Abstract
Background
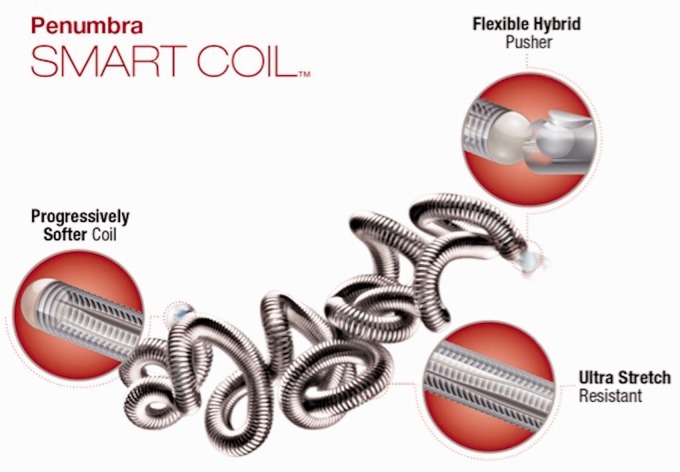
Penumbra SMART coils differ from traditional microcoils used for endovascular coil embolization of intracranial aneurysms (IAs) in that they (1) become progressively softer from their distal to proximal end, rather than being of uniform stiffness, (2) have a tight conformational structure, and (3) have a more robust stretch-resistance platform. These properties aid in preventing microcatheter prolapse and coil herniation during coil deployment and in filling small pockets of the aneurysm sac.
Objective/Methods
To determine the safety and efficacy of this device, the records of 17 consecutive patients with IAs treated with SMART coils were retrospectively analyzed.
Results
Thirteen female and four male patients were identified. Eleven patients presented with subarachnoid hemorrhage, four had recurrent aneurysms, and two had incidentally discovered aneurysms. Twelve aneurysms (two of which were recurrent) were treated with stand-alone coiling, three were treated with stent-assisted coiling, and two with flow diversion with adjuvant coiling. Microcatheter prolapse occurred in one case of a recurrent aneurysm, due to mechanical limitations imposed by a stent placed during prior coiling. Raymond-Roy Occlusion Classification (RROC) I or II occlusion was achieved in 12 aneurysms, including all 10 undergoing primary stand-alone coiling. Of the five RROC III occlusions, two were expected given treatment with flow diversion, while the other three occurred in complex, recurrent aneurysms. One patient suffered a thromboembolic complication of unclear clinical significance.
Conclusions
The Penumbra SMART coil is a safe and effective device for the endovascular treatment of IAs. Follow-up studies are required to establish long-term results.
Keywords: Endovascular coil embolization, intracranial aneurysm, subarachnoid hemorrhage
Introduction
Since the introduction of Guglielmi Detachable Coils (GDCs) to clinical neuroendovascular practice in 1995, endovascular coil embolization has emerged as the dominant treatment modality for ruptured and unruptured intracranial aneurysms (IAs) worldwide.1,2 The widespread utilization of endovascular techniques, however, has been met with concerns regarding increased rates of incomplete occlusion, recanalization, and retreatment relative to microsurgical clip occlusion.3,4 To mitigate these concerns and improve angiographic and neurological outcomes, coil technology has evolved over the past two decades.
The original GDCs were composed of bare platinum, assumed a helical two-dimensional (2D) shape upon deployment, and had a constant “slinky” shape without protection from stretching.5,6 Subsequent design iterations and technological advancements included coils with 3D and complex conformational shapes, varying degrees of uniform stiffness, stretch-resistance, larger diameters, and “bioactive” coil coatings in an effort to increase the efficacy of endovascular coil embolization. The Penumbra SMART coil (Penumbra Inc; Alameda, CA) is a novel next-generation device that becomes progressively softer from its distal to proximal end and has a tighter loop configuration that is truer to the intended coil size. These designs are intended to prevent microcatheter prolapse from the aneurysm and allow for more deliberate coil selection to minimize coil herniation or streaming from aneurysm during deployment. SMART coils also have a more robust stretch-resistance platform, which allows for operator comfort in repositioning or removing coils prior to detachment. These properties will potentially allow for reduced intra-procedural events and improved long-term results. The purpose of this study is to report our early experience with SMART coils in the treatment of IAs, with a particular emphasis on the safety and efficacy of the device after initial endovascular coiling.
Methods
Patients and data collection
We performed an observational, retrospective, single-center study to assess the safety and efficacy of the Penumbra SMART coil in the treatment of IAs. Following institutional review board approval at Massachusetts General Hospital (MGH), the records of 17 patients with 17 IAs treated with SMART coils between August 2015 and January 2016 were retrospectively reviewed. The study sample was collected by reviewing the prospectively maintained MGH neuroendovascular database within the study period. All historical, clinical, radiographic, and follow-up information was obtained from the electronic medical record in accordance with the Health Insurance Portability and Accountability Act (HIPAA). The Raymond-Roy Occlusion Classification (RROC) was used to grade the degree of initial aneurysm occlusion, as follows: Class I, complete occlusion; Class II, residual neck; and Class III, residual aneurysm.7 RROC I and II occlusions were considered “good” angiographic outcomes, given recent reports detailing their favorable prognosis.8–10 Thromboembolic complications were assessed by directly comparing pre- and post-embolization cerebral angiograms.
Device details
The Penumbra SMART coil is constructed with proprietary SmartCore™ technology. The distal portion of the coil is soft and atraumatic, while the proximal 10%–50% of the coil becomes progressively softer along its length. The coils also have a tighter conformational structure and a more robust stretch-resistance platform. SMART coils are compatible with standard 0.010-inch microcatheter systems, and are available in standard, soft, and extra soft varieties (Figure 1).
Figure 1.
Penumbra SMART coil. Published with permission.
In the present series, the IAs treated with stent-assisted coiling were performed with the Low-Profile Intraluminal Support Device (LVIS Jr, MicroVention, Tustin, CA) and the IAs treated with flow diversion with adjunctive coiling used the Pipeline Embolization Device (Medtronic; Minneapolis, MN). Coil(s) from other vendors were used in addition to SMART coils when specific SMART coil sizes were not available (four cases).
Statistical analyses
Descriptive statistics were calculated for clinical and radiographic factors, using the mean as a measure of central tendency. Comparative statistics were not conducted. All analyses were performed using JMP Pro 12 (SAS; Cary, NC).
Results
Patient, aneurysm, and procedural data
Seventeen patients with 17 IAs were analyzed. Table 1 summarizes the patient, aneurysm, and procedural details of this cohort. The median age was 65.6 years, and 76.5% were female. A history of smoking and hypertension was confirmed in seven (41.1%) and nine (52.9%) patients, respectively. A family history notable for IAs was confirmed in one (5.9%) patient.
Table 1.
Characteristics of 17 intracranial aneurysms treated with Penumbra SMART coils.
| Characteristic | No. (%) |
|---|---|
| Patient data | |
| Total patients | 17 |
| Total aneurysms | 17 |
| Age (years) | 65.6a |
| Female | 13 (76.5) |
| History of smoking | 7 (41.1) |
| History of hypertension | 9 (52.9) |
| Family history of intracranial aneurysm(s) | 1 (5.9) |
| Aneurysm data | |
| Location | |
| ACoA | 7 (41.1) |
| PCoA | 4 (23.5) |
| OphA | 2 (11.8) |
| BA apex | 1 (5.9) |
| BA trunk | 1 (5.9) |
| MCA | 1 (5.9) |
| PcaA | 1 (5.9) |
| Aneurysm size (mm) | 7.3a |
| Small (<10 mm) | 12 (70.6) |
| Large (10–25 mm) | 5 (29.4) |
| Giant (>25 mm) | 0 |
| Neck size (mm) | 3.2a |
| Ruptured | 11 (64.7) |
| Recurrent | 4 (23.5) |
| Blebs or dome irregularity | 12 (70.6) |
| Procedural data | |
| Embolization | |
| Stand-alone coiling | 12b (70.6) |
| Balloon-assisted coiling | 0 |
| Stent-assisted coiling | 3 (17.6) |
| Flow diversionc with adjuvant coiling | 2 (11.8) |
| Microcatheter prolapse | 1 (5.9) |
| Intraprocedural rupture | 0 |
| Thromboembolic complication | 1 (5.9) |
ACoA: anterior communicating artery; BA: basilar artery; MCA: middle cerebral artery; PcaA: pericallosal artery; PCoA: posterior communicating artery; OphA: ophthalmic artery.
Mean. bOne recurrent aneurysm was initially treated with stent-assisted coiling. cPipeline Embolization Device.
The mean aneurysm size was 7.3 mm, with 12 (58.8%) aneurysms measuring less than 10 mm. The mean neck size was 3.2 mm. The most common aneurysm locations were the anterior communicating artery (41.1%), posterior communicating artery (23.5%), and ophthalmic artery (11.8%). Twelve (70.6%) aneurysms harbored blebs or dome irregularities. Of the 17 IAs, four (23.5%) were recurrent and 11 (64.7%) were ruptured.
Twelve (70.6%) aneurysms (two of which were recurrent) were treated with stand-alone coiling, three (17.6%) were treated with stent-assisted coiling (two of which were recurrent), and two (11.8%) with flow diversion with adjuvant coiling. Microcatheter prolapse occurred during the coiling of one (5.9%) recurrent aneurysm that had been initially treated with stent-assisted coiling. One (5.9%) patient with a high-grade subarachnoid hemorrhage (SAH) suffered a thromboembolic complication of unclear clinical significance. No patient experienced an intraprocedural rupture.11
Angiographic data
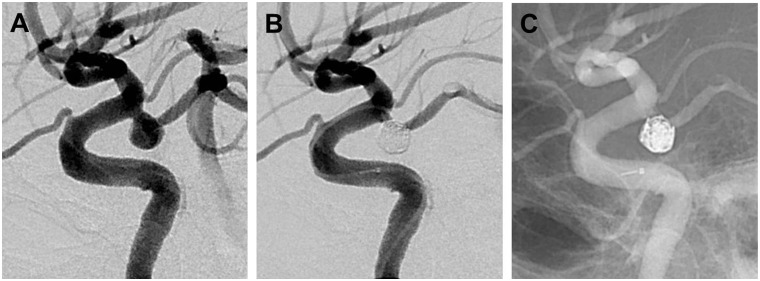
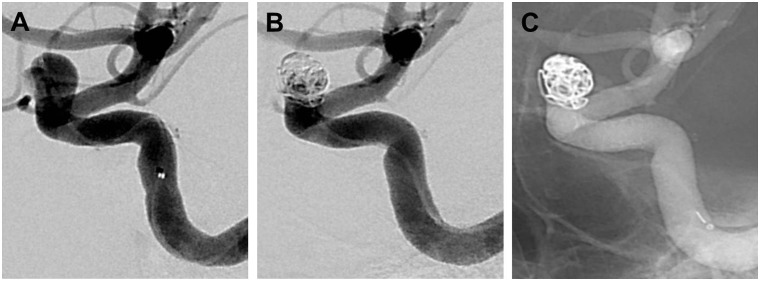
Table 2 lists the RROC designations for all 17 aneurysms at the time of SMART coil embolization. Of the 10 IAs undergoing primary treatment with stand-alone coiling, 10 (100%) had RROC I or II occlusions (Figure 2). Both (100%) IAs treated with flow diversion with adjunctive coiling had RROC III closures, given expected porous flow through the flow diverter and a loosely applied coil mass (Figure 3). Of the four complex, recurrent aneurysms, two were treated with stent-assisted coiling and two with stand-alone coiling; a RROC I or II occlusion was noted in one (25%) patient.
Table 2.
Angiographic outcomes of 17 intracranial aneurysms treated with Penumbra SMART coils.
| Treatment type | RROC I/II, no. (%) | RROC III |
|---|---|---|
| Primary | ||
| Stand-alone coiling | 10 (100) | 0 |
| Stent-assisted coiling | 1 (100) | 0 |
| Flow diversiona with adjuvant coiling | 0 | 2 (100) |
| Recurrent | 1 (25) | 3 (75) |
RROC: Raymond-Roy Occlusion Classification.
Pipeline Embolization Device.
Figure 2.
Lateral left internal carotid artery angiograms demonstrating a ruptured posterior communicating artery aneurysm before (a) and after ((b), (c)) Raymond-Roy Occlusion Classification I closure following Penumbra SMART coiling.
Figure 3.
Oblique lateral right internal carotid artery angiograms demonstrating an incidentally discovered bilobed ophthalmic artery aneurysm before (a) and after ((b), (c)) expected Raymond-Roy Occlusion Classification III closure following flow diversion with adjunctive Penumbra SMART coiling.
Clinical data
All six patients with unruptured aneurysms treated with SMART coil embolization had stable modified Rankin Scale (mRS) scores from admission to discharge. Of the 11 patients with ruptured aneurysms treated with SMART coil embolization, three patients died secondary to the severity of the initial hemorrhage. The remaining eight patients had stable or improved mRS scores from admission to discharge.
Discussion
Herein, we demonstrate the safety and efficacy of endovascular coiling of ruptured and unruptured IAs with next-generation Penumbra SMART coils. Seventeen patients underwent treatment of 17 IAs, 11 of which were ruptured and four of which were recurrent. The majority of IAs were located at the anterior or posterior communicating arteries (seven and four, respectively). Three aneurysms were treated with stent-assisted coiling and two with flow diversion with adjuvant coiling. Microcatheter prolapse occurred in one case of a recurrent aneurysm due to mechanical limitations imposed by a stent placed during prior endovascular treatment. RROC I or II occlusions were achieved in 12 cases, including all 10 aneurysms undergoing primary stand-alone coiling. Of the five RROC III occlusions, two were expected given treatment with flow diversion with adjuvant coiling, while the other three occurred in complex, recurrent aneurysms. One patient suffered a thromboembolic complication, while no patient suffered an intraprocedural rupture event.
Over the past two decades, microcoil technology has evolved significantly, with the current armamentarium of devices including those of different complex conformational shapes, varying degrees of uniform stiffness, larger diameters, and different metal alloys with “bioactive” coatings, all developed in an effort to increase angiographic and neurological outcomes following endovascular coil embolization. The importance of coil stiffness (or softness) is well recognized, and many coil manufacturers produce a range of microcoils of similar diameter and length with varying degrees of stiffness (but constant within one particular coil). Standard (i.e. stiffer) microcoils are typically deployed first in the embolization procedure and are used to create a frame within the aneurysm sac. Slightly softer microcoils are then used to fill the aneurysm sac within this initial coil frame, and even softer finishing microcoils are added last to occlude small pockets of persistent opacification not addressed by the framing and filling microcoils. The Penumbra SMART coil is unlike other microcoils in that the degree of stiffness varies not just among different microcoils but also within each microcoil from its distal to proximal end. This “progressively soft” design is intended to prevent microcatheter and microcoil prolapse from the aneurysm during treatment in order to improve angiographic and patient outcomes.
The efficacy of endovascular coiling of IAs has been well documented in several large-scale, randomized clinical trials, including the International Study of Unruptured Intracranial Aneurysms (ISUIA),12,13 the International Subarachnoid Aneurysm Trial (ISAT),14–17 and the Barrow Ruptured Aneurysm Trial (BRAT).18–20 Nevertheless, there remains a concern regarding the long-term durability of coil embolization as compared to clip occlusion. It is imperative to recognize that the vast majority of patients treated in these studies were done so with first- or second-generation endovascular devices. As further advances in microcoil technology are made, it is expected that both angiographic and neurological outcomes will continue to improve. The Penumbra SMART coil represents a critical development in the continued evolution of endovascular microcoil technology.
Conclusions
The Penumbra SMART coil is a safe and effective device for the endovascular treatment both of unruptured and ruptured IAs. Further prospective studies are necessary to determine the comparative efficacy of SMART coils to more traditional microcoils, with regard to packing density, degree of initial occlusion, recanalization, and retreatment.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ABP is a consultant for Penumbra Inc (Alameda, CA) and Covidien (Medtronic; Minneapolis, MN). CJS and CMT have no competing interests.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Brinjikji W, Rabinstein AA, Nasr DM, et al. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001–2008. AJNR Am J Neuroradiol 2011; 32: 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinjikji W, Lanzino G, Rabinstein AA, et al. Age-related trends in the treatment and outcomes of ruptured cerebral aneurysms: A study of the nationwide inpatient sample 2001-2009. AJNR Am J Neuroradiol 2013; 34: 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldron JS, Halbach VV, Lawton MT. Microsurgical management of incompletely coiled and recurrent aneurysms: Trends, techniques, and observations on coil extrusion. Neurosurgery 2009; 64(5 Suppl 2): 301–315. discussion 315–307. [DOI] [PubMed] [Google Scholar]
- 4.Owen CM, Montemurro N, Lawton MT. Microsurgical management of residual and recurrent aneurysms after coiling and clipping: An experience with 97 patients. Neurosurgery 2015; 62(Suppl 1): 92–102. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmi G, Viñuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg 1991; 75: 1–7. [DOI] [PubMed] [Google Scholar]
- 6.Guglielmi G, Viñuela F, Dion J, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg 1991; 75: 8–14. [DOI] [PubMed] [Google Scholar]
- 7.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001; 32: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 8.Mascitelli JR, Oermann EK, De Leacy RA, et al. Angiographic outcome of intracranial aneurysms with neck remnant following coil embolization. J Neurointerv Surg 2015; 7: 484–489. [DOI] [PubMed] [Google Scholar]
- 9.Mascitelli JR, Moyle H, Oermann EK, et al. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 2015; 7: 496–502. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton CJ, Torok CM, Rabinov JD, et al. Validation of the Modified Raymond-Roy classification for intracranial aneurysms treated with coil embolization. J Neurointerv Surg. Epub ahead of print 5 October 2015. DOI: 10.1136/neurintsurg-2015-012035. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton CJ, Walcott BP, Butler WE, et al. Neurological outcomes following intraprocedural rerupture during coil embolization of ruptured intracranial aneurysms. J Neurosurg 2015; 122: 128–135. [DOI] [PubMed] [Google Scholar]
- 12.Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med 1998; 339: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 13.Wiebers DO, Whisnant JP, Huston J, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362: 103–110. [DOI] [PubMed] [Google Scholar]
- 14.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet 2002; 360: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–817. [DOI] [PubMed] [Google Scholar]
- 16.Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol 2009; 8: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molyneux AJ, Birks J, Clarke A, et al. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015; 385: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg 2012; 116: 135–144. [DOI] [PubMed] [Google Scholar]
- 19.Spetzler RF, McDougall CG, Albuquerque FC, et al. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg 2013; 119: 146–157. [DOI] [PubMed] [Google Scholar]
- 20.Spetzler RF, McDougall CG, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg 2015; 123: 609–617. [DOI] [PubMed] [Google Scholar]