Abstract
Introduction
Single-volume reconstruction of three-dimensional (3D) digital subtraction angiography (DSA) can be effectively used for aneurysm assessment and planning of endovascular embolization. Unfortunately, post-embolization follow-up angiographic images can be obscured by artifact. The dual-volume reconstruction technique was developed in order to reduce artifact and enhance the visualization of the aneurysm, the parent vessel and side branches, and endovascular devices. The purpose of this study was to compare the reliability of dual- vs single-volume reconstruction of 3D DSA in evaluation of follow-up images after endovascular embolization of intracranial aneurysms.
Method
Four cerebrovascular neurosurgeons independently and blindly reviewed 20 randomly selected dual-and single-volume reconstructions of 3D DSAs demonstrating cerebral aneurysms treated with primary coil embolization, stent-assisted coil embolization, or Pipeline embolization. Five images were repeated for each modality (single and dual volume) in order to assess intra-rater reliability. The intraclass correlation coefficient was calculated as a measure of the overall inter-rater agreement. Cohen’s kappa value was used to assess repeat measurement consistency for each rater.
Results
Overall inter-rater agreement using dual- and single-volume reconstruction was 0.81 and 0.75, respectively. Dual-volume reconstruction resulted in superior agreement in assessing location, occlusion status, position of aneurysm recanalization or residual, status of nearby branches, presence of coil migration and presence of intravascular devices (stent or Pipeline).
Conclusion
Three-dimensional reconstruction is an important complementary imaging technique in evaluating the angioarchitecture of aneurysms and recanalization after endovascular embolization. Dual-volume reconstruction imaging was associated with superior inter- and intra-rater reliability.
Keywords: Dual-volume, single-volume, reconstruction, 3D, aneurysms
Introduction
Three-dimensional (3D) reconstruction of digital subtracted angiography (DSA) has become an indispensable complementary technique for evaluation of aneurysms, in addition to the standard two-dimensional (2D) DSA.1 The conventional single-volume reconstruction can be effectively used prior to endovascular embolization of aneurysms. However, the follow-up imaging can be obscured by artifact, making recanalization difficult to detect and intervention difficult to plan.2 The dual-volume reconstruction technique, in which two acquired data volumes are reconstructed separately and then fused together, was developed to minimize artifacts and show a greater level of detail related to the aneurysm, the parent vessel, side branches, and endovascular devices. Little evidence is available regarding the reliability of dual-volume reconstruction and whether this technique is superior to single-volume reconstruction. The purpose of this study was to compare reliability of both techniques in the evaluation of follow-up DSA images after endovascular embolization in seven categories, such as recanalization, which are essential for the evaluation of endovascularly treated aneurysms.
Methods
Rotational angiography with 3D reconstruction
Rotational angiography was performed using a motorized AXIOM Artis® C-arm (Siemens Healthcare, Erlangen, Germany) with 360-degree rotational run and acquisition of 256 images, and with contrast material injection rates of 3- to 4-ml per second, which results in a total of 15–20 ml of contrast in each artery. The image data were transferred to a Siemens LEONARDO® workstation (Siemens Healthcare, Erlangen, Germany), where reconstruction of 3D images is performed. A complete acquisition consisted of two rotational scans: The first scan collects subtraction masks, and the second acquires images during the passage of a contrast medium. Software reconstructions then enable extraction of residual bony structures, deletion of unnecessary vessels, zooming and rotation of the image, as well as enabling differentiation between contrast filling the vessels and endovascular devices to produce the dual-volume reconstruction images.
Observer reliability assessment
Four raters, all cerebrovascular neurosurgeons, independently reviewed 20 single-volume and 20 dual-volume reconstructions of 3D DSAs demonstrating cerebral aneurysms treated with primary coil embolization, stent-assisted coil embolization, or Pipeline embolization (PED, Covidien-Ev3, Plymouth, MN). The images used in the study were randomly selected from a cerebrovascular endovascular database. Seven factors were assessed by the reviewers, including location of the aneurysm, occlusion status of the aneurysm, position of the residual aneurysm in relation to the coil mass, status of the parent artery, status of the nearby branches, coil migration, and presence of intravascular assist devices. Occlusion status of the aneurysm was categorized using the Raymond-Roy Occlusion Classification3 (Table 1). Five scans of each modality (single and dual volume) were randomly repeated for intra-rater reliability assessment, for a total of 50 sets of images. Reviewers were blind to previous image interpretation and to all clinical information. Scans that were repeated were randomly inserted into the dataset. The necessary sample size was calculated using a method developed by Walter et al.4 that showed that increasing the number of raters (four in the present study) per participant will decrease the total number of observations required to achieve adequate sample size, a number determined to be sufficient for statistical analysis.
Table 1.
Variables assessed by the reviewers for evaluation of follow-up images.
| 1: Location of the aneurysm |
| 2: Aneurysm occlusion status |
| Complete obliteration |
| Residual neck |
| Residual aneurysm with contrast dye within coil interstices |
| Residual aneurysm with contrast dye flow along aneurysm wall |
| 3: Position of the residual (or recurrence) in relation to the coil mass |
| No residual |
| Above |
| Below |
| Left |
| Right |
| In front |
| Behind |
| 4: Status of the parent artery |
| Fully patent |
| Compromised < 50% |
| Compromised > 50% |
| 5: Status of the segmental or nearby branch arteries |
| Completely separated from aneurysm and patent |
| At least one narrowed or occluded |
| 6: Coil migration into the parent artery |
| No migration |
| Migrated but remained at parent artery side wall |
| Migrated and free floating inside the lumen |
| 7: Presence of intravascular assist device (Pipeline or stent) |
| Yes |
| No |
The intraclass correlation coefficient (ICC) was calculated as a measure of overall agreement on the tested variables between the four raters. Intraclass correlation greater than 0.75 is considered to have excellent agreement, with an ICC between 0.40 and 0.75 classified as fair to good, and less than 0.40 considered poor agreement. For intra-rater reliability analysis, Cohen’s kappa (κ) was used to assess repeat measurement agreement for each rater. Agreement measured by κ is interpreted as very high with κ values between 0.81 and 1.00, substantial with κ values between 0.61 and 0.80, moderate with κ values between 0.41 and 0.60, fair with κ values between 0.21 and 0.40, and poor with κ values between 0 and 0.20.5 All statistical analysis was performed using SPSS 21.0 (IBM Corp. Armonk, NY).
This study was approved by the institutional review board.
Results
Demographic factors
Images represent a total number of 20 aneurysms (median age 65.6 years, male-to-female ratio was 1:4). The aneurysms were located in the cavernous segment of the internal carotid artery in 10% of cases, ophthalmic segment in 15%, superior hypophyseal segment in 5%, posterior communicating artery segment in 15%, anterior communicating artery in 35%, middle cerebral artery in 5%, and basilar apex in 15%. Aneurysms were treated with coil embolization in 20%, stent-assisted coil embolization in 25%, and Pipeline with coil embolization in 55% (Table 2).
Table 2.
Baseline characteristics.
| Factor | Number |
|---|---|
| Gender | |
| Male | 4 (20%) |
| Female | 16 (80%) |
| Age (years; median) | 49–81 (65.6) |
| Location | |
| Internal carotid artery | |
| Cavernous | 2 (10%) |
| Ophthalmic | 3 (15%) |
| Superior hypophyseal | 1 (5%) |
| Posterior communicating | 3 (15%) |
| Anterior communicating artery | 7 (35%) |
| Middle cerebral artery | 1 (5%) |
| Basilar apex | 3 (15%) |
| Treatment | |
| Coiling | 4 (20%) |
| Stent-assisted coiling | 5 (25%) |
| Pipeline and coiling | 11 (55%) |
Inter-rater reliability
ICC was used to the assess the agreement between the four raters in evaluation of the selected variables for each aneurysm. The overall ICC using dual-volume and single-volume reconstruction images were 0.81 and 0.75, respectively. Dual-volume reconstruction had better agreement in evaluating aneurysm location (0.863 vs 0.842), occlusion status (0.578 vs 0.548), position of residual (0.504 vs 0.304), status of branches (0.554 vs 0.323), presence of coil migration 0 vs –0.12), and presence of assist devices (0.193 vs 0.019). Although the difference was most remarkable in assessing the position of the residual and the status of branches, there was still an overlap between the confidence intervals of ICCs of dual volume and single volume. Single-volume reconstruction had better agreement in evaluating the status of the parent artery (0.32 vs –0.033) (Table 3).
Table 3.
Inter-rater reliability.
| Factor | Single volume |
Dual volume |
||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| Location | 0.842 | 0.721 to 0.925 | 0.863 | 0.755 to 0.936 |
| Occlusion status | 0.548 | 0.326 to 0.755 | 0.578 | 0.359 to 0.775 |
| Position of residual | 0.304 | 0.095 to 0.564 | 0.504 | 0.285 to 0.723 |
| Status of parent artery | 0.32 | 0.113 to 0.575 | –0.033 | –0.104 to 0.113 |
| Status of branches | 0.323 | 0.116 to 0.578 | 0.554 | 0.339 to 0.758 |
| Coil migration | –0.12 | –0.054 to 0.128 | 0 | –0.108 to 0.201 |
| Assist devices | 0.019 | –0.112 to 0.248 | 0.193 | 0.007 to 0.457 |
| Overall | 0.75 | 0.692 to 0.804 | 0.81 | 0.753 to 0.851 |
ICC: intraclass correlation coefficient; CI: confidence interval.
Intra-rater reliability
Cohen’s κ was used to assess intra-rater reliability in assessing five randomly repeated images. Intra-rater reliability was perfect (κ = 1) in assessing all variables in two out of four raters and three out of four raters using single-volume and dual-volume reconstruction, respectively. Using single-volume reconstruction, intra-rater reliability was excellent (κ > 0.75) in assessing aneurysm location by raters 1 and 2. However, it was poor (κ = 0.05) in assessing position of residual in rater 1 compared to good (κ > 0.5) reliability using dual-volume reconstruction by the same rater (Table 4).
Table 4.
Intra-rater reliability.
| Rater | Location | Occlusion status | Position of residual | Status of parent artery | Status of branches | Coil migration | Assist devices |
|---|---|---|---|---|---|---|---|
| Single volume | |||||||
| Cerebrovascular surgeon 1 | 0.762 | 0.583 | 0.05 | 0 | 1 | 1 | 0 |
| Cerebrovascular surgeon 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cerebrovascular surgeon 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cerebrovascular surgeon 4 | 0.75 | 1 | 1 | 1 | 1 | 1 | 1 |
| Dual volume | |||||||
| Cerebrovascular surgeon 1 | 1 | 1 | 0.583 | 1 | 1 | 1 | 1 |
| Cerebrovascular surgeon 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cerebrovascular surgeon 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cerebrovascular surgeon 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Discussion
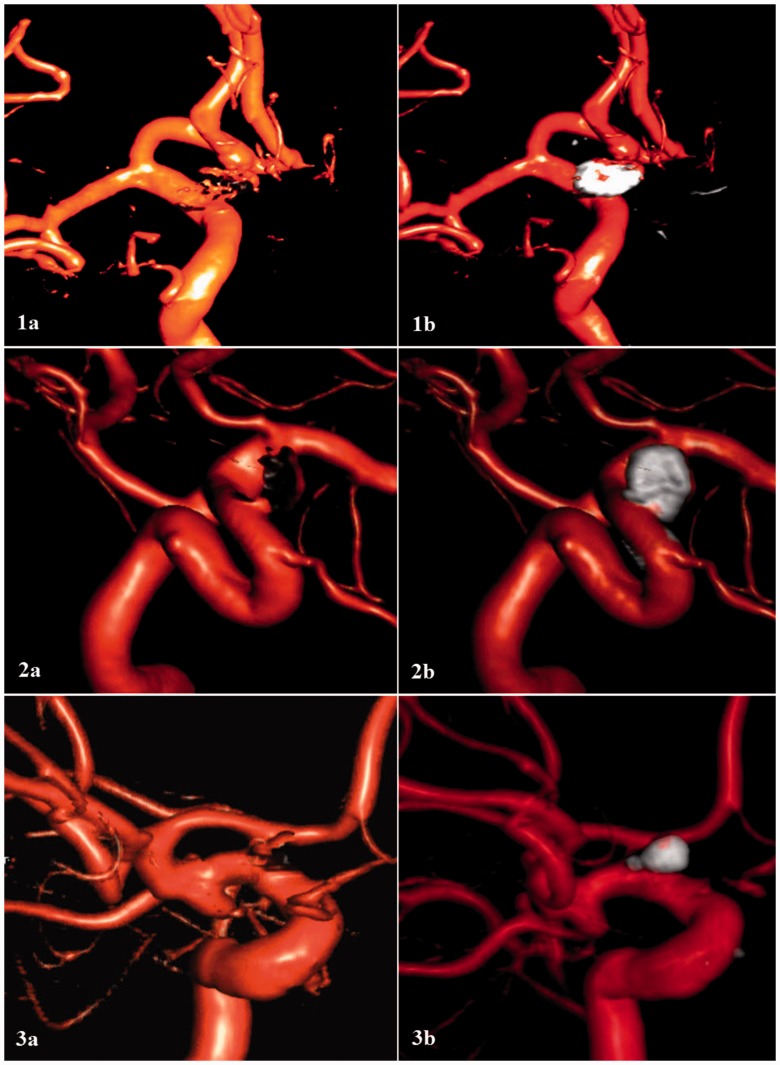
In the present study, single-volume and dual-volume reconstruction of 3D DSA were compared in follow-up evaluation of endovascularly treated aneurysms. Dual-volume reconstruction produced a higher overall agreement rate and improved reliability between the raters. Objective evaluation of both images showed better delineation of aneurysm location and relation to surrounding vasculature, occlusion status, and presence of coil migration using dual-volume reconstruction (Figure 1).
Figure 1.
Three-dimensional reconstruction of digital subtracted angiography using single-volume (Panels (a)) and dual-volume (Panels (b)) reconstruction imaging. Dual-volume reconstruction imaging was associated with an increased inter-rater reliability relating to the aneurysm location and its relation to surrounding vasculature (Panel 1), the presence of assist devices (Panel 2), and presence of coil migration (Panel 3).
Two-dimensional vs 3D angiography
Various image-processing techniques have been used in conjunction with cerebral angiography to improve visualization of vascular structures in diagnostic studies. The 2D DSA was a technological breakthrough that dramatically improved visualization of cerebral vessels, allowing real-time subtraction of bony topography. It has since been considered the most accurate imaging technique for evaluation of cerebrovascular anomalies. Nevertheless, 2D imaging is merely a representation of 3D structures and thus can impair optimal visualization and is associated with numerous limitations, including overlapping anatomy and vessel foreshortening.6 The development of 3D reconstruction techniques led to significant improvement in cerebral aneurysm diagnosis and treatment. Three-dimensional reconstruction enables 3D rotation of chosen structures, thereby enhancing the assessment of vessel length, vessel tortuosity, and bifurcation angles. Several studies have shown the clinical superiority of 3D imaging, when compared to 2D angiography, in delineation of vascular morphology and relationships and detection of small and ruptured aneurysms.2,6–17
Single-volume vs dual-volume reconstruction
Single-volume reconstruction depends on multiple image projections enabling the creation of a volumetric representation of the vessel. A complete acquisition consists of two rotational scans. The first scan collects subtraction masks (mask run), and the second one acquires images during the passage of a contrast medium (contrast run). Both scans are then fused and reconstructed as one volume. However, with the exponential increase in neurointerventional techniques, it has become crucial to evaluate not just the vessels, but also the devices in post-treatment and follow-up studies.18 Further, an accurate position of the residual aneurysm in relation to surrounding vessels and intravascular devices is critical to planning appropriate treatment. In dual-volume reconstruction, the acquired data volumes (mask run and contrast run) are reconstructed separately and then fused together, with each volume (e.g. a separate volume for the vessels and another for the devices) being modifiable individually to give the desired contrast, windowing, and color coding. This allows for more reliable localization of the aneurysm and any recurrence or residual in follow-up studies, and may improve intervention planning.
Reliability
Reliability of image interpretation is critically important when assessing aneurysm recanalization. High variability will complicate the detection of recanalization/residual or progressive recanalization. Dual-volume reconstruction results in improved inter- and intra-rater reliability, reducing the chance of missing subtle progressive recanalization. Similarly, more reliable assessment will prevent labeling stable recanalization as progressive, which may result in potentially unnecessary procedures.
Conclusion
Three-dimensional reconstruction is an important complementary imaging technique in evaluating the angioarchitecture of aneurysms and recanalization after endovascular embolization. Dual-volume reconstruction imaging provides the advantage of distinctly visualizing the endovascular devices, resulting in superior inter- and intra-rater reliability. In our practice we currently use dual-volume reconstruction for all aneurysms that have been previously treated with endovascular techniques (coils, stents, or Pipeline).
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Pedicelli A, Desiderio F, Esposito G, et al. Three-dimensional rotational angiography for craniotomy planning and postintervention evaluation of intracranial aneurysms. Radiol Med (Torino) 2013; 118: 415–430. [DOI] [PubMed] [Google Scholar]
- 2.Cieściński J, Serafin Z, Strześniewski P, et al. DSA volumetric 3D reconstructions of intracranial aneurysms: A pictorial essay. Pol J Radiol Pol Med Soc Radiol 2012; 77: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke J Cereb Circ 2001; 32: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 4.Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med 1998; 17: 101–110. [DOI] [PubMed] [Google Scholar]
- 5.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 6.Green NE, Chen SY, Messenger JC, et al. Three-dimensional vascular angiography. Curr Probl Cardiol 2004; 29: 104–142. [DOI] [PubMed] [Google Scholar]
- 7.Abe T, Hirohata M, Tanaka N, et al. Clinical benefits of rotational 3D angiography in endovascular treatment of ruptured cerebral aneurysm. AJNR Am J Neuroradiol 2002; 23: 686–688. [PMC free article] [PubMed] [Google Scholar]
- 8.Gailloud P, Oishi S, Murphy K. Three-dimensional fusion digital subtraction angiography: New reconstruction algorithm for simultaneous three-dimensional rendering of osseous and vascular information obtained during rotational angiography. AJNR Am J Neuroradiol 2005; 26: 908–911. [PMC free article] [PubMed] [Google Scholar]
- 9.Guberina N, Lechel U, Forsting M, et al. Dose comparison of classical 2-plane DSA and 3D rotational angiography for the assessment of intracranial aneurysms. Neuroradiology 2016; 58: 673–678. [DOI] [PubMed] [Google Scholar]
- 10.Hirai T, Korogi Y, Suginohara K, et al. Clinical usefulness of unsubtracted 3D digital angiography compared with rotational digital angiography in the pretreatment evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2003; 24: 1067–1074. [PMC free article] [PubMed] [Google Scholar]
- 11.Hochmuth A, Spetzger U, Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am J Neuroradiol 2002; 23: 1199–1205. [PMC free article] [PubMed] [Google Scholar]
- 12.Jalali A, Srinivasan VM, Chinnadurai P, et al. Two-color 3D-3D fusion of selective rotational cerebral angiograms: A novel approach to imaging in cerebrovascular neurosurgery. J Neurointerventional Surg. Epub ahead of print 16 November 2015. DOI: 10.1136/neurintsurg-2015-011963. [DOI] [PubMed] [Google Scholar]
- 13.Ogilvy CS, Chua MH, Fusco MR, et al. Stratification of recanalization for patients with endovascular treatment of intracranial aneurysms. Neurosurgery 2015; 76: 390–395. discussion 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podlesek D, Meyer T, Morgenstern U, et al. Improved visualization of intracranial vessels with intraoperative coregistration of rotational digital subtraction angiography and intraoperative 3D ultrasound. PloS One 2015; 10: e0121345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi WY, Li YD, Li MH, et al. 3D rotational angiography with volume rendering: The utility in the detection of intracranial aneurysms. Neurol India 2010; 58: 908–913. [DOI] [PubMed] [Google Scholar]
- 16.Sugahara T, Korogi Y, Nakashima K, et al. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2002; 23: 1545–1552. [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij WJ, Peluso JP, Sluzewski M, et al. Additional value of 3D rotational angiography in angiographically negative aneurysmal subarachnoid hemorrhage: How negative is negative? AJNR Am J Neuroradiol 2008; 29: 962–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooij WJ, Sprengers ME, de Gast AN, et al. 3D rotational angiography: The new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 2008; 29: 976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]