Abstract
Objective
The purpose of this study is to demonstrate our experience in endovascular reconstruction of carotid dissections using the Wingspan Stent System™ (Boston Scientific, Natick, MA, USA), a device we use because of its high radial force and its navigation in extreme curves.
Methods
We treated 11 consecutive patients with acute ischemic stroke due to carotid dissection with the Wingspan stent, in the cervical carotid artery.
Results
Functional evaluation revealed that 10 of the 11 patients were independent at 3 months post surgery and that the 11 stents used were found to be patent at the 6-month follow-up digital subtraction angiography (DSA).
Conclusions
The Wingspan stent is an alternative to classic carotid stents and flow diverters for the treatment of cervical internal carotid artery (ICA) dissection associated with ectasias or large loops. The device remains patent over the long term and it is not associated with arterial wall complications.
Keywords: brain, carotid stent, carotid dissection, internal carotid artery, stent, stroke, Wingspan stent
Introduction
Approximately 2% of ischemic strokes can be caused by carotid dissections, but they represent up to 20% of ischemic strokes in patients < 45 years old. With the widespread use of endovascular treatment of ischemic strokes, it is necessary to understand this disease and its therapeutic options, including the standards for anti-platelet or anti-coagulation treatment, and its associated complications.1–4 Endovascular treatment is a therapeutic alternative in patients with acute ischemic stroke secondary to dissection with carotid occlusion, whether or not it is associated with intracranial thromboembolism.5,6
Endovascular treatment of the dissection is limited by the curves and elongation that, in the majority of cases, make reconstruction with conventional carotid stents impossible. As an alternative, some authors have recommended the systematic use of flow diverters.7 One alternative is to use stents designed for intracranial use in areas of elongation and curves of the cervical and petrous carotid.8–10 The primary problem with the use of intracranial stents is the low radial force that occasionally does not keep the vascular lumen open properly; and if the caliber of the artery is greater than the sizes of the devices used for intracranial treatment.
The purpose of this study is to demonstrate our experience in endovascular reconstruction of carotid dissections using the Wingspan Stent System™ (Boston Scientific, Natick, MA, USA). We use this device because of its high radial force and its good navigation in extreme curves.11
Materials and methods
We retrospectively recorded 11 consecutive patients with acute ischemic stroke due to carotid dissection, with or without intracranial thromboembolism, between January 2010 and April 2015. Within this period, another five carotid dissections were done as treatment, but we did not use the Wingspan stent system in them. The criteria for endovascular treatment in the acute phase were: A modified rankin scale (mRS) score ≤ 1 pre-stroke, occlusion of the internal carotid artery (ICA) with or without intracranial obstruction, age > 18 years, with National Institute of Health Stroke Scale (NIHSS) ≥ 6 and Alberta Stroke Program Early CT score (ASPECTS) ≥ 6.12 Patients received treatment with tissue plasminogen activator (rtPA) in the first 4.5 hours of the onset of symptoms, if this would not delay the access to the endovascular treatment.
We collected data on the epidemiological characteristics of the patients, type of stent used, endovascular treatment characteristics, anti-platelet protocol used, functional prognosis using the mRS scale at the onset of symptoms, and imaging tests upon follow-up of the stent using digital subtraction angiography (at 6 months).
Results
Of the 11 patients studied (Table 1), nine were < 65 years of age and 10 had at least one cardiovascular risk factor (mean age: 58.09 years old; range: 42–71 years old; 72.7% were male gender).
Table 1.
Epidemiological characteristics and vascular risk factors.
| Patient No. | Age (yrs) | Gender | HBP | Dyslipidemia | Tobacco smoking | Diabetes |
|---|---|---|---|---|---|---|
| 1 | 48 | male | no | yes | yes | no |
| 2 | 64 | male | yes | no | yes | no |
| 3 | 51 | female | yes | no | yes | no |
| 4 | 42 | male | yes | yes | yes | no |
| 5 | 56 | male | no | no | yes | no |
| 6 | 53 | female | no | no | yes | no |
| 7 | 68 | male | yes | yes | yes | no |
| 8 | 64 | male | yes | yes | yes | no |
| 9 | 60 | female | yes | yes | no | no |
| 10 | 62 | male | no | no | no | no |
| 11 | 71 | male | yes | no | no | no |
HBP: High blood pressure; No: number; yrs: years
Epidemiological characteristics and medical antecedents
The 11 patients studied had a spontaneous dissection of the ICA with ischemic stroke and an NIHSS score > 8 points (main NHISS value: 13.6; range: 8–20). Of the 11 patients, seven had an associated intracranial thromboembolism (Table 2).
Table 2.
Neurological deficit, location and cause of the dissection.
| Patient No. | NIHSS | Cause | Location of the occlusion |
|---|---|---|---|
| 1 | 20 | Spontaneous | Left cervical ICA with intracranial thrombus (M3) |
| 2 | 11 | Spontaneous | Left cervical ICA with intracranial thrombus (M2) |
| 3 | 13 | Spontaneous | Right cervical ICA |
| 4 | 8 | Spontaneous | Left cervical ICA with intracranial thrombus (M2) |
| 5 | 11 | Spontaneous | Left cervical ICA with intracranial thrombus (M2) |
| 6 | 16 | Spontaneous | Right cervical ICA with intracranial thrombus (M2) |
| 7 | 17 | Spontaneous | Right cervical ICA |
| 8 | 18 | Spontaneous | Right cervical ICA with intracranial thrombus (T) |
| 9 | 14 | Spontaneous | Right cervical ICA |
| 10 | 10 | Spontaneous | Left cervical ICA |
| 11 | 12 | Spontaneous | Left cervical ICA with intracranial thrombus (M2) |
ICA: iInternal Carotid Artery; NIHSS: National institute of Health Stroke Scale; M2: Middle cerebral artery 2 Segment; M3: Middle cerebral artery 3 Segment; T: thrombus (a clot)
Vessel occlusion and clinical evaluation
Reopening of the dissection and resolution of the intracranial thrombus, if present, was achieved in all patients with a thrombolysis in cerebral infarction (TICI) grading system of IIb or III. For intraoperative anti-platelet therapy in the 11 patients, we used IIb to IIIa inhibitors in five patients, double anti-platelet therapy with intravenous lysine acetylsalicylate (LAS) and oral clopidogrel in four patients, and single anti-platelet therapy with LAS in two patients. All patients were treated with aspirin and clopidogrel post-operatively, for 24 hours. Functional evaluation revealed that 10 of the 11 patients were independent at 3 months, with a mRS of ≤ 2, and the 11 stents used were found to be patent at the 6-month follow-up DSA (Table 3).
Table 3.
Technical characteristics including antiplatelet therapy, and clinical and angiographic follow-up at 6 months.
| Patient No. | Anti-platelet protocol | Device and associated intracranial technique | TICI | Postoperative hemorrhage | Stent used | mRS at 3 months | Follow-up arteriogram at 6 months Stent patency | Follow-up arteriogram at 6 months Stent displacement |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 g of LAS IV + 300 mg Clopidogrel PO | SOLITAIRE | 2B | NO | Wingspan 4.5 × 20 mm Wingspan 4.5 × 20 mm | 0 | YES | YES |
| 2 | 1 g of LAS IV + Abciximab | SOLITAIRE | 3 | NO | Wingspan 4.5 × 20 mm | 0 | YES | NO |
| 3 | 1 g of LAS IV | - | 3 | NO | Wingspan 4.5 × 20 mm Wingspan 4 × 20 mm Wallstent 5 × 30 mm | 3 | YES | YES |
| 4 | 1 g of LAS IV + 300 mg Clopidogrel PO | SOLITAIRE | 3 | NO | Wingspan 4.5 × 25 mm Wingspan 4 × 20 mm Wallstent 5 × 30 Wallstent 7 × 30 | 0 | YES | YES |
| 5 | 1 g of LAS IV + Tirofiban | SOLITAIRE | 3 | NO | Wingspan 4.5 × 20 mm Wingspan 4.5 × 15 mm | 2 | YES | NO |
| 6 | 1 g of LAS IV + 300 mg Clopidogrel PO | SOLITAIRE | 2B | NO | Wingspan 4.5 × 25 mm Wingspan 4.5 × 20 mm | 1 | YES | NO |
| 7 | 1 g of LAS IV + 300 mg Clopidogrel PO | - | 3 | NO | Wingspan 4.5 × 25 mm Wallstent 5 × 30 mm | 1 | YES | YES |
| 8 | 1 g of LAS IV + Tirofiban | SOLITAIRE | 3 | NO | Wingspan 4.5 × 25 mm Wingspan 4.5 × 25 mm | 1 | YES | NO |
| 9 | 1 g of LAS IV + Tirofiban | - | 3 | NO | Wingspan 4.5 × 25 mm | 0 | YES | NO |
| 10 | 1 g of LAS IV + Tirofiban | - | 3 | NO | Wingspan 4.5 × 20 mm Wingspan 4.5 × 15 mm Wallstent 5 × 30 mm | 0 | YES | YES |
| 11 | 1 g of LAS IV | SOLITAIRE | 2B | NO | Wingspan 4.5 × 20 mm | 0 | YES | NO |
IV: intravenous; LAS: lysine acetylsalicylate; mos: months; mRS: modified Rankin Scale; PO: by mouth
Discussion
Carotid revascularization in a patient with obstruction of the ICA due to dissection is technically complicated, due to the presence of vascular elongation and large cervical curves that impede proper positioning of classic carotid stents. One alternative is the placement of flow diverters or intracranial stents to keep the arterial lumen patent (Figure 1).7–9
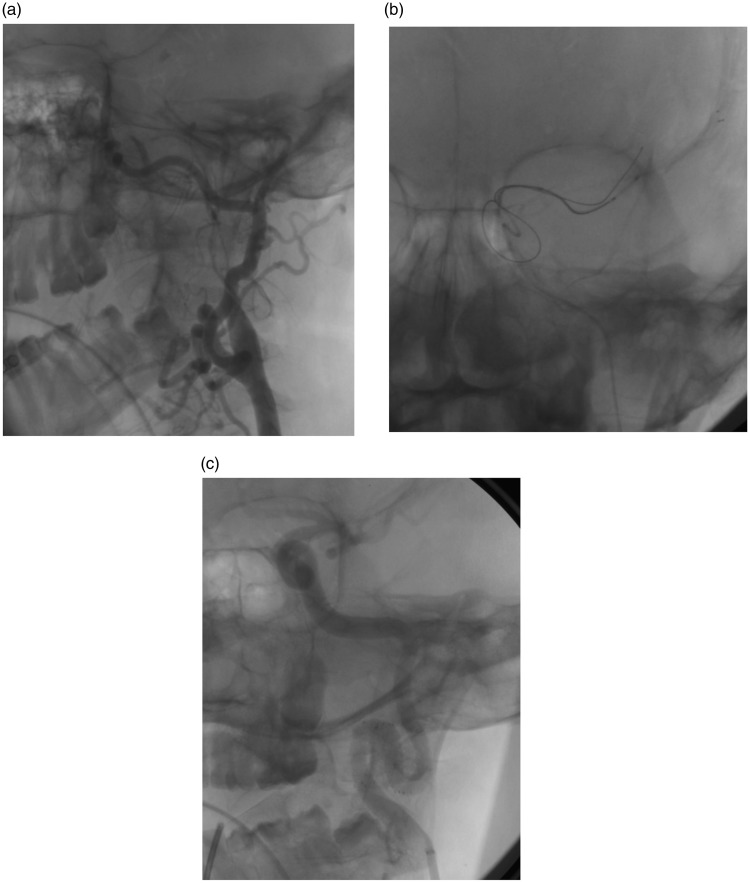
Figure 1.
Dissection in the cervical internal carotid artery, in a loop (a). It is associated with the presence of a thrombus in the M2 branch. Intracranial thrombus is extracted by a Solitaire (Medtronic, Minneapolis, USA) (b). Subsequently, two Wingspan stents were implanted in the dissection (c).
In our experience, the use of the Wingspan stent is a good alternative, due to its radial force that keeps the lumen of the artery patent without the need to dilate prior to or following stent placement. Another characteristic is its navigability through the arterial loops, without the need for distal access in small caliber branches. The primary problem is the small size of the stents, which are designed for a lumen of ≥ 5 mm, which results in repositioning and displacement of the material being seen at the 6-month follow-up, when treating the arterial dissection. Another problem with this device is its length, a maximum of 20 mm, which requires placement of several stents in the diseased segment, with the risk of distal and proximal displacement of a previously placed stent that has not fully adapted to a large-caliber artery (Figure 2).
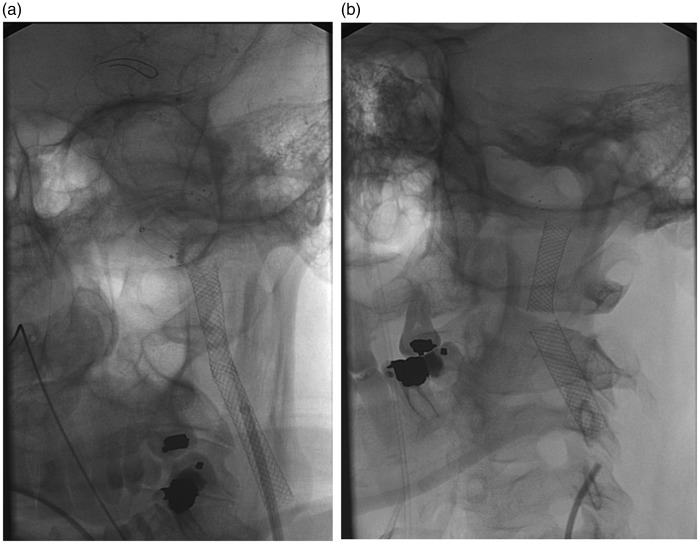
Figure 2.
Displacement of stents with healing of arterial dissection (a). The devices adapted to the new arterial caliber without complications at 6 months (b).
Nevertheless, in the small series shown, we achieved the proper positioning of the device in all cases, and the arterial lumen remained patent in all patients after 6 months, with no associated thromboembolic complications.
The anti-platelet regimen is heterogeneous. With the widespread use of intra-arterial treatment of stroke, the anti-platelet protocol in our center is done with a single 1-gram dose of intravenous LAS preoperatively and at 24 hours; and then we start two oral anti-platelet drugs, depending on the results of the imaging studies. A good option is probably intravenous (i.v.) infusion of IIb-IIIa inhibitors. In any case, this small series cannot be used to make conclusions on a more appropriate protocol, since none of the patients had hemorrhages in the post-operative period nor thromboembolic complications.
The excellent clinical progress of the patients demonstrated that endovascular techniques for the treatment of patients with ischemic stroke and occlusion of a large vessel are safe and effective, including when used outside of the strict regimens of current clinical guidelines. This will probably convert them into the techniques of choice, when faced with multiple pathologies such as symptomatic occlusion due to dissection of the ICA.
Conclusions
The Wingspan stent is an alternative to classic carotid stents and flow diverters for the treatment of cervical ICA dissection associated with ectasias or large loops. This device remains patent over the long term and it is not associated with arterial wall complications.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Ahlhelm F, Benz RM, Ulmer S, et al. Endovascular treatment of cervical artery dissection: Ten case reports and review of the literature. Interv Neurol 2012; 1: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xianjun H, Zhiming Z. A systematic review of endovascular management of internal carotid artery dissections. Interv Neurol 2013; 1: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: A systematic review of the literature. Neurosurgery 2011; 68: 856–866. [DOI] [PubMed] [Google Scholar]
- 4.Donas KP, Mayer D, Guber I, et al. Endovascular repair of extracranial carotid artery dissection: Current status and level of evidence. J Vasc Interv Radiol 2008; 19: 1693–1698. [DOI] [PubMed] [Google Scholar]
- 5.Mourand I, Brunel H, Vendrell JF, et al. Endovascular stent-assisted thrombolysis in acute occlusive carotid artery dissection. Neuroradiology 2010; 52: 135–140. [DOI] [PubMed] [Google Scholar]
- 6.Lavallée PC, Mazighi M, Saint-Maurice JP, et al. Stent-assisted endovascular thrombolysis versus intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke 2007; 38: 2270–2274. [DOI] [PubMed] [Google Scholar]
- 7.Brzezicki G, Rivet DJ, Reavey-Cantwell J. Pipeline embolization device for treatment of high cervical and skull base carotid artery dissections: Clinical case series. J Neurointerv Surg 2015. Jun 18. [DOI] [PubMed] [Google Scholar]
- 8.Ansari SA, Thompson BG, Gemmete JJ, et al. Endovascular treatment of distal cervical and intracranial dissections with the neuroform stent. Neurosurgery 2008; 62: 636–646. [DOI] [PubMed] [Google Scholar]
- 9.Juszkat R, Liebert W, Stanisławska K, et al. Extracranial internal carotid artery dissection treated with self-expandable stents: A single-centre experience. Cardiovasc Intervent Radiol 2015; 38: 1451–1457. [DOI] [PubMed] [Google Scholar]
- 10.Ohta H, Natarajan SK, Hauck EF, et al. Endovascular stent therapy for extracranial and intracranial carotid artery dissection: Single-center experience. J Neurosurg 2011; 115: 91–100. [DOI] [PubMed] [Google Scholar]
- 11.Krischek O, Miloslavski E, Fischer S, et al. A comparison of functional and physical properties of self-expanding intracranial stents (Neuroform3, Wingspan, Solitaire, Leo+, Enterprise). Minim Invasive Neurosurg 2011; 54: 21–28. [DOI] [PubMed] [Google Scholar]
- 12.Powers WJ, Derdeyn CP, Biller J, et al. Guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]