic antagonists, angiotensin inhibitor, or nondihydropyridine calcium channel antagonists to lower blood pressure are not well established.
Cervical pseudoaneurysms
Cervical pseudoaneurysms arise from a disruption within the arterial wall that allows blood to stream into the surrounding tissue to form a sac in communication with the arterial lumen, and generally result from dissections. Such dissections are primarily the result of neck trauma, iatrogenic vascular injury, or microvascular procedures in the general population. More often, it is after the dissection has healed that the pseudoaneurysm takes place between the tunica media and adventitia.
Several case reports have been published documenting the presence and treatment of cervical pseudoaneurysms in patients with inheritable connective-tissue diseases.74–77 In the general population, pseudoaneurysms of the extracranial carotid artery are very rare, and account for less than 1% of all arterial aneurysms. These are generally not prone to rupture because of the thick layer of adventitia seen in the cervical carotid vasculature. Conservative management with antiplatelet therapy and serial imaging is generally the preferred treatment modality; however, both surgical and endovascular intervention can be used in the treatment of high-risk cervical pseudoaneurysms.78 In general, the rupture risk of these pseudoaneurysms is very low. The main risks associated with these lesions are acute ischemic stroke and mass effect. Endovascular repair techniques reported include stenting with flow diversion or covered stents and/or coil embolization. Open surgical repair of these aneurysms through the use of interposition grafts has also been reported (Figure 5).
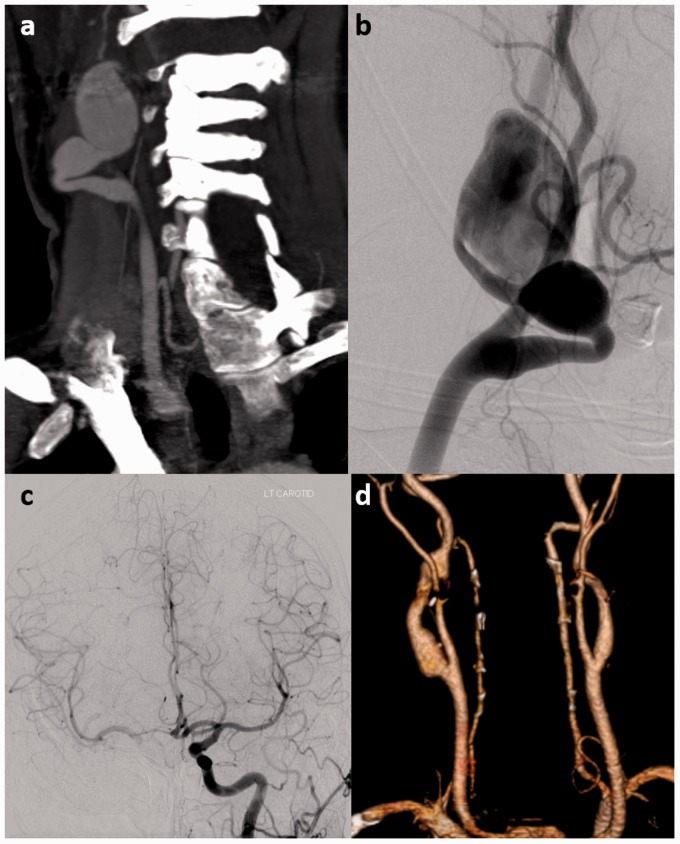
Figure 5.
A 38-year-old female with neurofibromatosis type 1 (NF1) presented with a large pulsatile mass in her right neck. (a) Computed tomography angiography (CTA) showed multiple pseudoaneurysms of the left internal carotid artery (ICA). (b) A cerebral angiogram was performed for balloon test occlusion prior to surgical repair of the pseudoaneurysms. The neck angiogram demonstrated the large ICA pseudoaneurysms. (c) Cerebral angiography with a balloon in the right ICA and left ICA injection shows filling of the right middle cerebral artery (MCA) and anterior cerebral artery (ACA) territories across the patient’s anterior communicating artery (Acom). (d) Surgical resection of the pseudoaneurysms with placement of a saphenous vein interposition graft was performed with no complications. Postoperative CTA shows patency of the saphenous vein graft.
Other neurovascular manifestations
NF1 and Moyamoya disease (MMD)
The occurrence of MMD in NF1 is rare, but encountered in the form of various case reports of MMD in pediatric populations.79–89 A retrospective analysis of 197 children with NF1, 168 of whom underwent cranial MRI, found that four (2.3%) were diagnosed with MMD. It is suggested that the neurofibromin in the endothelium and smooth muscle of the blood vessels in these patients may play an important role in the development of this vascular abnormality.24,90,91 MMD in pediatric patients with NF1 is usually unilateral and often involves anterior vascular territories.92 Although most patients are asymptomatic, subsequent clinical and radiologic worsening is likely to occur with events such as TIAs, infarcts, seizures with headache, and intracranial hemorrhage being the consequence.90,93,94 For this reason, the literature urges that although MMD may be generally rare in NF1, MRA or other imaging modalities be used because of MMD and its asymptomatic and progressive nature.91,95,96
A retrospective review of 39 patients with both NF1 and MMD, 32 of whom underwent surgical revascularization with pial synangiosis and 18 of whom had radiographic evidence of prior stroke at the time of MMD diagnosis, concluded that the clinical, radiographic, and angiographic features of MMD seen in the NF1 population are comparable to primary MMD.95 The authors of this study recommended treatment of children with MMD and NF1 with surgical revascularization. Not only is this method shown to be safe in NF1 patients, it is also protective against further ischemic and neurological damage, with a 27-fold reduction in stroke rate.97 In addition, the literature contains several cases of later-onset MMD in adult populations.98–104 An example of MMD associated with NF1 is provided in Figure 6.
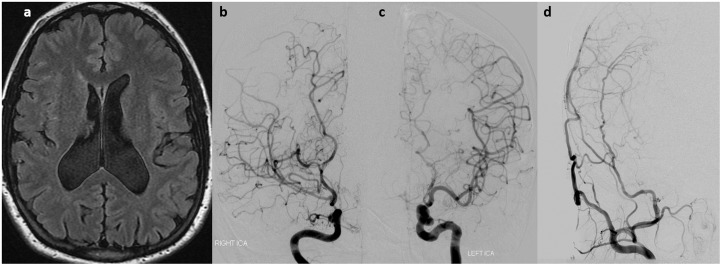
Figure 6.
A 23-year-old female with neurofibromatosis type 1 (NF1) presented with multiple episodes of left-sided hand symptoms thought to be related to opercular hypoperfusion. (a) Fluid-attenuated inversion recovery magnetic resonance imaging (FLAIR MRI) shows some high signal in the sulci of the right operculum and frontal lobe. (b) Right internal carotid artery (ICA) injection shows a high-grade stenosis of the supraclinoid right ICA and marked prominence of the middle cerebral artery (MCA) lenticulostriates. There is complete obliteration of the right anterior cerebral artery (ACA). (c) Left ICA injection shows mild narrowing of the supraclinoid left ICA and obliteration of the left A1. There is minimal, if any, prominence of the lenticulostriates on this side. (d) Postoperative cerebral angiogram following right superficial temporal artery to middle cerebral artery (STA-MCA) bypass shows excellent filling of the right MCA territory from the STA bypass.
A recent population-based, case controlled study addressed the risk of cerebrovascular events requiring hospitalization among patients with NF1.105 Of the 21,378 patients diagnosed with NF1 between 1998 and 2009 in this study, NF1 was associated with a younger mean age (41 versus 48) of strokes, despite a lower prevalence of stroke risk factors in adults. Although pediatric and adult NF1 patients were significantly more likely to be diagnosed with any stroke than the general population (1.2:1), the odds of ischemic stroke (3.4:1) and intracerebral hemorrhage among the hemorrhagic stroke group (8.1:1) were dramatically elevated in the pediatric population. In adults, the odds of intracerebral hemorrhage among hemorrhagic strokes (1.9:1) were also elevated. Thus, this study concluded that the likelihood of any type of stroke is significantly increased in NF1 patients compared to the general population.
NF1 and vertebro-vertebral fistulas
Although vascular involvement in the setting of NF1 is commonly reported, coexistence with vertebral arteriovenous fistulae (AVF) is rare. There are several case reports indicating AVF in NF1 patients.70,106–128 There are no systematic studies to date conducted on the prevalence and risk factors of AVF in NF1 patients. However, the association seems to be strong enough that every patient presenting with a rare vertebral-jugular fistula should be suspected to have NF1. These fistulae often form as a result of a vertebral artery pseudoaneurysm rupturing into the surrounding venous plexus. Fistulae between muscular branches of the vertebral artery and the surrounding venous plexus can also occur.
The primary method of treatment is endovascular embolization with coils or liquid embolic agents.112,113,117,120,121,129 A method that has shown lower success rates is the trapping of fistulas, owing to numerous potential feeding arteries that can complicate the procedure.108 In some more complicated cases, direct surgery, or a combination of surgery and endovascular embolization, may be necessary.111,116,119,123–127
EDS and Marfan syndrome and CCF
In a study conducted by Pepin et al., 44 out of the 419 patients with EDS IV had arterial complications of the central nervous system, with the most frequent being CCF.40,130 There are several case studies that present CCF in EDS,131–138 with some discussing difficulties and death associated with the treatment of EDS IV patients with extreme vessel fragility.130,139–149 CCF have also been reported in the setting of Marfan syndrome.
Because of the high risk associated with endovascular treatment of CCF in EDS IV patients, it is recommended that all spontaneous CCF cases be approached with the possibility of EDS IV in mind.150 Given the well-known vascular fragility of these patients, even a simple catheter angiography can lead to disastrous consequences in EDS IV151 and therefore should be considered only if endovascular therapy is considered. As an alternative to transfemoral endovascular treatment, the ipsilateral carotid artery and internal jugular vein can be surgically exposed for direct insertion of endovascular sheaths. This allows for a reduction in risks associated with arterial dissections that often accompany transfemoral access in EDS IV patients.133,135,143,152 Asymptomatic CCF can be managed conservatively through manual compression of the medial canthus (Figure 7).
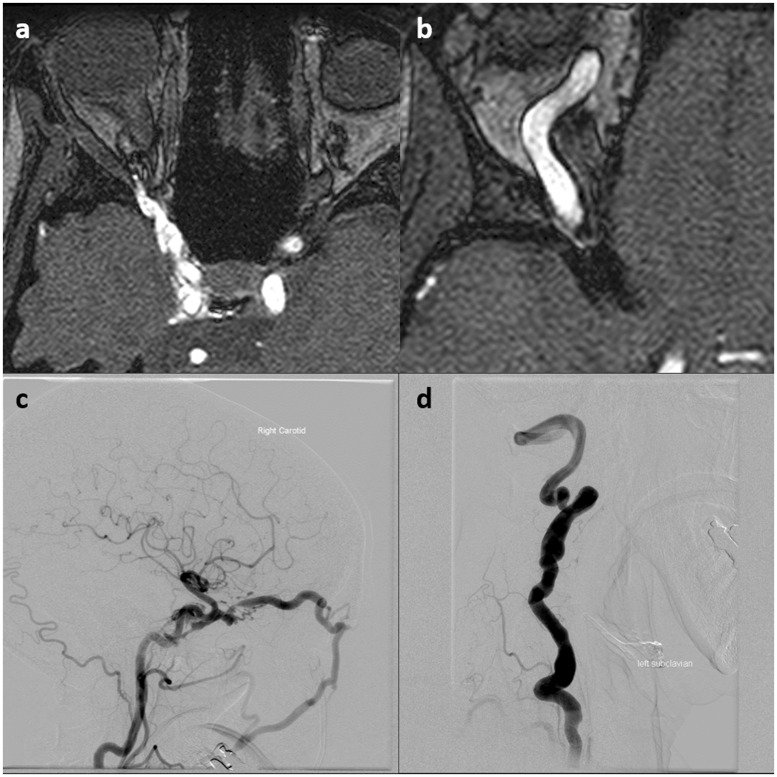
Figure 7.
Carotid-cavernous fistula (CCF) in a 47-year-old male with Marfan syndrome. ((a) and (b)) Time of flight magnetic resonance angiography (TOF MRA) shows high signal in the right cavernous sinus and marked enlargement of the superior ophthalmic vein, findings consistent with a CCF. (c) Right internal carotid artery (ICA) cerebral angiography shows a large direct CCF with early filling of the cavernous sinus and engorgement of the superior ophthalmic and facial veins. (d) Note the marked tortuosity and dolichoectasia of the left vertebral artery in this patient.
Conclusions
Although there are various case studies reporting neurovascular findings in connective-tissue diseases, there is a general lack of case control studies investigating the exact prevalence of these findings in these patient populations. Furthermore, little is known regarding the risks and benefits of various conservative, endovascular, and surgical management techniques in caring for patients with these lesions. Large multicenter registries may be helpful in the future in better characterizing the pathophysiology, prevalence, and ideal treatment options of neurovascular lesions in patients with connective-tissue diseases.
Acknowledgment
No portion of the contents of this paper has been presented previously.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366: 1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruno L, Tredici S, Mangiavacchi M, et al. Cardiac, skeletal, and ocular abnormalities in patients with Marfan’s syndrome and in their relatives. Comparison with the cardiac abnormalities in patients with kyphoscoliosis. Br Heart J 1984; 51: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010; 47: 476–485. [DOI] [PubMed] [Google Scholar]
- 4.Dietz HC, McIntosh I, Sakai LY, et al. Four novel FBN1 mutations: Significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics 1993; 17: 468–475. [DOI] [PubMed] [Google Scholar]
- 5.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: Implications for dissecting aortic aneurysm. Am J Cardiol 1977; 39: 13–20. [DOI] [PubMed] [Google Scholar]
- 6.Schlatmann TJ, Becker AE. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol 1977; 39: 21–26. [DOI] [PubMed] [Google Scholar]
- 7.Trotter SE, Olsen EG. Marfan’s disease and Erdheim’s cystic medionecrosis. A study of their pathology. Eur Heart J 1991; 12: 83–87. [DOI] [PubMed] [Google Scholar]
- 8.Collins MJ, Dev V, Strauss BH, et al. Variation in the histopathological features of patients with ascending aortic aneurysms: A study of 111 surgically excised cases. J Clin Pathol 2008; 61: 519–523. [DOI] [PubMed] [Google Scholar]
- 9.Hirst AE, Gore I. Editorial: Is cystic medionecrosis the cause of dissecting aortic aneurysm? Circulation 1976; 53: 915–916. [DOI] [PubMed] [Google Scholar]
- 10.Beighton P, De Paepe A, Steinmann B, et al. Ehlers–Danlos syndromes: Revised nosology, Villefranche, 1997. Ehlers–Danlos National Foundation (USA) and Ehlers–Danlos Support Group (UK). Am J Med Genet 1998; 77: 31–37. [DOI] [PubMed] [Google Scholar]
- 11.Beighton P, de, Paepe A, Danks D, et al. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet 1988; 29: 581–594. [DOI] [PubMed] [Google Scholar]
- 12.Callewaert B, Malfait F, Loeys B, et al. Ehlers–Danlos syndromes and Marfan syndrome. Best Pract Res Clin Rheumatol 2008; 22: 165–189. [DOI] [PubMed] [Google Scholar]
- 13.De Paepe A, Malfait F. The Ehlers–Danlos syndrome, a disorder with many faces. Clin Genet 2012; 82: 1–11. [DOI] [PubMed] [Google Scholar]
- 14.Eder J, Laccone F, Rohrbach M, et al. A new COL3A1 mutation in Ehlers–Danlos syndrome type IV. Exp Dermatol 2013; 22: 231–234. [DOI] [PubMed] [Google Scholar]
- 15.Pierard GE, Pierard-Franchimont C, Lapière CM. Histopathological aid at the diagnosis of the Ehlers–Danlos syndrome, gravis and mitis types. Int J Dermatol 1983; 22: 300–304. [DOI] [PubMed] [Google Scholar]
- 16.Lammert M, Friedman JM, Kluwe L, et al. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol 2005; 141: 71–74. [DOI] [PubMed] [Google Scholar]
- 17.Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am J Med Genet A 2010; 152A: 327–332. [DOI] [PubMed] [Google Scholar]
- 18.Stephens K, Kayes L, Riccardi VM, et al. Preferential mutation of the neurofibromatosis type 1 gene in paternally derived chromosomes. Hum Genet 1992; 88: 279–282. [DOI] [PubMed] [Google Scholar]
- 19.DeBella K, Szudek J, Friedman JM. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics 2000; 105: 608–614. [DOI] [PubMed] [Google Scholar]
- 20.Korf BR. Diagnostic outcome in children with multiple cafe au lait spots. Pediatrics 1992; 90: 924–927. [PubMed] [Google Scholar]
- 21.Gutmann DH, Rasmussen SA, Wolkenstein P, et al. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology 2002; 59: 759–761. [DOI] [PubMed] [Google Scholar]
- 22.Listernick R, Ferner RE, Piersall L, et al. Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology 2004; 63: 1944–1946. [DOI] [PubMed] [Google Scholar]
- 23.Lewis RA, Gerson LP, Axelson KA, et al. von Recklinghausen neurofibromatosis. II. Incidence of optic gliomata. Ophthalmology 1984; 91: 929–935. [DOI] [PubMed] [Google Scholar]
- 24.Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology 2005; 64: 553–555. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton SJ, Friedman JM. Insights into the pathogenesis of neurofibromatosis 1 vasculopathy. Clin Genet 2000; 58: 341–344. [DOI] [PubMed] [Google Scholar]
- 26.Norton KK, Xu J, Gutmann DH. Expression of the neurofibromatosis I gene product, neurofibromin, in blood vessel endothelial cells and smooth muscle. Neurobiol Dis 1995; 2: 13–21. [DOI] [PubMed] [Google Scholar]
- 27.Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005; 37: 275–281. [DOI] [PubMed] [Google Scholar]
- 28.LeMaire SA, Pannu H, Tran-Fadulu V, et al. Severe aortic and arterial aneurysms associated with a TGFBR2 mutation. Nat Clin Pract Cardiovasc Med 2007; 4: 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacCarrick G, Black JH, 3rd, Bowdin S, et al. Loeys–Dietz syndrome: A primer for diagnosis and management. Genet Med 2014; 16: 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Laer L, Dietz H, Loeys B. Loeys–Dietz syndrome. Adv Exp Med Biol 2014; 802: 95–105. [DOI] [PubMed] [Google Scholar]
- 31.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355: 788–798. [DOI] [PubMed] [Google Scholar]
- 32.Aalberts JJ, van den Berg MP, Bergman JE, et al. The many faces of aggressive aortic pathology: Loeys–Dietz syndrome. Neth Heart J 2008; 16: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maleszewski JJ, Miller DV, Lu J, et al. Histopathologic findings in ascending aortas from individuals with Loeys–Dietz syndrome (LDS). Am J Surg Pathol 2009; 33: 194–201. [DOI] [PubMed] [Google Scholar]
- 34.Kim ST, Brinjikji W and Kallmes DF. Prevalence of intracranial aneurysms in patients with connective tissue diseases: A retrospective study. Am J Neuroradiol. Epub ahead of print 18 March 2016. DOI: 10.3174/ajnr.A4718. [DOI] [PMC free article] [PubMed]
- 35.Conway JE, Hutchins GM, Tamargo RJ. Marfan syndrome is not associated with intracranial aneurysms. Stroke 1999; 30: 1632–1636. [DOI] [PubMed] [Google Scholar]
- 36.Schievink WI. Genetics of intracranial aneurysms. Neurosurgery 1997; 40: 651–662. discussion 662–663. [DOI] [PubMed] [Google Scholar]
- 37.van den Berg JS, Limburg M, Hennekam RC. Is Marfan syndrome associated with symptomatic intracranial aneurysms? Stroke 1996; 27: 10–12. [DOI] [PubMed] [Google Scholar]
- 38.Neil-Dwyer G, Bartlett JR, Nicholls AC, et al. Collagen deficiency and ruptured cerebral aneurysms. A clinical and biochemical study. J Neurosurg 1983; 59: 16–20. [DOI] [PubMed] [Google Scholar]
- 39.Debette S, Germain DP. Neurologic manifestations of inherited disorders of connective tissue. Handb Clin Neurol 2014; 119: 565–576. [DOI] [PubMed] [Google Scholar]
- 40.Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers–Danlos syndrome type IV, the vascular type. N Engl J Med 2000; 342: 673–680. [DOI] [PubMed] [Google Scholar]
- 41.North KN, Whiteman DA, Pepin MG, et al. Cerebrovascular complications in Ehlers–Danlos syndrome type IV. Ann Neurol 1995; 38: 960–964. [DOI] [PubMed] [Google Scholar]
- 42.Conway JE, Hutchins GM, Tamargo RJ. Lack of evidence for an association between neurofibromatosis type I and intracranial aneurysms: Autopsy study and review of the literature. Stroke 2001; 32: 2481–2485. [DOI] [PubMed] [Google Scholar]
- 43.Schievink WI, Riedinger M, Maya MM. Frequency of incidental intracranial aneurysms in neurofibromatosis type 1. Am J Med Genet A 2005; 134A: 45–48. [DOI] [PubMed] [Google Scholar]
- 44.Hughes BD, Powers CJ, Zomorodi AR. Clipping of a cerebral aneurysm in a patient with Loeys–Dietz syndrome: Case report. Neurosurgery 2011; 69: E746–E755. discussion E755. [DOI] [PubMed] [Google Scholar]
- 45.Kellner CP, Sussman ES, Donaldson C, et al. Cerebral arterial angioplasty in a patient with Loeys–Dietz syndrome. J Neurointerv Surg 2015; 7: e2. [DOI] [PubMed] [Google Scholar]
- 46.Levitt MR, Morton RP, Mai JC, et al. Endovascular treatment of intracranial aneurysms in Loeys–Dietz syndrome. J Neurointerv Surg 2012; 4: e37. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra A, Westesson PL. Loeys–Dietz syndrome. Pediatr Radiol 2009; 39: 1015. [DOI] [PubMed] [Google Scholar]
- 48.Rahme RJ, Adel JG, Bendok BR, et al. Association of intracranial aneurysm and Loeys–Dietz syndrome: Case illustration, management, and literature review. Neurosurgery 2011; 69: E488–E492. discussion E492–E493. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues VJ, Elsayed S, Loeys BL, et al. Neuroradiologic manifestations of Loeys–Dietz syndrome type 1. AJNR Am J Neuroradiol 2009; 30: 1614–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schievink WI, Link MJ, Piepgras DG, et al. Intracranial aneurysm surgery in Ehlers–Danlos syndrome Type IV. Neurosurgery 2002; 51: 607–611. discussion 611–613. [PubMed] [Google Scholar]
- 51.Colby GP, Lin LM, Zeiler SR, et al. Curative reconstruction of a cerebral aneurysm by flow diversion with the Pipeline embolisation device in a patient with Loeys–Dietz syndrome. BMJ Case Rep 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee VH, Brown RD, Jr, Mandrekar JN, et al. Incidence and outcome of cervical artery dissection: A population-based study. Neurology 2006; 67: 1809–1812. [DOI] [PubMed] [Google Scholar]
- 53.Debette S, Leys D. Cervical-artery dissections: Predisposing factors, diagnosis, and outcome. Lancet Neurol 2009; 8: 668–678. [DOI] [PubMed] [Google Scholar]
- 54.Sztajzel R, Hefft S, Girardet C. Marfan’s syndrome and multiple extracranial aneurysms. Cerebrovasc Dis 2001; 11: 346–349. [DOI] [PubMed] [Google Scholar]
- 55.Harrer JU, Sasse A, Klötzsch C. Intimal flap in a common carotid artery in a patient with Marfan’s syndrome. Ultraschall Med 2006; 27: 487–488. [DOI] [PubMed] [Google Scholar]
- 56.Schievink WI, Parisi JE, Piepgras DG, et al. Intracranial aneurysms in Marfan’s syndrome: An autopsy study. Neurosurgery 1997; 41: 866–870. discussion 871. [DOI] [PubMed] [Google Scholar]
- 57.Maski KP, Sengupta S, Silvera M, et al. Intracranial artery dissection in an adolescent with Marfan syndrome. Pediatr Neurol 2011; 45: 39–41. [DOI] [PubMed] [Google Scholar]
- 58.Kubo Y, Ogasawara K, Kurose A, et al. Ruptured cerebral fusiform aneurysm with mucopolysaccharide deposits in the tunica media in a patient with Marfan syndrome. J Neurosurg 2009; 110: 518–520. [DOI] [PubMed] [Google Scholar]
- 59.Dohle C, Baehring JM. Multiple strokes and bilateral carotid dissections: A fulminant case of newly diagnosed Ehlers–Danlos syndrome type IV. J Neurol Sci 2012; 318: 168–170. [DOI] [PubMed] [Google Scholar]
- 60.Mondon K, de Toffol B, Georgesco G, et al. Ehlers Danlos type IV syndrome presenting with simultaneous dissection of both internal carotid and both vertebral arteries [article in French]. Rev Neurol (Paris) 2004; 160(4 Pt 1): 478–482. [DOI] [PubMed] [Google Scholar]
- 61.Kurata A, Oka H, Ohmomo T, et al. Successful stent placement for cervical artery dissection associated with the Ehlers–Danlos syndrome. Case report and review of the literature. J Neurosurg 2003; 99: 1077–1081. [DOI] [PubMed] [Google Scholar]
- 62.Sengoku R, Sato H, Honda H, et al. Skin collagen abnormalities in a Japanese patient with extracranial internal carotid artery dissection followed by extracranial vertebral artery dissection [article in Japanese]. Rinsho Shinkeigaku 2006; 46: 140–143. [PubMed] [Google Scholar]
- 63.Ellis RJ, Salehin M, Zhou R, et al. Type IV Ehlers–Danlos syndrome presenting as recurrent, bilateral carotid dissections. BMJ Case Rep 2012; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chassin-Trubert Contreras AM. Carotid dissection associated with Ehlers–Danlos syndrome. Report of one case [article in Spanish]. Rev Med Chil 2013; 141: 392–395. [DOI] [PubMed] [Google Scholar]
- 65.Brandt T, Orberk E, Weber R, et al. Pathogenesis of cervical artery dissections: Association with connective tissue abnormalities. Neurology 2001; 57: 24–30. [DOI] [PubMed] [Google Scholar]
- 66.Brandt T, Hausser I, Orberk E, et al. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol 1998; 44: 281–285. [DOI] [PubMed] [Google Scholar]
- 67.Ulbricht D, Diederich NJ, Hermanns-Lê T, et al. Cervical artery dissection: An atypical presentation with Ehlers–Danlos-like collagen pathology? Neurology 2004; 63: 1708–1710. [DOI] [PubMed] [Google Scholar]
- 68.Peyre M, Ozanne A, Bhangoo R, et al. Pseudotumoral presentation of a cervical extracranial vertebral artery aneurysm in neurofibromatosis type 1: Case report. Neurosurgery 2007; 61: E658, discussion E658. [DOI] [PubMed] [Google Scholar]
- 69.Hiramatsu H, Matsui S, Yamashita S, et al. Ruptured extracranial vertebral artery aneurysm associated with neurofibromatosis type 1. Case report. Neurol Med Chir (Tokyo) 2012; 52: 446–449. [DOI] [PubMed] [Google Scholar]
- 70.Sampei T, Yugami H, Sumii T, et al. A case of neurofibromatosis type 1 associated with arteriovenous fistula caused by re-bleeding of a vertebral dissecting aneurysm [article in Japanese]. No Shinkei Geka 1999; 27: 927–931. [PubMed] [Google Scholar]
- 71.Goshgarian C, Lugo A, Salazar R. Proximal paraparesis due to aortic dissection extending into bilateral carotid arteries in a patient with Loeys–Dietz syndrome. J Clin Neurosci 2013; 20: 1790–1792. [DOI] [PubMed] [Google Scholar]
- 72.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Developed in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography. Catheter Cardiovasc Interv 2013; 81: E76–E123. [DOI] [PubMed] [Google Scholar]
- 73.Markus HS, Hayter E, Levi C, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): A randomised trial. Lancet Neurol 2015; 14: 361–367. [DOI] [PubMed] [Google Scholar]
- 74.Zuccoli G, Guidetti D, Nicoli F, et al. Carotid and vertebral artery dissection: Magnetic resonance findings in 15 cases. Radiol Med 2002; 104: 466–471. [PubMed] [Google Scholar]
- 75.Hamasaki O, Ikawa F, Hidaka T, et al. Extracranial internal carotid artery pseudoaneurysm associated with neurofibromatosis type 1 treated with endovascular stenting and coil embolization. Vasc Endovascular Surg 2014; 48: 176–179. [DOI] [PubMed] [Google Scholar]
- 76.Lennington BR, Laster DW, Moody DM, et al. Traumatic pseudoaneurysm of ascending cervical artery in neurofibromatosis: Complication of chiropractic manipulation. AJNR Am J Neuroradiol 1980; 1: 269–270. [PMC free article] [PubMed] [Google Scholar]
- 77.Detwiler K, Godersky JC, Gentry L. Pseudoaneurysm of the extracranial vertebral artery. Case report. J Neurosurg 1987; 67: 935–939. [DOI] [PubMed] [Google Scholar]
- 78.Fankhauser GT, Stone WM, Fowl RJ, et al. Surgical and medical management of extracranial carotid artery aneurysms. J Vasc Surg 2015; 61: 389–393. [DOI] [PubMed] [Google Scholar]
- 79.El-Koussy M, Lovblad KO, Steinlin M, et al. Perfusion MRI abnormalities in the absence of diffusion changes in a case of moyamoya-like syndrome in neurofibromatosis type 1. Neuroradiology 2002; 44: 938–941. [DOI] [PubMed] [Google Scholar]
- 80.Tan RM, Chng SM, Seow WT, et al. ‘Moya’ than meets the eye: Neurofibromatosis type 1 associated with Moyamoya syndrome. Singapore Med J 2008; 49: e107–e109. [PubMed] [Google Scholar]
- 81.Vargiami E, Sapountzi E, Samakovitis D, et al. Moyamoya syndrome and neurofibromatosis type 1. Ital J Pediatr 2014; 40: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Golomb MR, Smith JL. Poor wound healing after pial synangiosis in 2 children with moyamoya vasculopathy associated with neurofibromatosis type 1. J Child Neurol 2014; 29: NP101–NP104. [DOI] [PubMed] [Google Scholar]
- 83.Arita H, Narita Y, Ohno M, et al. Management of glioblastoma in an NF1 patient with moyamoya syndrome: A case report. Childs Nerv Syst 2013; 29: 341–345. [DOI] [PubMed] [Google Scholar]
- 84.Nzwalo H, Santos V, Gradil C, et al. Caucasian familial moyamoya syndrome with rare multisystemic malformations. Pediatr Neurol 2013; 48: 240–243. [DOI] [PubMed] [Google Scholar]
- 85.Ullrich NJ, Zimmerman M, Smith E, et al. Association of rapidly progressive moyamoya syndrome with bevacizumab treatment for glioblastoma in a child with neurofibromatosis type 1. J Child Neurol 2011; 26: 228–230. [DOI] [PubMed] [Google Scholar]
- 86.Tatli B, Ekici B, Sencer A, et al. Clinical features, prothrombotic risk factors, and long-term follow-up of eight pediatric Moyamoya patients. J Clin Neurol 2012; 8: 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barrall JL, Summers CG. Ocular ischemic syndrome in a child with moyamoya disease and neurofibromatosis. Surv Ophthalmol 1996; 40: 500–504. [DOI] [PubMed] [Google Scholar]
- 88.Inoue T, Matsushima T, Fujii K, et al. Akin moyamoya disease in children [article in Japanese]. No Shinkei Geka 1993; 21: 59–65. [PubMed] [Google Scholar]
- 89.Kwong KL, Wong YC. Moyamoya disease in a child with neurofibromatosis type-1. J Paediatr Child Health 1999; 35: 108–109. [PubMed] [Google Scholar]
- 90.Cairns AG, North KN. Cerebrovascular dysplasia in neurofibromatosis type 1. J Neurol Neurosurg Psychiatry 2008; 79: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 91.Duat-Rodríguez A, Carceller Lechón F, López Pino MÁ, et al. Neurofibromatosis type 1 associated with moyamoya syndrome in children. Pediatr Neurol 2014; 50: 96–98. [DOI] [PubMed] [Google Scholar]
- 92.Rea D, Brandsema JF, Armstrong D, et al. Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics 2009; 124: e476–e483. [DOI] [PubMed] [Google Scholar]
- 93.Lin N, Baird L, Koss M, et al. Discovery of asymptomatic moyamoya arteriopathy in pediatric syndromic populations: Radiographic and clinical progression. Neurosurg Focus 2011; 31: E6. [DOI] [PubMed] [Google Scholar]
- 94.Ulrich PT, Januschek E. Revascularisation surgery and long-term follow-up in juvenile Moyamoya syndrome: A retrospective analysis. Acta Neurochir Suppl 2011; 112: 39–43. [DOI] [PubMed] [Google Scholar]
- 95.Han C, Yang WZ, Zhang HT, et al. Clinical characteristics and long-term outcomes of moyamoya syndrome associated with neurofibromatosis type 1. J Clin Neurosci 2015; 22: 286–290. [DOI] [PubMed] [Google Scholar]
- 96.Ghosh PS, Rothner AD, Emch TM, et al. Cerebral vasculopathy in children with neurofibromatosis type 1. J Child Neurol 2013; 28: 95–101. [DOI] [PubMed] [Google Scholar]
- 97.Koss M, Scott RM, Irons MB, et al. Moyamoya syndrome associated with neurofibromatosis type 1: Perioperative and long-term outcome after surgical revascularization. J Neurosurg Pediatr 2013; 11: 417–425. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi K, Morofuji Y, Horie N, et al. A case of neurofibromatosis type 1 complicated with repeated intracerebral hemorrhage due to quasi-Moyamoya disease. J Stroke Cerebrovasc Dis 2015; 24: e109–e113. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto Y, Kodama K, Yokoyama S, et al. A pleural solitary fibrous tumor, multiple gastrointestinal stromal tumors, Moyamoya disease, and hyperparathyroidism in a patient associated with NF1. Case Rep Surg 2015; 2015: 375416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siqueira Neto JI, Silva GS, De Castro JD, et al. Neurofibromatosis associated with moyamoya arteriopathy and fusiform aneurysm: Case report [article in Portuguese]. Arq Neuropsiquiatr 1998; 56: 819–823. [DOI] [PubMed] [Google Scholar]
- 101.Hattori S, Kiguchi H, Ishii T, et al. Moyamoya disease with concurrent von Recklinghausen’s disease and cerebral arteriovenous malformation. Pathol Res Pract 1998; 194: 363–369. [DOI] [PubMed] [Google Scholar]
- 102.Gracia CM, Bittencourt PC, Mazer S, et al. Neurofibromatosis and extensive intracranial arterial occlusive disease (moyamoya disease). Report of a case [article in Portuguese]. Arq Neuropsiquiatr 1986; 44: 395–400. [DOI] [PubMed] [Google Scholar]
- 103.Koc F, Yerdelen D, Koc Z. Neurofibromatosis type 1 association with moyamoya disease. Int J Neurosci 2008; 118: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 104.Horiguchi S, Mitsuya K, Watanabe R, et al. Pleomorphic xanthoastrocytoma and moyamoya disease in a patient with neurofibromatosis type 1—case report. Neurol Med Chir (Tokyo) 2011; 51: 310–314. [DOI] [PubMed] [Google Scholar]
- 105.Terry AR, Jordan JT, Schwamm L, et al. Increased risk of cerebrovascular disease among patients with neurofibromatosis type 1: Population-based approach. Stroke 2016; 47: 60–65. [DOI] [PubMed] [Google Scholar]
- 106.Murayama Y, Usami S, Abe T, et al. Transvenous Doppler guidewire sonographic monitoring during treatment of a complex vertebral arteriovenous fistula associated with neurofibromatosis type 1. Neuroradiology 1999; 41: 328–333. [DOI] [PubMed] [Google Scholar]
- 107.Kamiyama K, Endo S, Horie Y, et al. Neurofibromatosis associated with intra- and extracranial aneurysms and extracranial vertebral arteriovenous fistula [article in Japanese]. No Shinkei Geka 1985; 13: 875–880. [PubMed] [Google Scholar]
- 108.Higa G, Pacanowski JP, Jr, Jeck DT, et al. Vertebral artery aneurysms and cervical arteriovenous fistulae in patients with neurofibromatosis 1. Vascular 2010; 18: 166–177. [DOI] [PubMed] [Google Scholar]
- 109.Hughes DG, Alleyne CH., Jr Rare giant traumatic cervical arteriovenous fistula in neurofibromatosis type 1 patient. BMJ Case Rep 2012; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kāhārā V, Lehto U, Ryymin P, et al. Vertebral epidural arteriovenous fistula and radicular pain in neurofibromatosis type I. Acta Neurochir (Wien) 2002; 144: 493–496. [DOI] [PubMed] [Google Scholar]
- 111.Saito A, Takahashi T, Ezura M, et al. Intercostal arteriovenous fistula associated with neurofibromatosis manifesting as congestive myelopathy: Case report. Neurosurgery 2007; 61: E656–E657. discussion E657. [DOI] [PubMed] [Google Scholar]
- 112.Koenigsberg RA, Aletich V, Debrun G, et al. Cervical vertebral arteriovenous fistula balloon embolization in a patient with neurofibromatosis type 1. Surg Neurol 1997; 47: 265–273. [DOI] [PubMed] [Google Scholar]
- 113.Anegawa S, Hayashi T, Torigoe R, et al. Symptomatic arteriovenous fistula in a patient with neurofibromatosis type I [article in Japanese]. No Shinkei Geka 1997; 25: 373–378. [PubMed] [Google Scholar]
- 114.Wada K, Ohtsuka K, Terayama K, et al. Neurofibromatosis with spinal paralysis due to arteriovenous fistula. Arch Orthop Trauma Surg 1989; 108: 322–324. [DOI] [PubMed] [Google Scholar]
- 115.Kubota T, Nakai H, Tanaka T, et al. A case of intracranial arteriovenous fistula in an infant with neurofibromatosis type 1. Childs Nerv Syst 2002; 18: 166–170. [DOI] [PubMed] [Google Scholar]
- 116.Tanaka T, Hasegawa Y, Kanki T, et al. Combination of intravascular surgery and surgical operation for occipital subcutaneous arteriovenous fistula in a patient with neurofibromatosis type I [article in Japanese]. No Shinkei Geka 2002; 30: 309–313. [PubMed] [Google Scholar]
- 117.Johnson CE, Russell EJ, Huckman MS. Resolution of spinal epidural vascular pseudotumor following balloon occlusion of a postoperative vertebral arteriovenous fistula. Neuroradiology 1990; 31: 529–532. [DOI] [PubMed] [Google Scholar]
- 118.Schievink WI, Piepgras DG. Cervical vertebral artery aneurysms and arteriovenous fistulae in neurofibromatosis type 1: Case reports. Neurosurgery 1991; 29: 760–765. [DOI] [PubMed] [Google Scholar]
- 119.Roth TC, Manness WK, Hershey BL, et al. Complex vertebral arteriovenous fistula and ruptured aneurysm in neurofibromatosis: A therapeutically challenging case. Skull Base Surg 2000; 10: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siddhartha W, Chavhan GB, Shrivastava M, et al. Endovascular treatment for bilateral vertebral arteriovenous fistulas in neurofibromatosis 1. Australas Radiol 2003; 47: 457–461. [DOI] [PubMed] [Google Scholar]
- 121.Hori Y, Goto K, Ogata N, et al. Diagnosis and endovascular treatment of vertebral arteriovenous fistulas in neurofibromatosis type 1. Interv Neuroradiol 2000; 6: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maheshwari S, Kale HA, Desai SB, et al. Magnetic resonance imaging findings in an unusual case of atlanto axial dislocation and vertebral artery-vein fistulas in a patient of neurofibromatosis-1. Australas Radiol 2002; 46: 316–318. [DOI] [PubMed] [Google Scholar]
- 123.Hauck EF, Nauta HJ. Spontaneous spinal epidural arteriovenous fistulae in neurofibromatosis type-1. Surg Neurol 2006; 66: 215–221. [DOI] [PubMed] [Google Scholar]
- 124.Guzel A, Tatli M, Er U, et al. Surgical treatment of cervical arteriovenous fistula in a patient with neurofibromatosis type 1. A case report. Neuroradiol J 2007; 20: 566–569. [DOI] [PubMed] [Google Scholar]
- 125.Hasegawa H, Bitoh S, Katoh A, et al. Bilateral vertebral arteriovenous fistulas and atlantoaxial dislocation associated with neurofibromatosis—case report. Neurol Med Chir (Tokyo) 1989; 29: 55–59. [DOI] [PubMed] [Google Scholar]
- 126.Paolini S, Colonnese C, Galasso V, et al. Extradural arteriovenous fistulas involving the vertebral artery in neurofibromatosis Type 1. J Neurosurg Spine 2008; 8: 181–185. [DOI] [PubMed] [Google Scholar]
- 127.Benndorf G, Assmann U, Bender A, et al. Vertebral arteriovenous fistula associated with neurofibromatosis type I misdiagnosed as a giant aneurysm. Interv Neuroradiol 2000; 6: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patro SN, Gupta AK, Arvinda HR, et al. Combined transarterial and percutaneous coiling of a spontaneous vertebrovertebral fistula associated with neurofibromatosis type 1. Case report. J Neurosurg 2009; 111: 37–40. [DOI] [PubMed] [Google Scholar]
- 129.Takegami T, Imai K, Umezawa K, et al. Endovascular trapping using a tandem balloon technique for a spontaneous vertebrovertebral fistula associated with neurofibromatosis type 1 [article in Japanese]. No Shinkei Geka 2012; 40: 705–709. [PubMed] [Google Scholar]
- 130.Desal HA, Toulgoat F, Raoul S, et al. Ehlers–Danlos syndrome type IV and recurrent carotid-cavernous fistula: Review of the literature, endovascular approach, technique and difficulties. Neuroradiology 2005; 47: 300–304. [DOI] [PubMed] [Google Scholar]
- 131.Nakagawa I, Park HS, Wada T, et al. A novel approach to the treatment of a direct carotid-cavernous fistula in a patient with Ehlers–Danlos syndrome type IV. BMJ Case Rep 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Linfante I, Lin E, Knott E, et al. Endovascular repair of direct carotid-cavernous fistula in Ehlers–Danlos type IV. BMJ Case Rep 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakagawa I, Park HS, Wada T, et al. A novel approach to the treatment of a direct carotid-cavernous fistula in a patient with Ehlers–Danlos syndrome type IV. J Neurointerv Surg 2016; 8: e2. [DOI] [PubMed] [Google Scholar]
- 134.Kim JG, Cho WS, Kang HS, et al. Spontaneous carotid-cavernous fistula in the type IV Ehlers–Danlos syndrome. J Korean Neurosurg Soc 2014; 55: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan A, Chaudhary N, Pandey AS, et al. Direct puncture of the highest cervical segment of the internal carotid artery for treatment of an iatrogenic carotid cavernous fistula in a patient with Ehlers–Danlos syndrome. J Neurointerv Surg 2012; 4: e29. [DOI] [PubMed] [Google Scholar]
- 136.Wang Q, Chen G. Endovascular treatment of bilateral multiple carotid-cavernous fistulas in a patient with Ehlers–Danlos syndrome. J Neurol Surg A Cent Eur Neurosurg 2013; 74(Suppl 1): e41–e44. [DOI] [PubMed] [Google Scholar]
- 137.Forlodou P, de Kersaint-Gilly A, Pizzanelli J, et al. Ehlers–Danlos syndrome with a spontaneous caroticocavernous fistula occluded by detachable balloon: Case report and review of literature. Neuroradiology 1996; 38: 595–597. [DOI] [PubMed] [Google Scholar]
- 138.Kanner AA, Maimon S, Rappaport ZH. Treatment of spontaneous carotid-cavernous fistula in Ehlers–Danlos syndrome by transvenous occlusion with Guglielmi detachable coils. Case report and review of the literature. J Neurosurg 2000; 93: 689–692. [DOI] [PubMed] [Google Scholar]
- 139.Horowitz J. Unusual presentation of a carotid-cavernous fistula in Ehlers–Danlos type IV [article in Hebrew]. Harefuah 2013; 152: 106–108, 122. [PubMed] [Google Scholar]
- 140.Lach B, Nair SG, Russell NA, et al. Spontaneous carotid-cavernous fistula and multiple arterial dissections in type IV Ehlers–Danlos syndrome. Case report. J Neurosurg 1987; 66: 462–467. [DOI] [PubMed] [Google Scholar]
- 141.Kashiwagi S, Tsuchida E, Goto K, et al. Balloon occlusion of a spontaneous carotid-cavernous fistula in Ehlers–Danlos syndrome type IV. Surg Neurol 1993; 39: 187–190. [DOI] [PubMed] [Google Scholar]
- 142.Farley MK, Clark RD, Fallor MK, et al. Spontaneous carotid-cavernous fistula and the Ehlers–Danlos syndromes. Ophthalmology 1983; 90: 1337–1342. [DOI] [PubMed] [Google Scholar]
- 143.Hollands JK, Santarius T, Kirkpatrick PJ, et al. Treatment of a direct carotid-cavernous fistula in a patient with type IV Ehlers–Danlos syndrome: A novel approach. Neuroradiology 2006; 48: 491–494. [DOI] [PubMed] [Google Scholar]
- 144.Usinskiene J, Mazighi M, Bisdorff A, et al. Fatal peritoneal bleeding following embolization of a carotid-cavernous fistula in Ehlers–Danlos syndrome type IV. Cardiovasc Intervent Radiol 2006; 29: 1104–1106. [DOI] [PubMed] [Google Scholar]
- 145.Chuman H, Trobe JD, Petty EM, et al. Spontaneous direct carotid-cavernous fistula in Ehlers–Danlos syndrome type IV: Two case reports and a review of the literature. J Neuroophthalmol 2002; 22: 75–81. [DOI] [PubMed] [Google Scholar]
- 146.Fox R, Pope FM, Narcisi P, et al. Spontaneous carotid cavernous fistula in Ehlers Danlos syndrome. J Neurol Neurosurg Psychiatry 1988; 51: 984–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bashir Q, Thornton J, Alp S, et al. Carotid-cavernous fistula associated with Ehlers–Danlos syndrome type IV. A case report and review of literature. Interv Neuroradiol 1999; 5: 313–320. [DOI] [PubMed] [Google Scholar]
- 148.Van Overmeire O, De Keukeleire K, van Langenhove P, et al. Carotid-cavernous fistula in Ehlers–Danlos syndrome by pure transvenous approach. Interv Neuroradiol 2006; 12: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Halbach VV, Higashida RT, Dowd CF, et al. Treatment of carotid-cavernous fistulas associated with Ehlers–Danlos syndrome. Neurosurgery 1990; 26: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 150.Kojima A, Saga I, Tomio R, et al. Aggressive change of a carotid-cavernous fistula in a patient with Ehlers–Danlos syndrome type IV. Interv Neuroradiol 2015; 21: 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tanaka T, Hayakawa M, Sadato A, et al. Transvenous embolization for carotid-cavernous fistula in a patient with vascular type of Ehlers–Danlos syndrome—direct superior ophthalmic vein approach: Case report. Neurol Med Chir (Tokyo) 2014; 54: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mitsuhashi T, Miyajima M, Saitoh R, et al. Spontaneous carotid-cavernous fistula in a patient with Ehlers–Danlos syndrome type IV—case report. Neurol Med Chir (Tokyo) 2004; 44: 548–553. [DOI] [PubMed] [Google Scholar]