Abstract
Tobacco use is a global pandemic that poses a substantial and costly health burden. There are some treatment options are available, but currently marketed smoking-cessation drugs lack high levels of efficacy, particularly in real-life settings. Consequently, there is a compelling need for more effective pharmacotherapies to aid smokers in maintaining long-term abstinence. Advances in the understanding of the mechanisms involved in nicotine dependence have recently been translated into new medications and vaccines that interfere with nicotine signaling, many of which are currently at an advanced stage of development. In the present article we review current and emerging pharmacotherapies for tobacco dependence, focusing on the mechanistic rationale for their potential anti-addiction efficacy, major findings in preclinical and clinical studies, and future research directions.
Introduction
Tobacco use is a global pandemic that affects an estimated 1.2 billion people and poses a substantial health burden. With approximately 5 million tobacco-related deaths annually, tobacco smoking is the leading cause of preventable premature mortality in the world [1]. Death is primarily caused by lung and other cancers, coronary heart disease, chronic obstructive pulmonary disease (COPD) and stroke, and also by infectious diseases [2–4]. The risk of serious disease diminishes rapidly after smoking cessation — ‘quitting’ — and permanent abstinence is known to reduce the risk of lung cancer, heart disease, chronic lung disease, stroke, and other cancers [5,6].
‘Offer help to quit tobacco use’ in people addicted to nicotine is one of the six proven policies identified by the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) to expand the fight against the tobacco epidemic [7]. In keeping with these recommendations, state governments (the FCTC has been endorsed by over 160 countries) are under an obligation to address and treat tobacco dependence in their primary healthcare services. Treatment for smoking cessation includes diverse methods from simple medical advice to pharmacotherapy, and evidence-based recommendations indicate that providing advice on smoking cessation is useful in helping smokers to quit [8]. Counseling is effective in treating tobacco dependence, and its effectiveness increases with treatment intensity. Two components of counseling are especially effective, and clinicians should use these when counseling patients making a quit attempt-practical counseling (problem solving/skills training), and social support delivered as part of the treatment [8]. Counseling and medication are each effective in treating tobacco dependence, but the combination of both is more effective than either alone, probably at least in part because counseling improves medication adherence. Thus, clinicians should encourage all individuals making a quit attempt to use both counseling and medication [8]. Moreover, treatments aimed at smoking cessation are among the most cost-effective interventions in healthcare [9].
Unfortunately, the powerful addictive qualities of nicotine create a huge hurdle, even for those with a strong desire to quit. Approximately 80% of smokers who attempt to quit on their own relapse within the first month of abstinence, and only ~3–5% remain abstinent at 6 months [10]. The pharmacologic effect of nicotine plays a crucial role in tobacco addiction [11], and therefore pharmacotherapy is important to address this component of tobacco dependence in order to improve success rates (Box 1).
Box 1. Neural pathways involved in nicotine addiction.
Addiction is a complex behavioral phenomenon with causes and effects that range from molecular mechanisms to social interactions. Essentially, the process of nicotine addiction begins with molecular interactions that alter the activity and metabolism of the neurons that are sensitive to nicotine. Over time this alters the properties of individual neurons and circuits, and this leads to complex behaviors including dependence, tolerance, sensitization, and craving.
Upon inhalation of cigarette smoke, nicotine passes into the bloodstream and, within seconds, crosses the blood-brain barrier to enter the brain. Nicotine binds principally to α4β2 and α7 nicotinic acetylcholine receptors (nAChRs) located on dopaminergic, glutamatergic and GABAergic neurons in the ventral tegmental area (VTA) of the midbrain, which in turn modulate the release of extracellular dopamine (DA) in the nucleus accumbens (NAcc). The release of DA in the NAc is responsible for the rewarding and addictive effects of nicotine.
The activity of DA neurons in the VTA is under tonic excitatory glutamatergic inputs predominantly from the prefrontal cortex, and tonic inhibitory GABAergic inputs from local GABAergic interneurons as well as from long-loop GABAergic projections from the NAcc. Endogenous ACh release from brainstem cholinergic neurons is also known to modulate the activity of the inhibitory GABAergic interneurons.
In the presence of nicotine concentrations similar to those found in the blood of smokers, the α4β2 nAChRs of the GABA interneurons rapidly desensitize, effectively inhibiting GABAergic inputs to DA neurons in the VTA. The α7 nAChRs located on presynaptic glutamatergic terminals do not desensitize to the same extent, and glutamatergic inputs are therefore enhanced as GABAergic inputs are depressed, thereby leading to a net increase in excitation of the DA neurons in the VTA.
In addition, chronic nicotine exposure could also increase endocannabinoid content in the VTA and the NAcc, and this could remove the tonic inhibitory GABAergic control on VTA DA neurons via CB1 receptors localized on VTA GABAergic neurons or their terminals, thus indirectly modulating NAcc DA release and nicotine reward.
Although much attention has focused on the VTA—NAcc pathway, many other brain sites that have not yet been extensively studied, and numerous neurochemical systems (including catecholamines, serotonin, neuropeptides, hypocretins), are also likely to contribute to nicotine reward and addiction.
Based on this model, diverse pharmacological agents that target acetylcholine, dopamine, glutamate, GABA, and endocannabinoid signaling systems have been proposed and studied for their potential use in the treatment of nicotine dependence. Furthermore, strategies to reduce the rate and the quantity of nicotine entry into the brain (i.e. nicotine vaccines), could be also of significant benefit.
In this article we review the available pharmacological treatments for tobacco dependence and discuss new smoking-cessation products in clinical development.
Present therapeutic options for nicotine addiction
Present clinical practice guidelines categorize pharmacotherapy for the treatment of tobacco dependence into first-line [nicotine replacement therapy (NRT), bupropion and varenicline] and second-line medications (including nortriptyline and clonidine), although these medications are also used in combination [8]. We will discuss monotherapy in detail, but combination pharmacotherapy is also addressed below. Compared to placebo alone, first-line medications are modestly effective, but they can substantially enhance the effect of counseling [8]. With the exception of varenicline, which has been shown to offer notable improvement in abstinence rates over bupropion [8], all first-line medications appear to be of similar efficacy, but there have been few direct comparisons. Second-line medications for treatment of tobacco dependence can be effective, but drug manufacturers have not sought approval from the US Food and Drug Administration (FDA) for this indication, and there are concerns about potential side effects. Second-line therapies are recommended by current guidelines for patients who are unresponsive to or unable to tolerate first-line agents. In addition to reducing withdrawal symptoms and craving, pharmacotherapy decreases the short-term reinforcing effects of tobacco after initial cessation. This can help to ease the process of learning new coping skills. The addition of a pharmacologic agent to a quit plan can have a positive psychological impact on those making cessation attempts.
NRT
NRT is the most common medication used to assist tobacco cessation [12]. Its primary mechanism of action is to replace partially the nicotine formerly obtained from tobacco smoking (Figure 1), and this aids smoking cessation by attenuating the reinforcing effects of nicotine delivered via tobacco, and therefore reduces the severity of withdrawal symptoms and cravings [13]. NRT also simultaneously reduces the psychogenic reward associated with smoking [14]. NRT does not completely eliminate all symptoms of withdrawal because the available delivery systems do not reproduce the rapid and high levels of nicotine achieved through tobacco use [15,16]. Differences in formulations (nicotine lozenge, gum, patch, nasal spray, and inhaler) could have a distinct impact upon either withdrawal symptoms or urges to smoke, but there is little direct evidence that one nicotine product is more effective than another. A Cochrane Review article recently found that all forms of NRT approximately double the likelihood of long-term abstinence from smoking [17]. Likewise, at least two large studies found that all forms of NRT tested (gum, patch, nasal spray, and inhaler) produced similar quit rates and were equally effective at reducing the frequency, duration, and severity of urges to smoke [18,19]. According to the US Public Health Service Guidelines meta-analyses, the nicotine nasal spray is slightly more effective than the standard dose patch or short-term gum [8]. Although not formally regulated as a pharmaceutical product, the electronic-cigarette (e-Cig) can also deliver nicotine. It is a battery-powered electronic device resembling a cigarette in which no tobacco or combustion is necessary for its operation. By supplying nicotine, e-Cigs can help smokers to remain abstinent or reduce their cigarette consumption. To date there is no formal demonstration supporting the efficacy and safety of these devices, but several large prospective studies are ongoing in Italy, New Zealand and USA [listed on the US National Institutes of Health (NIH) Clinical Trials website at http://clinicaltrials.gov/ and by the New Zealand Clinical Trials unit at http://www.ctru.auckland.ac.nz/index.php/research-programmes/addiction-research].
Figure 1.
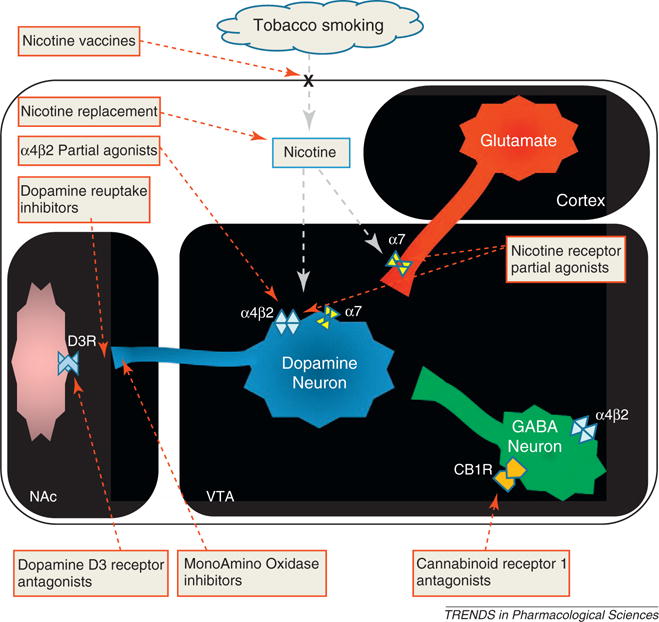
Simplified model of the nicotine—acetylcholine—glutamate—GABA—dopamine neural circuitry promoting nicotine reward, dependence and withdrawal, and the related mechanism-based pharmacological rationale for the treatment of nicotine dependence. Nicotine from cigarette smoke reaches the midbrain and binds to α4β2 and α7 nicotinic acetylcholine receptors (nAChRs) located on dopaminergic (blue), glutamatergic excitatory (red) and GABAergic inhibitory (green) neurons in the ventral tegmental area (VTA). This modulates the release of dopamine (DA) in the nucleus accumbens (NAc), which mediates the rewarding and addictive effects of nicotine. Based on this model, different pharmacological strategies have been proposed and studied for their potential use in the treatment of nicotine dependence.
In general, NRT is considered to be safe for most patients, with a relatively low rate of discontinuation due to adverse events [17–19]. Adverse events are generally formulation-specific, and depend on the delivery system used [20]. Contraindications or warnings for NRT include a history of myocardial infarction within the past 6 weeks, uncontrolled hypertension (or hypertension that emerges during treatment), severe dysrhythmia, or unstable angina. Despite a warning in the package insert, NRT has been found to be safe in smokers with cardiovascular disease, including those with recent myocardial infarction [21]. There is concern regarding the use of NRT in patients with uncontrolled diabetes mellitus because nicotine can impair insulin sensitivity, but the risks of NRT have to be weighed against the risk of continued smoking [22]. Because of the slower delivery of nicotine (and, in part, because NRT only partially addresses the reinforcing behavioral and social effects of smoking), NRT has been shown to have low liability for abuse and low dependence potential [23]. In addition, there is little to no withdrawal discomfort when patients discontinue NRT use [23].
Pre-cessation use of NRT (i.e. use for several weeks prior to tobacco cessation) has been reported in several small trials and meta-analyses to enhance smoking cessation success [24]. Possible mechanisms are reduced reward from smoking due to nicotinic acetylcholine receptor (nAChR) desensitization and extinction of the conditioned link between smoking and nicotine self-administration. A recent large clinical trial did not find benefit of pre-cessation NRT; however, this trial was conducted via a national quit-line so compliance with treatments was difficult to assess, and the dropout rate was high [25].
Bupropion
Bupropion hydrochloride, a drug chemically related to phenylethylamines, was initially developed and marketed as an antidepressant. Bupropion was subsequently found to be effective as a smoking-cessation aid, with sustained-release (SR) oral formulations preferred over immediate release. Bupropion SR (Zyban®, GlaxoSmithKline) is taken twice daily, and bupropion XL (Wellbutrin®, GlaxoSmithKline) is taken once daily. Recently, several generic versions of bupropion have been marketed for smoking cessation worldwide. Dosing with bupropion for 1 week before quitting is recommended so as to allow accumulation of blood levels of bupropion and its active metabolites.
The mode of action of bupropion in smoking cessation is not completely understood, but inhibition of neuronal reuptake of dopamine and a weak nAChR antagonist effect (Figure 1) are thought to contribute to the reported reduction in the severity of nicotine cravings and withdrawal symptoms [26,27]. A Cochrane Review article found that bupropion doubles the chances of quitting smoking compared with placebo [28]. Pooled analyses of studies with bupropion generally show quit-rates similar to those obtained with NRT [17,28]. This finding has been also confirmed by the meta-analyses of the US Public Health Service Guidelines [8]. Bupropion has been found to be equally effective in smokers with and without a history of depression [28]. The Roswell Park Cancer Institute is currently investigating the effects of extended pre-cessation bupropion for smoking cessation in Phase II studies (NIH Clinical Trials website).
The most common adverse events with bupropion are insomnia (30–40% of patients), and dry mouth (approx. 10% of patients) [28]. In a comparative trial, the incidence of nausea was similar with bupropion, NRT, and the combination of both, and all had approximately double the rate observed with placebo. Rates of discontinuation from clinical trials due to adverse events generally range from 7% to 12% [28]. A small risk of seizures has been observed; two large studies reported a seizure incidence of approximately 1 per 1000 [29,30]. Therefore, prescription is contraindicated in patients with a history of seizures. Bupropion is safe for use in patients with cardiovascular disease, although occasional increases in blood pressure have been reported in smokers with hypertension [31]. The prescribing information for bupropion carries a ‘black-box’ warning based on observations that antidepressants have increased the risk for suicidal ideation and behavior in children and adolescents with certain psychiatric disorders.
Varenicline
Pfizer’s varenicline (Chantix/Champix®), launched in 2006, became the first new prescription drug for smoking cessation in ~10 years. It is a partial agonist selective for the α4β2 nAChR subtypes in the ventral tegmental area of the brain (Figure 1). Varenicline has dual effects: partial stimulation of nAChRs, without creating the full effect of nicotine (agonist action), and blocking nAChRs, which prevents the nicotine from tobacco from reaching them (antagonist action) [32,33].
These effects provide relief from the cravings and withdrawal symptoms experienced during smoking cessation [32,33]. Furthermore, the drug could also reduce smoking satisfaction, thereby potentially reducing the risk of relapse. In two identically-designed randomized, doubleblind multicenter trials (which were placebo-controlled and active-controlled with bupropion-SR 150 mg twice daily), investigators demonstrated that, after one year, healthy smokers had a ~2.5 greater odds of quitting with varenicline 1 mg twice-daily compared with placebo, and approximately 1.7 times greater odds compared with bupropion [34,35]. The US Public Health Service Guidelines meta-analyses confirm this significant improvement in abstinence rates with varenicline over bupropion [8].
An evaluation of long-term maintenance treatment in patients who stopped smoking during 12-week open-label treatment with varenicline showed this agent offers significant advantages for relapse prevention over placebo after 6 months of treatment (OR, 2.48; 95% CI, 1.95–3.16) [36]. Unlike other pharmacotherapies, varenicline is associated with progressively increasing cessation rates over 12 weeks of treatment, presumably due to the antagonism of nicotine from cigarettes, resulting in less satisfaction from smoking.
Varenicline is generally well tolerated. The most commonly reported adverse effects are nausea, insomnia, gastrointestinal upsets and headache, but these were also commonly reported with placebo [34,35]. Just as for bupropion, the prescribing information for varenicline also carries a black-box warning highlighting an increased risk of psychiatric symptoms and suicidal ideation in patients reporting any history of psychiatric illness.
Varenicline is also safe and effective in patients with COPD and cardiovascular disease [37,38]. In a 12-month, multicenter, double-blind, placebo-controlled trial of 499 patients with mild-to-moderate COPD [37], 18.6% of the varenicline group ceased smoking versus 5.6% of the placebo group. In a multicenter, double-blind, placebo-controlled study of 714 smokers with stable cardiovascular disease [38], varenicline was three times more effective than placebo. The continuous abstinence rate at 12 months (confirmed by CO monitoring) was 19.2% in the varenicline group and 7.2% in the placebo group.
Nortriptyline
Nortriptyline is a second-generation tricyclic antidepressant used in the treatment of major depression. Nortriptyline has been studied in smoking-cessation studies at dosages of 75–100 mg/day [39]. A Cochrane Review metaanalysis of six randomized clinical trials indicated that nortriptyline treatment doubles the odds of smoking cessation, with an OR for abstinence of 2.14 (95% CI, 1.49–3.06) [39]. Thus, nortriptyline appears to be as effective as NRT or bupropion. Several theories regarding the effect of nortriptyline on tobacco dependence have been proposed, including its antidepressant action and its noradrenergic effects. However, there are no preclinical or clinical studies available to support any of these potential mechanisms [39].
There are a number of potential adverse effects of nortriptyline, including sedation, dizziness, insomnia, blurred vision, constipation, and nausea. Whereas these adverse events occur frequently in patients being treated for depression, they are rarely seen at the doses used for smoking cessation [39]. Despite this, the prescribing information for nortriptyline carries a black-box warning similar to that for bupropion and varenicline regarding an increased risk of suicidal ideation and behavior particularly among patients taking antidepressants. Caution should be exercised when considering nortriptyline for patients with cardiovascular disorders because it can increase the risk of dysrhythmia, hypertension, orthostatic hypotension, and tachycardia [40]. Because of the limited number and range of patients in whom nortriptyline has been evaluated for smoking cessation, the complete safety profile in these patients is unclear [40].
Clonidine
Clonidine is approved by the FDA only for the treatment of hypertension. However, it has been also shown to be effective in reducing symptoms of nicotine withdrawal, and for this reason it is listed as a second-line tobacco-cessation drug [8]. Its efficacy in smoking cessation is based on its ability to counteract CNS features of nicotine withdrawal, including craving and anxiety [41].
Both oral (0.15–0.45 mg/day) and transdermal patch (0.1–0.3 mg/day) formulations of clonidine have been shown to be effective aids for smoking cessation [42]. Pooled results from six randomized clinical trials demonstrated an approximate doubling of the rate of abstinence after at least 12 weeks of follow-up compared with placebo (OR, 1.89; 95% CI, 1.30–2.74) [42].
The Cochrane Review noted a high incidence of dose-dependent adverse events that are consistent with the central and systemic effects of the α2-adrenergic agonist activity of clonidine; these include significant sedation and postural hypotension [42]. Other dose-related adverse events with clonidine include dry mouth, bradycardia, dizziness and constipation. Caution should also be used when coadministering clonidine with β-blockers, calcium channel blockers, or digitalis.
Combination pharmacotherapy
Combinations of smoking-cessation medications appear to increase efficacy in smoking cessation compared to monotherapy [8,43]. Combinations that have been well-studied with proven benefit include the nicotine patch plus a more rapid release NRT such as gum, lozenge or spray, and bupropion plus NRT. The latter is approved for marketing as a combination therapy. However, the cost-effectiveness of this approach has not been clearly demonstrated. Combinations of nortiptyline plus NRT, varenicline plus bupropion, and varenicline plus NRT have or are being studied, but their efficacy and safety have not yet been established. Mechanisms underlying the benefit of combination NRT are thought to be a stable level of nicotine from the patch to relieve withdrawal symptoms plus the use of more rapid release preparations to deal with episodes of craving or other withdrawal symptoms. Combinations of other medications provide two different mechanisms for relief of withdrawal symptoms and/or antagonism of nicotine reinforcement from smoking relapses.
The tobacco-cessation pipeline
As discussed above, currently-marketed tobacco-cessation products increase the chance of quitting smoking. However, they lack high levels of efficacy, show wide variation in success rates across studies, and some are associated with significant adverse side effects. Consequently, there is a compelling need for more effective smoking-cessation drugs. In an effort to fill this gap a host of pharmaceutical companies and research institutions are researching novel smoking-cessation products that interfere with nicotine signaling, many of which are currently in clinical development (Table 1).
Table 1.
Overview of smoking-cessation products in clinical development
| Company/institution name | Product name | Drug type | Active ingredient | Trial phase | Description |
|---|---|---|---|---|---|
| Acrux | Nicotine MDTS | Nicotine receptor ligand | Nicotine | Phase I | Metered dose skin spray delivery technology |
| Aradigm | ARD1600 | Nicotine receptor ligand | Nicotine | Phase I | Aerosolized, inhaled nicotine developed using AERx inhalation technology |
| NAL Pharmaceuticals | NAL2771 | Nicotine receptor ligand | Nicotine | Phase I | New nicotine 24 h matrix patch |
| NAL Pharmaceuticals | NAL2762 | Nicotine receptor ligand | Nicotine | Phase II | NAL2762 is being developed as a nicotine orally dissolving film (ODF) for smoke cessation. |
| Sopharma AD | Tabex | Nicotinic Receptor Partial Agonists | Cytisine | Phase III | Cytisine is a natural alkaloid with partial agonist activity at the α4 β2 nicotinic receptor. |
| Merck & Co | MK0364 | Cannabinoid-1 receptor (CB1R) antagonism | Taranabant | Phase II | Taranabant acts by reducing the food intake and increasing energy expenditure and fat oxidation. MK-0364 is being developed as an aid for smoking cessation. |
| GlaxoSmithKline | GSK598809 | Dopamine D3 antagonist | Not available | Phase I | Antagonizing dopamine selectively at the D3 receptor disrupts nicotine conditioned effects. GW598809 has been developed for smoking cessation and drug addiction. |
| Evotec | EVT 302 | MAO-B inhibitor | Not available | Phase II | EVT 302 could increase dopamine levels in the brain by preventing the metabolism of dopamine by MAO-B, thus enhancing dopaminergic transmission. |
| National Institute on Drug Abuse | Selegiline | MAO-B inhibitor | Selegiline | Phase II | Selegiline enhances dopaminergic transmission in the brain by preventing the metabolism of dopamine by MAO-B. Both transdermal and oral formulations are under investigation, as aids in smoking cessation. |
| Celtic Pharmaceuticals | TA-NIC | Therapeutic vaccine | Nicotine butyric acid covalently linked to recombinant cholera toxin B | Phase II | Safety and smoking abstinence rate of 2 doses of TA-NIC compared to placebo in 525 patients enrolled in three arms |
| Cytos Biotechnology/Novartis | NIC002 | Therapeutic vaccine | Recombinantly produced virus-like protein | Phase II | Safety and smoking abstinence rate of NIC002 in 200 cigarette smokers motivated to quit with a reformulated vaccine with fewer side-effects. |
| Independent Pharmaceutica | Niccine | Therapeutic vaccine | Not available | Phase II | Ability of Niccine to prevent relapse in 355 smokers that have recently stopped smoking with the aid of a smoking-cessation drug and counseling. |
| GlaxoSmithKline/Nabi Biopharmaceuticals | NicVAX | Therapeutic vaccine | 3′-Aminomethyl nicotine conjugated to recombinant Pseudomonas exoprotein A | Phase III | Evaluate NicVAX as an aid to smoking cessation for long term abstinence (by subject self-report and carbon monoxide confirmation). |
| Nabi Biopharmaceuticals | NicVAX + Champix | Combination product | 3-Aminomethyl nicotine hapten + varenicline | Phase II | Co-administration of NicVAX with varenicline as a powerful aid to smoking cessation and relapse prevention. |
| Cary Pharmaceuticals | QuitPak | Combination product | Bupropion HCl + mecamylamine | Phase I | QuitPak contains mecamylamine and bupropion hydrochloride. |
| Boston University | D-cycloserine | Broad-spectrum antibiotic | Cycloserine | Phase II | Cycloserine, a second-line, broad-spectrum antibiotic, enhances cognitive behavioral therapy. D-cycloserine is being developed for the smoking cessation. |
| GlaxoSmithKline | GW468816 | Glycine receptor antagonist | Not available | Phase II | GW468816 is a glycine receptor antagonist being developed for smoking cessation. |
| Roswell Park Cancer Institute | Bupropion HCl RPCI | Antidepressant | Bupropion HCl | Phase II | Bupropion hydrochloride is being developed as an oral formulation for smoking cessation in extended pre-cessation studies. |
| Somaxon Pharmaceuticals | Nalmefene | Opioid receptor antagonist | Nalmefene | Phase II | Nalmefene is an opioid receptor antagonist developed for smoking cessation. |
| University of Chicago | Naltrexone | Opioid receptor antagonist | Naltrexone HCl | Phase II | Prevents binding of the opiate agonists to opioid receptors. Naltrexone is being developed as tablet formulation for smoking cessation in women. |
Novel pharmaceutical nicotine products
ARD-1600 (Aradigm Corporation) is an inhaled aerosolized nicotine developed for the treatment of smoking cessation using AERx inhalation technology. The AERxEssence palm-size inhaler delivers consistent doses of small droplet aerosols to the deep lung for systemic uptake of nicotine. A Phase I trial of 18 adult male smokers demonstrated that using the AERx Essence inhaler results in very rapid absorption of nicotine into the bloodstream and appears to be associated with acute reduction of craving for cigarettes (http://www.aradigm.com/products_1600.html) Blood levels of nicotine rose much more rapidly following a single-breath inhalation compared to published data on other approved nicotine delivery systems. A substantial and consistent reduction in mean craving scores was observed as early as 5 min after inhalation of the nicotine solution and did not return to pre-dose baseline during the 4 h of subsequent monitoring. No serious adverse reactions were reported in the study.
Nicotine MDTS (Acrux Limited) is being developed using metered-dose skin-spray delivery technology. This formulation has been optimized to deliver higher amounts of nicotine across human skin than can be achieved with standard NRT patches. This is presently in Phase I clinical trials in Australia (details on the Acrux website at http://www.acrux.com.au).
Another interesting nicotine formulation in clinical development is NAL2762 (NAL Pharmaceuticals Ltd), a nicotine orally-dissolving film (ODF) for smoking cessation. This is presently in Phase II clinical trials [NAL Pharmaceuticals exhibitor abstract at the American Association of Pharmaceutical Scientists (AAPS) meeting 2009; http://www.aaps.org/meetings/annualmeet/am09/index.asp].
nAChR partial agonists
As discussed above, nicotinic ligands with partial agonist activity at specific brain nicotinic receptor subtypes (Figure 1) have the potential to optimize benefit and minimize adverse effects. A number of partial agonists have been synthesized or purified and evaluated as possible smoking-cessation treatments [44]. Three examples mentioned in recent publications are dianacline, sazetidine-A and cytisine [45,46].
Cytisine is a natural alkaloid found in plants such as Cytisus laburnum [47]. It is a structural analog of nicotine and a partial agonist at the α4β2 nAChR. Cytisine also has high affinity for other nAChR subtypes, the therapeutic consequence of which is unknown. Cytisine has been used for smoking cessation in central and eastern European countries for many years, although controlled clinical-trial data on efficacy are lacking [47]. Cytisine has a short halflife, requiring frequent daily dosing. Furthermore, cytisine has relatively poor brain penetration, requiring high doses and potentially limiting efficacy. An advantage of cytisine is that it is inexpensive to manufacture, which could lead to greater accessibility by smokers, particularly in developing countries. In 2007 Sopharma was granted registration of the first original Bulgarian product, Tabex (now on the market in the Republic of Serbia), and clinical trials are ongoing in Europe.
Cannabinoid receptor 1 antagonism
The cannabinoid receptor system is thought to indirectly inhibit the dopamine-mediated rewarding properties of food and tobacco. Functionally, chronic nicotine exposure appears to activate the brain endocannabinoid system in limbic regions, and the cannabinoid receptor 1 (CB1R) of the GABA interneurons in the VTA could play a key role in this interaction [48] (Box 1 and Figure 1). It has therefore been proposed that CB1R antagonists might have value in smoking-cessation therapy [49,50].
Rimonabant is a CB1R antagonist with demonstrated efficacy as an anti-obesity drug and smoking-cessation treatment [48,51]. However, because of FDA concerns regarding the safety profile of rimonabant, the manufacturer withdrew the New Drug Application (NDA) in 2007 (details at http://en.sanofi-aventis.com). Sanofi-Aventis had been developing surinabant for smoking cessation. In 2008, Sanofi-Aventis discontinued development of the drug, which had reached Phase II trial. MK-0364 (Merck & Co Inc) was also being developed as an aid for smoking cessation. MK-0364 contains taranabant, a CB1R inverse agonist, which acts by reducing food intake and increasing energy expenditure and fat oxidation. In a recent large Phase II randomized clinical trial, 8-week treatment with MK0364 did not improve smoking cessation and was associated with increased incidence of psychiatric adverse events, gastrointestinal discomfort, and flushing [52].
Dopamine D3 antagonists
The dopamine D3 receptor is significantly involved in mechanisms of dependence on nicotine and other drugs. Antagonizing dopamine selectively at the D3 receptor disrupts these nicotine-mediated effects and could represent a novel therapeutic approach for smoking cessation (Figure 1). GlaxoSmithKline has recently completed Phase I clinical testing of an investigational dopamine D3 antagonist, GSK598809, for smoking cessation and drug addiction, and is now launching a Phase II trial to establish whether GSK598809 can help to reduce relapse in people who have recently stopped smoking (NIH Clinical Trials website).
Monoamine oxidase inhibitors
Several monoamine oxidase type B (MAO-B) inhibitors are under investigation as therapies for smoking cessation. This class of drug is thought to increase dopamine levels in the brain by preventing the metabolism of dopamine by MAO-B, thus enhancing dopaminergic transmission and reducing nicotine-withdrawal symptoms [53] (Figure 1).
Evotec has developed EVT 302, an orally active, potent, highly selective and reversible MAO-B inhibitor, as an aid to smoking cessation. Phase I safety and tolerability trials of EVT 302 were successfully completed but in a recent Phase II proof-of-concept study, 8-week treatment with EVT 302 failed to demonstrate any significant improvement in cessation rate (details at http://www.evotec.com).
Selegiline is a selective and irreversible MAO-B inhibitor that is used in conjunction with levodopa to alleviate symptoms associated with Parkinson’s disease (PD) [54]. PD is characterized by loss of dopamine-producing cells and treatment with selegiline helps retention of stored dopamine by inhibiting its breakdown. The USA National Institute on Drug Abuse (NIDA) is investigating both oral and transdermal formulations of selegiline as aids in smoking cessation. Several small-scale studies have shown that selegiline is effective in reducing withdrawal symptoms and increasing abstinence compared with placebo. In one study, 10 mg oral selegiline decreased craving during abstinence and reduced smoking satisfaction during smoking [55]. In another study, oral selegiline (5 mg twice daily) increased the trial endpoint (8 week) 7-day-point prevalent abstinence by threefold [56]. In a third study, oral selegiline (plus nicotine patch) doubled the 52-week continuous abstinence rate compared with nicotine patch alone, although the difference was not significant due to small subject numbers [57]. Unfortunately, the first large double-blind, placebo-controlled randomized trial of oral selegiline for smoking cessation failed to show improvement in smoking-abstinence rates [58]. Nonetheless, NIDA is currently carrying out a number of Phase II trials on selegiline in the USA.
Nicotine vaccines
One of the most active areas of the tobacco-dependence pipeline is the development of therapeutic vaccines. Nicotine vaccines work by causing the immune system to produce antibodies directed against the nicotine obtained from tobacco smoking, thus reducing the rate and quantity of nicotine entry into the brain [59] (Figure 1). This reduces the pleasure and other rewarding effects produced by nicotine. It is hoped that nicotine vaccines will interrupt the reward-inducing effects of nicotine to assist patients in preventing relapse. The nicotine molecule itself is not immunogenic because it is too small to be recognized by the immune system, and nicotine vaccines under development therefore comprise nicotine conjugated to a larger carrier protein. Examples include a bacterial exoprotein (a protein at the external surface of bacteria) as in NicVAX by Nabi Biopharmaceuticals/GSK, a virus-like-particle (recombinantly produced virus shells containing no viral genetic information) as in NIC002 by Cytos Biotechnology/Novartis, and a recombinant cholera toxin as in TA-NIC by Celtic Pharmaceuticals.
If successful, nicotine vaccines will contribute to the fight against tobacco addiction in an innovative way. Nicotine vaccines could have an important advantage in that these can have a prolonged effect on the immune system (for 6–12 months), and this could reduce the relapse rate. Another advantage of nicotine vaccines is that daily administration of the drug is not required; only occasional booster shots are needed to maintain an adequate antibody titer. However, there has been inconsistency in the degree of antibody response; some people do not achieve adequate antibody titres (see below). Possible disadvantages of nicotine vaccines include the necessity for multiple injections and the time delay before an effective immune response is achieved.
In 2007 Celtic Pharmaceuticals Holdings LP began a large Phase II trial of its developmental nicotine vaccine, TA-NIC, to assess safety and smoking abstinence rates at 6 months (NIH Clinical Trials website). Enrollment of up to 175 patients in each of three arms of the study (placebo arm and two dose levels of the vaccine) has been completed, but the results have not yet been announced. Initial experience with a Phase I trial of TA-NIC showed a substantially greater 12-month self-reported quit rates among those receiving the vaccine (19% and 38% in the 250 μg and 1000 μg TA-NIC groups, respectively) than in those receiving placebo (8%). A booster given at 32 weeks produced a substantial and sustained increase in nicotine-specific antibodies in both groups receiving 250 μg and 1000 μg TA-NIC (details at http://hugin.info/133161/R/982993/146255.pdf)
Independent Pharmaceutica AB is developing Niccine, a proprietary vaccine designed to prevent and treat nicotine dependence. In 2008, Independent announced that enrollment of 355 smokers into a Phase II clinical trial with Niccine had been completed (details at http://www.independentpharma.com). The primary goal of this multi-center study is to demonstrate the ability of the vaccine to prevent relapse in smokers that have recently stopped smoking with the aid of smoking-cessation drugs and counseling.
Earlier Phase II trials with NIC002 (also known as Nicotine QB or CYT002-NicQB) from Cytos Biotechnology demonstrated that the vaccine promoted and sustained tobacco abstinence in smokers who achieved high levels of antibodies [60]. However, side-effects (including flu-like symptoms) occurred in 69.4% of subjects. In 2007, Cytos Biotechnology entered into a licence agreement with Novartis and, in 2008, Novartis began a new Phase II trial in 200 cigarette smokers with a reformulated vaccine with fewer side effects. However, interim analysis showed that the primary endpoint (continuous abstinence from smoking from weeks 8 to 12 after start of treatment) was not achieved, possibly because NIC002 failed to induce sufficiently high antibody titres (http://www.cytos.com).
Nabi Biopharmaceuticals has announced positive results from Phase II trials of NicVAX [61]. NicVAX vaccine was safe and well-tolerated, and generated high anti-nicotine antibody levels. In patients vaccinated with Nic-VAX there was an observable correlation between antibody levels and the ability of patients to stop smoking. Indeed, statistically significant numbers of patients treated with NicVAX have been able to cease smoking and remain abstinent over the long-term. NicVAX has now entered Phase III clinical trials (NIH Clinical Trials website).
Concluding remarks
Cigarette smoking creates an addiction that is difficult to break. Smokers trying to quit have to cope simultaneously with the psychological and pharmacologic aspects of tobacco dependence. The pharmacologic effects of nicotine play a crucial role in tobacco addiction, and therefore pharmacotherapy is important to improve success rates. Currently- marketed smoking-cessation products (such as NRT, buproprion and varenicline) increase the likelihood that smokers quit smoking, particularly if combined with counseling programs. Unfortunately, these programs lack high levels of efficacy, particularly in real-life settings [62]. This reflects the chronic relapsing nature of tobacco dependence, and not physician inadequacy nor failure of their patients, but more effective smoking-cessation interventions are clearly needed.
Improved understanding of the mechanisms involved in nicotine dependence has recently been translated into new treatments. The success of varenicline as the first partial agonist selective for α4β2 nAChR subtypes opens new opportunities for using partial-agonist agents to target other important receptor subtypes involved in nicotine signaling. Moreover, vaccine approaches to treatment of nicotine dependence are developing rapidly, and nicotine vaccines could substantially influence the way healthcare practitioners provide smoking-cessation treatment. Substantial research on new pharmacological approaches is currently ongoing and the results are eagerly awaited.
Despite these developments, more effort should be devoted towards identifying new molecular targets, testing innovative approaches, and establishing the best use of what it is already available. In relation to this latter point, acknowledging smokers’ preferences regarding the route and schedule of administration and the identification of individual characteristics that predict successful responses to these treatments are highly desirable [63]. Smokers worldwide are in great need of more effective tobacco-dependence treatments; this unmet need should be a major priority for academic institutions and the pharmaceutical industry.
Acknowledgments
R.P. is supported by the University of Catania, Italy. N.B. was supported in part by a US Public Health Service grant, DA02277 from the National Institute on Drug Abuse.
Footnotes
Conflict of interest
R.P. has received lecture fees from Pfizer and GlaxoSmithKline and a research grant from Pfizer; he has also served as a consultant to Pfizer and Global Health Alliance for the treatment of tobacco dependence. N.B. serves as a consultant to Pfizer and has consulted in the past with several other pharmaceutical companies that are developing smoking-cessation medications.
References
- 1.World Health Organization (WHO) Tobacco or Health: a Global Status Report. World Health Organization; 1997. pp. 1–32. [Google Scholar]
- 2.Doll R, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519–1528. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. The Health Consequences of Amoking: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 4.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;20:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. The Health Benefits of Smoking Cessation. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. (DHHS Publication No. (CDC) 90-8516). [Google Scholar]
- 6.Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation. Circulation. 1997;96:1089–1096. doi: 10.1161/01.cir.96.4.1089. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Global Report on the Global Tobacco Epidemic; Implementing Smoke-Free Environments. World Health Organization; 2009. http://www.who.int/tobacco/mpower/2009/gtcr_download/en/index.html. [Google Scholar]
- 8.Fiore MC, et al. Treating tobacco use and dependence: 2008 update. US Dept of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 9.Parrott S, et al. Guidance for commissioners on the cost effectiveness of smoking cessation interventions. Thorax. 1998;53(Suppl 5):2, S1–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes JR, et al. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George TP. Medication Treatments for Nicotine Dependence. CRC/Taylor & Francis; 2007. [Google Scholar]
- 13.Gross J, Stitzer ML. Nicotine replacement: ten-week effects on tobacco withdrawal symptoms. Psychopharmacology (Berl) 1989;98:334–341. doi: 10.1007/BF00451684. [DOI] [PubMed] [Google Scholar]
- 14.Foulds J, et al. Advances in pharmacotherapy for tobacco dependence. Expert Opin Emerg Drugs. 2004;9:39–53. doi: 10.1517/eoed.9.1.39.32951. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL. Nicotine replacement therapy: what has been accomplished — can we do better? Drugs. 1993;45:157–170. doi: 10.2165/00003495-199345020-00001. [DOI] [PubMed] [Google Scholar]
- 16.Johansson CJ, et al. Absolute bioavailability of nicotine applied to different nasal regions. Eur J Clin Pharmacol. 1991;41:585–588. doi: 10.1007/BF00314989. [DOI] [PubMed] [Google Scholar]
- 17.Stead LF, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Hajek P, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033–2038. doi: 10.1001/archinte.159.17.2033. [DOI] [PubMed] [Google Scholar]
- 19.Tønnesen P, Mikkelsen KL. Smoking cessation with four nicotine replacement regimes in a lung clinic. Eur Respir J. 2000;16:717–722. doi: 10.1034/j.1399-3003.2000.16d25.x. [DOI] [PubMed] [Google Scholar]
- 20.Henningfield JE, et al. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. [DOI] [PubMed] [Google Scholar]
- 21.Joseph AM, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335:1792–1798. doi: 10.1056/NEJM199612123352402. [DOI] [PubMed] [Google Scholar]
- 22.Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis. 2003;45:405–413. doi: 10.1053/pcad.2003.00103. [DOI] [PubMed] [Google Scholar]
- 23.West R, et al. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 2000;149:198–202. doi: 10.1007/s002130000382. [DOI] [PubMed] [Google Scholar]
- 24.Shiftman S, Ferguson SG. Nicotine patch therapy prior to quitting smoking: a meta-analysis. Addiction. 2008;103:557–563. doi: 10.1111/j.1360-0443.2008.02138.x. [DOI] [PubMed] [Google Scholar]
- 25.Bullen C, et al. Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction. 2010;105:1474–1483. doi: 10.1111/j.1360-0443.2010.02989.x. [DOI] [PubMed] [Google Scholar]
- 26.Miller DK, et al. Bupropion inhibits nicotine-evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine and from rat hippocampal slices preloaded with [3H]norepinephrine. J Pharmacol Exp Ther. 2002;302:1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- 27.Jorenby D. Clinical efficacy of bupropion in the management of smoking cessation. Drugs. 2002;62(Suppl 2):25–35. doi: 10.2165/00003495-200262002-00003. [DOI] [PubMed] [Google Scholar]
- 28.Hughes JR, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Dunner DL, et al. A prospective safety surveillance study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry. 1998;59:366–373. doi: 10.4088/jcp.v59n0705. [DOI] [PubMed] [Google Scholar]
- 30.Boshier A, et al. Evaluation of the safety of bupropion (Zyban SR) for smoking cessation from experience gained in general practice use in England in 2000. Eur J Clin Pharmacol. 2003;59:767–773. doi: 10.1007/s00228-003-0693-0. [DOI] [PubMed] [Google Scholar]
- 31.Tonstad S, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24:946–955. doi: 10.1016/s0195-668x(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 32.Coe JW, et al. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 33.Rollema H, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Gonzales D, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Jorenby DE, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 36.Tonstad S, et al. Effect of maintenance therapy with varenicline for smoking cessation. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 37.Tashkin DP, et al. Effects ofvarenicline on smoking cessation in mild-to-moderate COPD: a randomized controlled trial. Chest. 2010 doi: 10.1378/chest.10-0865. [DOI] [PubMed] [Google Scholar]
- 38.Rigotti NA, et al. Efficacy and safety of varenicline for smoking cessation in patients with CVD. Circulation. 2010;121:221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes JR, et al. Nortriptyline for smoking cessation: a review. Nicotine Tob Res. 2005;7:491–499. doi: 10.1080/14622200500185298. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 2nd. American Psychiatric Association; 2000. [PubMed] [Google Scholar]
- 41.Gourlay SG, Benowitz NL. Is clonidine an effective smoking cessation therapy? Drugs. 1995;50:197–207. doi: 10.2165/00003495-199550020-00001. [DOI] [PubMed] [Google Scholar]
- 42.Gourlay SG, et al. Clonidine for smoking cessation. Cochrane Database Syst Rev. 2004:CD000058. doi: 10.1002/14651858.CD000058.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebbert JO, et al. Combination pharmacotherapy for stopping smoking: what advantages does it offer? Drugs. 2010;70:643–650. doi: 10.2165/11536100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogg RC, Bertrand D. Partial agonists as therapeutic agents at neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;73:459–468. doi: 10.1016/j.bcp.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Rollema H, et al. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin ED, et al. Sazetidine-A, a selective alpha4beta2 nicotinic receptor desensitizing agent and partial agonist, reduces nicotine self-administration in rats. J Pharmacol Exp Ther. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- 47.Etter JF, et al. Cytisine for smoking cessation: a research. Drug Alcohol Depend. 2008;92:3–8. doi: 10.1016/j.drugalcdep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Cohen C, et al. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005;81:387–395. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 49.Cohen C, et al. SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 50.Cohen C, et al. Nicotine-associated cues maintain nicotineseeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- 51.Van Gaal LF, et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 52.Morrison MF, et al. Randomized, controlled, double-blind trial of taranabant for smoking cessation. Psychopharmacology (Berl) 2010;209:245–253. doi: 10.1007/s00213-010-1790-2. [DOI] [PubMed] [Google Scholar]
- 53.Lewis A, et al. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Gerlach M, et al. Pharmacology of selegiline. Neurology. 1996;47:S137–S145. doi: 10.1212/wnl.47.6_suppl_3.137s. [DOI] [PubMed] [Google Scholar]
- 55.Houtsmuller EJ, et al. Effects of selegiline (l-deprenyl) during smoking and short-term abstinence. Psychopharmacology (Berl) 2002;163:213–220. doi: 10.1007/s00213-002-1152-9. [DOI] [PubMed] [Google Scholar]
- 56.George TP, et al. A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biol Psychiatry. 2003;53:136–143. doi: 10.1016/s0006-3223(02)01454-3. [DOI] [PubMed] [Google Scholar]
- 57.Biberman R, et al. A randomized controlled trial of oral selegiline plus nicotine skin patch compared with placebo plus nicotine skin patch for smoking cessation. Addiction. 2003;98:1403–1407. doi: 10.1046/j.1360-0443.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 58.Weinberger AH, et al. A double-blind, placebo-controlled, randomized clinical trial of oral selegiline hydrochloride for smoking cessation in nicotine-dependent cigarette smokers. Drug Alcohol Depend. 2010;107:188–195. doi: 10.1016/j.drugalcdep.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maurer P, Bachmann MF. Vaccination against nicotine: an emerging therapy for tobacco dependence. Expert Opin Investig Drugs. 2007;16:1775–1783. doi: 10.1517/13543784.16.11.1775. [DOI] [PubMed] [Google Scholar]
- 60.Cornuz J, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS ONE. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatsukami DK, et al. Safety and immunogenicity ofa nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Casella G, et al. Therapeutic advances in the treatment of nicotine addiction: present and future. Ther Adv ChronicDis. 2010;1:95–106. doi: 10.1177/2040622310374896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caponnetto P, Polosa R. Common predictors of smoking cessation in clinical practice. Respir Med. 2008;102:1182–1192. doi: 10.1016/j.rmed.2008.02.017. [DOI] [PubMed] [Google Scholar]


