Abstract
B lymphocytes are essential for an efficient immune response against a variety of pathogens. A large fraction of hematologic malignancies is of B cell origin suggesting that development and activation of B cells need to be tightly regulated. In recent years, increasing evidence has emerged demonstrating that microRNAs (miRNAs) -- a class of non-coding RNAs that control gene expression -- are involved in the regulation of B cell development and function. Here, we provide an overview of the current knowledge on the role of miRNAs and their relevant targets in B cell development, B cell activation and B cell malignant transformation.
Keywords: miRNAs, B cell development, B cell function, B cell lymphomas
miRNAs Are Essential for Gene Regulation
MicroRNAs (miRNAs) are a class of short non-coding RNAs of ~22 nucleotides that function as guide molecules in RNA silencing (see Glossary). In this form of gene regulation, the RNA-induced silencing complex (RISC) is guided to target messenger RNAs (mRNAs) via base complementarity between the miRNA and the 3′ untranslated regions (UTRs) of target transcripts, thus leading to mRNA degradation or translational repression [1]. Because many protein-coding transcripts are subject to RNA silencing, miRNAs play important regulatory roles in numerous biological pathways. Biogenesis of individual miRNAs is tightly controlled in a cell-type and -temporal manner and their deregulation has been associated with many human diseases, including lymphomas [2]. While initially described in the early 1990s [3, 4], the true importance of miRNAs in the regulation of gene expression was not fully appreciated until the characterization of this class of non-coding RNAs across several species and cell types in the last fifteen years [5–7]. Recent advances in gene expression profiling and the use of conditional gene targeting to dissect the role of individual miRNAs in a cell type-specific manner has allowed immunologists to shed new light on the contribution of miRNAs in hematopoiesis and lymphomagenesis.
Regulation of B Cell Development
In mammals, B cell lymphopoeisis is a tightly regulated process in which early hematopoietic progenitors differentiate into B lymphocytes following a set of ordered rearrangements at immunoglobulin (Ig) loci. Ig proteins, generated by sequential V(D)J recombination events at immunoglobulin heavy chain (IgH) and light chain (IgL) loci occurring at distinct stages of B cell development, play a critical role -- along with transcription factors, in regulating this process. The carefully orchestrated, step-wise process ensures that each B lymphocyte acquires a single, unique B cell antigen receptor (BCR). Once the nascent B cells undergo negative selection to ensure that self-reactive clones are eliminated, newly minted immunocompetent B cells exit from the bone marrow (BM) into the periphery (Figure 1, Key Figure) [8]. Upon encountering cognate antigen (Ag) in secondary lymphoid organs, B cells will undergo somatic hypermutation (SHM) along the variable regions of IgH and IgL loci, contributing to the enhanced affinity of Igs for Ag. Class switch recombination (CSR) leads to a change in the constant region of an IgH, resulting in the expression of different classes of antibodies, and allowing for distinct antibody function [9]. Ig gene rearrangement, SHM and CSR enable the generation of a highly diverse antibody repertoire, necessary to provide specific protection from a wide range of potential pathogens. The exact nature of the intracellular interactions between the BM microenvironment and the developing lymphocytes, as well as the precise signaling pathways involved in positive and negative selection of immature B cells remain to be fully elucidated. Over the past few years, conditional gene targeting and high-throughput RNA-sequencing (RNA-seq) have provided novel insight into the role of individual miRNAs and their relevant targets in B cell development. Given that a single miRNA may influence the posttranscriptional processing of multiple mRNA targets and significantly alter whole signaling networks [10], understanding the contribution of complex miRNA-mRNA regulatory networks may provide new insight into basic B cell biology and lymphomagenesis and yield potential therapeutic applications for different malignancies. In this review, we summarize our current knowledge of the contribution of miRNAs to B cell lineage commitment, early B cell development, B cell maturation and function, as well as malignant transformation.
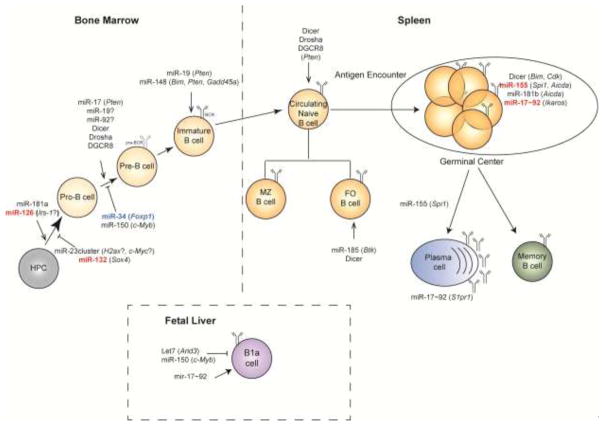
Key Figure, Figure 1.
miRNAs Can Regulate All Stages of Mammalian B Cell Development and Activation
miRNAs are Differentially Expressed at Distinct Stages of B Cell Development, Maturation and Activation
Since the discovery of miRNAs, numerous researchers have attempted to understand the roles of these non-coding RNAs in mammalian cells. Early studies of miRNAs in mammalian hematopoietic cells focused on mapping the landscape of miRNAs expression to establish whether the newly discovered mode of posttranscriptional regulation played a role in immune cell development [11–16]. These cloning and sequencing studies allowed the identification of miRNAs that were abundantly or uniquely expressed in different cell types and at distinct stages of development [14]. In addition, ubiquitously expressed miRNAs were also identified [14]. Differential expression of miRNAs throughout the hematopoietic organs revealed by early cloning and transcriptional expression analysis (Northern blotting) approaches suggested a role for miRNAs in hematopoiesis [12]. For instance, miR-181 and miR-142 were found to be selectively expressed in murine mature B lymphocytes [12]. Further studies in mouse cells confirmed that several miRNAs were expressed at different levels, depending on the stage of B cell development [13, 15]. Recently, an atlas of miRNAs in human B cells was constructed [11, 16]. For example, data from these reports identified new miRNAs and potentiated future analysis of individual miRNAs using gene targeting approaches. However, this methodology has several limitations: for instance, Northern blot analysis or miRNA arrays often fail to detect low abundance miRNAs, while sequencing studies similarly suffer from the potential bias that can result from depth of sequencing, the exact library preparation protocol used (which often eliminates some miRNAs) [17, 18]. In addition, inconsistencies as to what precisely constitutes a novel miRNA – features such as the exact length, requirement for identification of both mature strands and conservation across species -- contributes to the challenge of high throughput profiling of non-coding RNAs.
miRNAs in B Cell Lineage Commitment: miR-126, miR23a, miR-132
Commitment of hematopoietic stem and progenitor cells (HSPCs) to the B cell lineage is regulated by several transcription factors, including Ikaros and immunoglobulin enhancer-binding factors E12/E47 (E2A), which have been implicated in lymphoid lineage fate decisions [19]. Early B cell factor-1 (EBF1) and paired box protein 5 (Pax5) are also important for further differentiation into the B cell lineage in both human and mouse [20, 21]. Recently, miRNAs have been suggested to modulate B cell fate commitment via regulation of several of these key transcriptional networks. For example, overexpression of miR-181 using a retroviral vector was demonstrated to induce preferential differentiation of murine lineage negative (lin−) BM cells toward the B cell lineage in vivo and in vitro [12]. However, mice lacking miR-181 exhibited a modest impairment in B cell development only [22, 23], suggesting that the impact of miR-181 in B cell development remains to be elucidated. In addition, overexpression of miR-126 retrovirus in an immature B cell line derived from an acute lymphoblastic leukemia patient, and in mouse lin− HSPCs induced B cell differentiation through the regulation of insulin regulatory subunit-1 (IRS-1), an inhibitor of differentiation and proliferation [24]. However, how IRS-1 regulates B cell differentiation remains undetermined [24]. This study suggests that miR-126 plays a critical role in B cell lineage commitment; yet, these conclusions are based on overexpression of this miRNA well above physiological levels (with miR-126 transcripts expressed 600 fold higher than in control cells) and thus, have to be carefully scrutinized [24]. By contrast, retroviral overexpression of the miR-23a cluster (encoding miR-23a, miR-27a and miR-24-2) in mouse primary HSPCs repressed development of CD19 positive B cells in vitro and prevented B cell hematopoiesis upon BM reconstitution of lethally irradiated animals [25]. The authors of this study have speculated that changes in the expression of histone variant H2AX (involved in mediating double-stranded DNA breaks), and transcription factor c-Myc (another known target of miR-24) contribute to the repression of murine B cell fate commitment [25].
Enforced expression of another miRNA, miR-132, in murine HSPCs used for BM reconstitution experiments, inhibited the differentiation of pro-B cells, the earliest stage of B cell development [26]. While miR-132 deficient animals lacked any discernable B cell phenotype at steady-state conditions, B cell development was blocked at the pro-B cell stage following depletion of mature B cells. Sox4 was identified as the target of miR-132, and consistent with these findings, B cell development at the pro-B cell stage could be rescued by expression of a Sox4 mutant lacking miR-132-binding sites or in mice deficient for miR-132 [26]. These results suggest that miR-132 can negatively regulate early B cell development via direct regulation of Sox4, a factor important for the expression of recombination activating gene 1 (RAG1), a critical enzyme in VDJ rearrangement and necessary for B cell development [26]. Together, these studies highlight the essential role of miRNAs in B cell lineage commitment.
miRNAs in Early B Cell Development
Once cells are committed to the B cell lineage, nascent pro-B cells upregulate RAG enzymes, rearrange their IgH loci and progress to the pre-B cell stage where IgL rearrangement takes place (Key Figure, Figure 1). The critical role of miRNAs in the regulation of the pro-B to pre-B cell transition was first highlighted by the characterization of a near complete block in B cell development at the pro-B stage in mice that lacked miRNAs in developing B cells [27]. This observation was made in animals in which the critical enzyme for miRNA biogenesis, Dicer, was ablated conditionally using Mb1-Cre (an allele encoding Cre recombinase under the B cell-specific promoter of the Igα signaling subunit of the BCR) [27]. More recently, Mb1-Cre-mediated genetic ablation of Drosha [28], and Dgcr8 [28, 29] -- enzymes constituting the microprocessor complex (and therefore necessary for miRNA biogenesis)-- yielded a similar block in early B cell lymphopoeisis in mice [28, 29]. These studies revealed that the global absence of miRNAs during the earliest stages of B cell development led to a block in the pro-B to pre-B transition, caused by a defect in survival and proliferation of these cells (Key Figure, Figure 1) [27–29].
miR-17~92
Early studies in mice have indicated that the miR-17~92 cluster directly regulates the pro-apoptotic protein Bim, which is known to be critical for maintenance of B cell survival during early hematopoiesis. Analysis of Bim 3′UTR reveals several sites that closely match the seed sequence of miR-17~92 miRNA [27, 30]. The putative link between miR-17~92 and Bim was established based on the observation that Bim transcript levels were elevated in B cells from animals in which the miR-17~92 cluster is ablated in the germline or from B cells devoid entirely of all miRNAs [27, 30]. A recent study, examined B cell development in mice lacking miR-17~92 and two paralogue clusters, miR-106a~363 and miR-106b~25, that encode miRNAs sharing the same seed sequence [31]. The authors noted a block in B cell development and increased apoptosis reminiscent to what had been observed upon ablation of Dicer [31]. However, Bim upregulation was not observed in these triple knock-out B cells and the observed developmental block could not be rescued by expression of the anti-apoptotic factor Bcl2 alone, suggesting that Bim was not responsible for apoptosis in these cells [31]. Moreover, in vitro expression of the entire miR-17~92 cluster, (or of miR-17 alone) in HSPCs from these mice was sufficient to rescue B cell development. This study highlighted the importance of miR-17 in controlling the pro-B to pre-B cell transition, as well as a supporting role of miR-19 and miR-92 in orchestrating early B cell development. However the relevant direct targets of these miRNAs in early B cell development remain to be identified [31].
While the miR-17~92 cluster is highly expressed in developing murine B lymphocytes, other miRNAs associated with lymphocyte function, must be maintained at a low level throughout B cell hematopoiesis. For instance, miR-150 is expressed at low levels in murine pro-B and pre-B cells, while expression of this miRNA increases dramatically at later stages of B cell development [32, 33]. Mice ectopically expressing miR-150 (from a miR-150 transgene [32] or following BM reconstitution with HSPCs transduced with a vector encoding miR-150 [33]) have been shown to exhibit a block in early B cell development due to induced cell death in pro-B and pre-B cells [32, 33]. This effect of miR-150 appears to be due to downregulation of its target gene, the transcription factor c-Myb based on the analysis of murine B cells either deficient in miR-150 or carrying a transgene that results in over-expression of this miRNA[32, 33]. Indeed, loss of one copy of c-Myb resulted in similar decreased levels of this key transcription factor as those ovbserved from ectopic expression of miR-150, as well as in a similar block in B cell development in the transgenic animals [32]. By the same token, ectopic expression of miR-34 in BM progenitors has been reported to lead to reduced numbers of pre-B cells in BM reconstitution experiments in mice [34]. Specifically, B cell development was shown to be rescued by co-expression of a vector expressing the miR-34 target forkhead box P1 (Foxp1) with a mutated miR-34 binding site in the 3′UTR in murine bone marrow [34]. This suggested that suppression of the early B cell transcriptional regulator FoxP1 by miR-34 [35], might be responsible for the observed developmental block at the pro- to pre-B cell transition [34].
Collectively, these data demonstrate that expression of a specific set of miRNAs in developing BM B cells can be critical for the orchestration of proper B cell development (Key Figure, Figure 1). Indeed, early lymphocyte development is characterized by carefully coordinated DNA rearrangement and proliferation. MiRNAs provide an additional layer of control for the events occurring at this critical stage of B cell development. While some of the relevant targets of these miRNAs have been identified, the full repertoire of direct targets and signals triggering the induction of these miRNAs remain to be thoroughly elucidated.
miRNAs in Central Tolerance
During normal B cell development, immature B cells expressing the newly formed BCR, undergo negative selection, eliminating cells that express a self-reactive antibody. This checkpoint is known as central tolerance, and is important for preventing the development of autoimmunity [36]. Recent studies have shown that miRNAs play a role in the regulation of this critical checkpoint.
miR-17~92
One of the animal models used to study central tolerance is that of IgMb-macroself mice which ubiquitously express a membrane-bound antigen reactive to the IgH constant region of IgM, the surface BCR expressed on immature B lymphocytes [37]. IgMb-macroself mice lack mature peripheral B cells due to induced cell death, as a consequence of central tolerance [37]. Conditional overexpression of a transgene encoding miR-17~92 in murine B cells using CD19-Cre (Cre recombinase under the B cell-specific cd19 promoter) led to the rescue of B cell development and maturation in the presence of IgMb-macroself antigen [31]. In addition, BM chimera reconstitution experiments of lethally-irradiated IgMb-macroself recipients with WT BM cells that were transduced with lentiviral vectors encoding the full length miR-17~92 cluster, or with specific members of the miR-17~92 miRNA family, allowed the identification of miR-19 as the critical miRNA in the regulation of B cell selection/survival [31]. Indeed miR-19 expression was sufficient to rescue B cell development in this context, while its absence did not allow the other members of the cluster to rescue B cell development [31]. Moroever, using photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) and luciferase reporter assays, the authors identified phosphatase and tensin homolog (Pten), a negative regulator of phosphoinositide-3 kinase (PI3K) [38], as a target of miR-19 in immature murine B cells [31]. Ablation of Pten in a IgMb-macroself background led to a near complete rescue of B cell development, highlighting the role of the miR-19~92/PI3K axis in negative selection of developing B lymphocytes [31]. In addition, IgL receptor editing -- another molecular mechanism contributing to tolerance -- was increased in miR-17~92 transgenic mice relative to control animals, and decreased in miR-17~92-deficient B cells relative to wild-type B lymphocytes [31]. Thus, miR-17~92, and in particular miR-19, appear to regulate central tolerance and receptor editing in immature B cells in mice, through the control of Pten expression [31].
Once immature B cells leave the BM and enter the spleen, they become transitional B cells that give rise to mature B lymphocytes [39]. Inducible ablation of Dicer (and thus all miRNAs) in transitional B cells has been documented to lead to a reduction of PI3K signaling and apoptosis in these cells [40]; these defects could be rescued by reducing levels of Pten in vivo, in mice [40]. Specifically, by using CD19-deficient mice where PI3K signaling is diminished [41], the authors showed that decreased levels of c-Myc (known to be regulated by PI3K), led to reduced miR-19 expression and increased Pten mRNA and protein levels [40]. This result suggests a regulatory loop where BCR signaling via PI3K induces c-Myc expression, in turn activating miR-17~92 expression, which presumably contributes to Pten-mediated inhibition of PI3K, and thus contributing to the regulation of central tolerance [40]. Taken together, these results underscore the role of the miR-17~92 cluster in the regulation of B cell central tolerance and the fundamental role this cluster plays in maintaining immune homeostasis.
miR-148a
Another miRNA that appears to be critical for the regulation of B cell tolerance is miR-148a which is expressed at low levels in immature murine B cells [13]. Overexpression of miR-148a has been shown to potentiate the survival of WEHI-231 immature B cells following BCR activation with anti-IgM antibodies in vitro; this suggests that regulation of target gene(s) by miR-148a might be critical for maintaining cell survival at the immature B cell stage [42]. Analysis of differentially expressed genes upon ectopic expression of miR-148 in a murine lymphoma cell line identified growth arrest and DNA-damage-inducible protein Gadd45α (an autoimmune suppressor implicated in the regulation of MAPK pathway[43]), as well as Bim and Pten, as putative direct targets of miR-148 [42]. Indeed, BM reconstitution of lethally-irradiated IgMb-macroself mice with BM from Gadd45α– or Bim-deficient mice, or from mice lacking Pten selectively in B cells, allowed B cell maturation to proceed, despite strong engagement of the antigen receptor, which would normally arrest B cell development at the immature B cell stage [42]. This report concluded that miR-148 might be critical for the regulation of apoptosis in immature B cells upon BCR activation in mice, and furthermore that miR-148a might regulate B cell survival by targeting Gadd45α, Bim and Pten proteins [42].
Overall, these data suggest that miRNAs play an essential role in the regulation of cell death of immature and transitional B cells, thus contributing to negative selection, as well as to the regulation of humoral immune responses and autoimmunity (Key figure, Figure 1).
miRNAs in Mature B Cells
Studies examining the landscape of non-coding RNAs in distinct hematopoietic lineages and analysis of RNAi deficient B cells, solidified the fact that B lymphocytes have a distinct repertoire of miRNAs, which contribute to B cell lineage commitment and guide early B lymphocyte development. B cells deficient in miRNAs as a consequence of lineage specific ablation of Dicer, Drosha or Dgcr8, can be partially rescued by expression of a pro-survival Bcl2 transgene in mice [27, 28]. The B lymphocytes that develop in the absence of miRNAs were shown to express low levels of surface Ig as a consequence of ongoing receptor editing, mediated by inappropriately expressed RAG1/2 recombinase enzymes [28]. IgL secondary rearrangement is typically restricted to pre-B or immature B cell stages, taking place if the newly-minted BCR is autoreactive or out-frame [44]. Aberrant RAG expression was observed in miRNA-deficient peripheral B cells, which was attributed to increased Pten levels in these cells, presumably leading to the dampening of PI3K/Akt signaling, and the eventual upregulation of Forkhead box O1 (FoxO1), a known regulator of Rag expression [28]. These results suggest that miRNAs can contribute to the regulation of RAG expression in mature B lymphocytes and that they appear to be necessary in order to prevent lgL rearrangement in mature B cells [28]. This may constitute a key regulatory step to ensure the maintenance of genomic stability of mature B lymphocytes by blocking aberrant genomic rearrangements that could lead to malignant lymphomagenesis [45, 46].
miR-185
Ablation of Dicer in immature B cells using CD19-Cre mice was reported to lead to the preferential development of marginal zone (MZ) B cells with fewer follicular (FO) B cells in the spleen [47]. The differentiation of immature B cells into MZ B cells or into FO B cells is controlled, at least in part, by the strenghth of BCR signaling, with FO B cell differentiation requiring a stronger BCR signaling [48]. Moreover, differential gene expression and miRNA profiling of MZ and FO B cells identified miR-185 and its putative target, Bruton’s tyrosine kinase (Btk) as a signaling axis that could be important in the determination of MZ versus FO B cell development [47]. In this study, miR-185 was expressed at a higher level in FO B cells compared to MZ B cells. Consequently, Btk expression was increased in Dicer-deficient FO B cells, but not in MZ miRNA-deficient B cells. These data suggested that the shift in B cell maturation observed in Dicer-deficient B cells could be dependent on the strenghth of BCR signaling regulated by Btk, downstream of miR-185 [47]. Moreover, the antibody repertoire of B cells from Dicerfl/fl CD19-Cre mice was skewed towards self-reactive BCR specificities when compared to littermate controls, with aged female Dicer-deficient mice producing antibodies against a variety of self-antigens, including single- and double-stranded DNA, and developing an autoimmune phenotype with immune-complex deposition and kidney lymphocyte infiltration [47]. These results thus support a role for miRNAs in the differentiation of mature B cells (Key Figure, Figure 1) and possibly, in preventing the production of autoantibodies and autoimmunity.
miRNAs in Terminal Differentiation of B Cells
Following encounter with their cognate antigens, mature B cells enter the germinal center (GC) and futher differentiate into plasma cells (PC) or memory B cells [21, 49]. Deletion of Dicer in activated murine B cells using Cre under the promoter of aicda, the gene encoding activation-induced cytidine deaminase (AID), an enzyme critical for the GC reaction [50], has been shown to result in impaired CSR, reduced affinity maturation and deficient GC formation[51]. Ablation of miRNA biogenesis in GC B cells has also been shown to lead to a block in plasma cell and memory cell differentiation [51]. Furthermore, miRNA-deficient GC B cells have been found to exhibit defects in survival and proliferation, which have been attributed to elevated levels of Bim and higher transcript levels of several inhibitors of cyclin-dependent kinases (CDK), with greater than 10-fold higher expression of Cdkn1c and Cdkn2brelative to controls [51]. Thus, Dicer-dependent miRNAs also appear to be necessary for the orchestration of proper GC B cell responses and to be required for terminal differentiation of B lymphocytes into long lived plasma cells.
miR-155
In mice, expression of miR-155 (encoded within the non-coding RNA B cell integration cluster (Bic) gene) is induced during B cell activation; for instance, mice deficient in miR-155 present a defect in CSR into the IgG1 isotype [52]. In addition, these mice harbor fewer B cells in their GCs, which produce reduced amounts of two cytokines important for GC formation, namely, tumor necrosis factor (TNF) and lymphotoxin-α (LT- α), relative to GC B cells from control animals [53]. Another study has described crippled GC and T-cell independent extrafollicular responses, with compromised generation of plasma and memory B cells in miR-155-deficient mice [54]. BM chimeras and in vitro experiments have revealed that defective antibody responses to attenuated Salmonella immunizations in miR-155-deficient mice, were in fact, B cell intrinsic, and due at least in part, to impaired differentiation or survival of plasmablasts and memory B cells [54]. Furthermore, luciferase reporter assays have suggested that such plasmablast defects in miR-155-deficient animals were due to a direct regulation of Sfpi1 by miR-155 (Sfpi1 encodes the transcription factor, PU.1, important in hematopoietic development [55]) [54]. Indeed, the critical role of the miR-155-PU.1 axis in late B cell development has been supported by a study employing mice that lacked the miR-155 binding site in the Sfpi1 3′UTR [56]. Analysis of these animals revealed that miR-155-mediated regulation of PU.1 contributed to an early extrafollicular response, and moreover, that direct regulation of PU.1 by miR-155 was dispensable for the maintenance of the GC response [56]. Given the precise gene targeting approach employed to target the miR-155 binding site in the Sfpi1 3′ UTR only, it is likely that the decreased number of GCs previously observed in miR-155-deficient animals may have been due to the impact of miR-155 on other putative targets. Indeed, comparison of genes differentially expressed in PU.1 mutant B cells and miR-155-deficient B cells compared to wild-type B cells, suggests that most of the miR-155 effects that have been observed in activated B cells may be due to miR-155-mediated regulation of PU.1 [56]. However, which targets of miR-155 are responsible for the regulation of GC responses remains an open question.
Of clinical relevance, miR-155 was recently found to be expressed at higher levels in B cells from patients with rheumatoid arthritis (RA) compared to healthy individuals [57]. In vitro stimulation of peripheral blood B cells from healthy individuals by a variety of B cell activator and survival factors (including anti-IgM, CD40L, or BAFF) readily led to induction of miR-155 expression in vitro, suggesting that miR-155 expression in human B cells, as in murine mature B cells, could be induced upon B cell stimulation [57]. Furthermore, in vitro inhibition of miR-155 in peripheral B cells from RA patients using an in vitro transfection system, resulted in increased PU.1 expression. Thus, This study suggested that miR-155 might be potentiallycontrolling auto-antibody production in RA patients, and possibly regulating PU.1 expression [57]. However, these findings warrant further and robust investigation.
AID has been identified as another direct target of miR-155 in activated splenic B cells in mice [58, 59]. In vivo and in vitro activated B cells from mice carrying various copy numbers of a transgene harboring the Aicda locus with a mutation in the miR-155 binding site of the Aicda 3′UTR, expressed higher levels of AID protein compared to control mice expressing a transgene with an unmutated 3′UTR [59]. Mice carrying AID with a 3′ UTR deficient for the miR-155 binding site, exhibited impaired SHM, as evidenced from fewer high affinity antibodies in the sera of NP-CGG immunized animals relative to control animals, as well as by the presence of increased numbers of mutations at non-Ig loci such as Bcl6, an oncogene known to be hypermutated in GC lymphomas [59]. In another mouse model that targeted the 3′UTR of Aicda (also mutated at the miR-155 binding site), in-vitro stimulation of B cells led to increased expression and half-life of Aicda mRNA and consequently, to elevated AID protein expression with increased CSR; the study thus suggested that miR-155 might control Aicda mRNA degradation (vs stability) in activated B cells [58]. While no impact on SHM at the Ig locus has been observed in animals harboring a mutant Aicda 3′UTR, increased oncogenic chromosome translocations between Igh and My --shown to be induced by AID [60]—have been detected in these mice relative to Aicda+/− controls [58]. This suggests that miR-155 deficiency might contribute to translocations associated with B cell lymphomagenesis [58]. Together, these robust studies implicate miR-155 in modulating terminal differentiation of B cells, and further propose that regulation of AID by miR-155 may be important in the induction of lymphoma-relevant translocations [53, 54, 56–59].
miR-181b and miR-17~92
In terms of AID regulation, miR-181b has been shown to act as a negative regulator of CSR in in vitro high-throughput screens using CSR as a readout, and was found to antagonize murine Aicda mRNA expression in luciferase reporter assays, suggesting that AID constituted a direct target of miR-181b [61]. Aicda mRNA contains several putative binding sites for miR-181b, and the data indicated that AID activity was regulated by miR-181b [61]; this has led to the hypothesis that since B cell lymphoma-associated genomic translocations are frequently attributed to aberrant AID activity, miR-181b expression may be important in preventing B cell malignant transformation [61].
A study examining the functional role of the miR-17~92 cluster in late B cells revealed its potential importance in plasma cells [62] (thus, important not only in early B cell development and tolerance [30, 31, 40]). Indeed, conditional deletion of this cluster in mature B cells using CD19-Cre mice resulted in increased plasma cell homing to the BM upon immunization with a T-independent antigen, suggesting that this miRNA cluster regulated homing of plasma cells to their natural niche [62]. Speciffically, the data indicated that the sphingosine 1-phosphate receptor 1 (S1PR1), a receptor for S1P, was a target of different members of the miR-17~92 cluster in B cells [62]. The S1PR1-S1P signaling pathway had been previously shown to be critical for immune cell migration from secondary lymphoid organs to the BM [63]. Conditional ablation of one copy of s1pr1 in CD19 positive B cells reversed the phenotype of miR-17~92 deficient plasma cells, where homing of plasma cells to the BM was restored to the degree resembling that of control animals [62]. The report concluded that miR-17~92 could affect homing of plasma cells in the BM by reducing the levels of S1PR1 on plasma cells[62]. This finding is important, as homing to the BM allows for the long-term longevity of plasma cells, key to enabling efficient immune responses [64]. Moreover, ablation of miR-17~92 in antigen-activated B cells led to reduced CSR into the IgG2c isotype as a consequence of upregulated Ikaros mRNA and protein expression[62]. In vitro luciferase assays also revealed that Ikaros (Ikzf1) was specifically targeted by miR-92 [62]. As IgG2a/c is the most frequently found isotype in lupus patients, ablation of miR-17~92 in B cells in a lupus mouse model (Ptpn6fl/fl CD19-Cre mice lacking Shp1 tyrosine phosphatase B cells), led to attenuation of autoimmunity (reduced deposition of Ig to kidneys and fewer autoantibodies compared to Ptpn6fl/fl CD19-Cre mice expressing miR-17~92) [62]. This study suggests that investigating the therapeutic potential of miR-17~92 in lupus treatment might be considered [62].
Overall, several miRNAs have recently been shown to regulate B cell responses upon immunization, with miR-155, miR-181 and the omnipresent miR-17~92 cluster, being most intimately linked to the regulation of GC responses and terminal differentiation (Key Figure, Figure 1). In light of the fact that the majority of B cell malignancies arise from GC or post-GC B cells, it is reasonable to suppose that miRNA-mediated regulation of B cell differentiation and function might embody an important layer of control. In the context of GC B cells, miRNAs seem to be important for the regulation of adaptive immunity against pathogens and might also mediate critical steps to keep SHM and CSR in check, thus reducing the probability of translocation occurrences that could contribute to malignant transformation.
Regulation of B1 Cell Development by miRNAs
B1a cells are a subset of B lymphocytes constituting the main source of “natural” antibodies that are poly-reactive, and frequently self-reactive. This compartment of B cells is more innate-like as opposed to the conventional B2 cells and is largely restricted to pleural and peritoneal cavities in rodents [65]. The existance of B1 cells in humans is highly controversial; however, CD5 positive chronic lymphocytic leukemia (CLL) is thought to be primarily derived from B1-like lymphocytes, largely bearing a germline, unmutated, polyreactive antibody repertoire, and suggesting the existence of this innate-like B cell population in humans [66, 67]. B1a cells are mostly generated from fetal liver (FL) HSPCs, and the compartment is thought to be self-replenishing in adult mice; however, the regulation of B1a cell development is poorly understood [65]. Adult murine BM HSPCs or pro-B cells, ectopically expressing the protein Lin28b-- a negative regulator of the let-7 miRNA family-- have been more prone to giving rise to B1a cells than conventional B2 cells when injected into immunodeficient recipient mice [68, 69]. Conversely, ectopic let-7 expression in FL murine pro-B cells, has led to a dramatic skewing of B cell development towards the conventional B2 subtype, at the expense of B1a cells [70]. The authors of this study identified the transcription factor Arid3a (also know as Bright) -- previously implicated in the regulation of IgH expression and B cell responses [71]-- as being differentially expressed in fetal and adult pro-B cells [70]. Transfer of adult BM pro-B cells overexpressing Arid3a into immunodeficient mice led to increased B1a cell differentiation whereas knock-down of Arid3a using short hairpin RNA (shRNA) in fetal pro-B cells led to reduced B1a cell development [70]. These data suggested that Lin28b could positively regulate B1a differentiation by targetting let-7 mRNA, which in turn could regulate Arid3a [70]. MiR-150 knockout mice exhibited an increase in B1a cells in the spleen and peritoneal cavity, whereas ectopic expression of miR-150, as well as deletion of one allele of Myb, -- c-Myb, a putative target of miR-150 -- led to reduced numbers of B1 cells, suggesting that miR-150 might also negatively regulate B1a cell development through the upregulation of c-Myb [32].
Mice conditionally expressing two copies of a human miR-17~92 transgene in B and T cells exhibited increased numbers of B1a cells in the spleen and peritoneal cavity compared to control mice [72]. Moreover, B cell-specific ablation of miR-17~92 using CD19-Cre mice has been shown to lead to reduced B1a cell numbers in the peritoneal cavity [62]. Development of murine B1a cells indeed depends on the expression of CD19 and on the activation of PI3K via CD19 signaling [73, 74]. Furthermore, miR-17~92 overexpression can partially rescue B1a development in the peritoneal cavity of mice lacking CD19, possibly as a consequence of PI3K activation [40]. Together, these data suggest that miR-17~92 might influence B1a cell development and/or maintenance by regulating PI3K signaling, but further studies are required to fully validate this hypothesis.
In fact, B1 development remains to be fully elucidated, but recent studies probing the contribution of miRNAs in regulating B1 vs B2 lineage commitment have contributed to our understanding of putative signaling pathways modulating the differentiation of this enigmatic B cell subset (Key Figure, Figure 1). While the exact role of these enigmatic cells in humans is not fully clear, characteristics of this subset of B lymphocytes, namely, the constitutive production of broadly reactive antibodies, renders this lineage relevant to research in diverse areas, including autoimmunity and transplantation. Further studies investigating the role of miRNAs in B1 lineage commitment and function may yield novel insight not only into miRNA function, but also into the intrinsic biology of this poorly understood B cell subset.
miRNAs in B Cell Lymphomagenesis
There is increasing evidence implicating miRNAs in B cell lymphomagenesis, both in human and mouse. The majority of known miRNAs have been shown to be downregulated in tumor cells for various lymphomas [75, 76]; however, several miRNAs known to be important in B cell development, survival and function, can also be overexpressed in B cell lymphomas [72, 77–88].
miR-17~92
miR-17~92, encoded by Chromosome 13 open reading frame 25 (C13orf25) located in human chromosome 13q31, is amplified in many solid tumors and hematopoietic malignancies, with the most common types being non-Hodgkin lymphoma and diffuse large B-cell lymphoma (DLBCL) [89]. Expression of miRNAs from this cluster has been found to be increased in human B cell lymphoma primary samples and cell lines carrying the 13q31 amplicon [81]. Retroviral overexpression of miR-17~92 in a mouse model of B cell lymphoma (Eμ-Myc, where expression of the oncogene c-Myc is driven by the B cell-specific IgH enhancer Eμ [90]) was reported to accelerate the onset and severity of the tumors [81]. This cluster has thus been described as an oncogenic miRNA cluster, or oncomiR-1 [81]. Moreover, mice expressing two copies of a human miR-17~92 transgene in the B- and T-cell compartments, present signs of lymphoproliferative disease, with an expansion of B1a cells, GC B cells, as well as a reduction in MZ B cells, in 5-week- to 20-week-old mice [72]. These mice also develop autoimmune disease with age, characterized by an elevated production of autoantibodies, and infiltration of lymphocytes into non-lymphoid organs, including salivary glands, lungs and to a lesser extent, kidneys [72]. Ex vivo, B cells from these mice exhibit increased proliferation and are resistant to cell death relative to control animals [72]. Moreover, mice lacking one copy of each of the Bim and Pten targeted genes, recapitulate some of the features of miR-17~92 transgenic mice with age, including increased numbers of GC B cells; consequently, the effects of miR-17~92 overexpression appear to be mediated, at least in part, by decreased levels of Bim and Pten (miR-17~92 targets) [72].
In another study, conditional deletion of miR-17~92 in a murine miR-17~92fl/fl Eμ-Myc lymphoma cell line using tamoxifen-inducible Cre-Ert2, led to an increase in apoptosis and delayed tumorigenicity when injected into nude mice [84]. In vitro and in vivo analysis using retroviral vectors expressing miR-17~92 mutant alleles, each lacking or expressing different members of this cluster, led to the identification of miR-19a and miR-19b as being crucial for the survival and tumorigenicity of Eμ-Myc lymphoma cells [84]. The effect of miR-19 was primarily attributed to its direct regulation of Pten levels, as knockdown of Pten in miR-17~92-deficient cells rescued the phenotypes, with reduced apoptosis in vitro, and faster tumor formation when injected into recipient mice when compared to miR-17~92-deficient cells [84]. Moreover, in one study, upregulated miR-19a expression has been reported in samples from CLL patients, correlating with low levels of PTEN in the tumor cells [77]. While the above studies demonstrate the importance of miR-17~92 in B cell tumorigenesis, they do not directly establish the role of miR-17~92 as a driver of B cell lymphomagenesis. B-cell specific overexpression of this cluster using CD19-Cre mice, has been led to lymphomagenesis, seemingly derived from GC B cells and post-GC plasmablasts, and phenotypically resembling human lymphomas, including DLBCL [82]. In addition, PAR-CLIP analysis of human immortalized B cells and analysis of in vitro activated FO B cells from miR-17~92-overexpressing transgenic mice has suggested that the cell cycle regulator E2F3, in addition to Bim, might be direct targets of this cluster; indeed, their protein levels are reduced in cells overexpressing miR-17~92, which could contribute to increased proliferation and survival of these B cells [82]. Moreover, transgenic expression of miR-17~92 in murine FO B cells has led to repression of Pten and Phlpp2, two negative regulators of PI3K, resulting in increased PI3K signaling [82]. In addition, NF-κB deubiquitinases CYLD and A20, which negatively regulate canonical NF-κB signaling, can induce NF-κB downstream of the BCR in miR-17~92-overexpressing B cells, and thus, have also been proposed as targets of oncomiR-1, [82]. Tumor-bearing mice treated with chemical inhibitors of either PI3K (AZD8055) or NF-κB (BMS-345541), have in fact exhibited reduced numbers of tumors and prolonged survival relative to controls; this suggests that miR-17~92 could be important for both tumor development and maintenance [82].
Mice bearing a conditional deletion of miR-17~92 induced by CD19-Cre on a λ-Myc background develop fewer lymphomas than mice carrying λ–Myc transgene alone (reminiscent of human Burkitt’s lymphoma), most of them originating from early B cell precursors [82]. The majority of tumors found in these mice maintain expression of miR-17~92, and thus, may have developed prior to Cre expression, suggesting a selective advantage of miR-17~92-expressing tumors [82]. In another overexpression model of this oncomiR cluster, Eμ-miR-17-92 transgenic mice developed B cell malignancy resembling human CLL and DLBCL, by 12–18 months [87]. Microarray analysis of B cells in these mice suggested that PI3K was downregulated in these cells [87], a finding that differs from data suggested another previously cited model [82]. Of note, in a recent study, miR-17~92 was overexpressed in a small proportion of mouse hematopoietic cells with Cre expression under the vascular and visceral smooth muscle cell and cardiac muscle promoter Sm22a (which unexpectedly, also led to Cre activity in a few hematopoietic cells within the BM) [79]. This system revealed that miR-17~92 overexpression in only a few cells in the BM-- including stem cells—led to lymphoma development, suggesting that inappropriate expression of this oncomir in a small subset of cells is sufficient to promote lymphomagenesis [79]. These animals developed lymphoproliferative disease that was primarily characterized by the expansion of different subsets of B cells, as the malignancies resembled marginal zone lymphomas, but also occasionally, exhibited DLBCL-like appearance [79].
miR-155, miR-132, miR-126, and miR-34
Overexpression of other miRNAs has been associated with different B cell lymphomas. High expression of miR-155 has been associated with CLL [78] and DLBCL among other human B cell lymphomas [80] although the direct target genes of miR-155 that may contribute to miR-155-mediated malignant transformation remain to be identified. Expression of miR-132 could be induced in CLL patient B cells upon BCR stimulation, and it is believed that miR-132 may contribute to severity of the disease [83, 86, 88]. However, overexpression of miR-132 on a Eμ-Myc background in mice, resulted in improved animal survival when compared to Eμ-Myc mice, suggesting that miR-132 may yield a protective effect, at least in this mouse model [26]. Mice overexpressing miR-126 in lin− HSPCs, have developed precursor B cell leukemia [85]. Indeed, RNA-seq analyses of human CD34+ progenitor cells and murine B cell acute lymphoblastic leukemia (B-ALL) cells overexpressing or lacking miR-126, suggested that this miRNA could modulate the expression of genes implicated in the p53 tumor suppressor signaling pathway, where cyclin-dependent kinase inhibitor 2a-interacting protein, Cdkn2aip, was validated as a direct target of miR-126 in a luciferase reporter assay [85]. The leukemia in miR-126 overexpressing mice can regress if expression of miR-126 is abolished, demonstrating that the malignant cells may be addicted to miRNA-126 expression[85]. However, regression can be delayed upon expression a dominant-negative p53 mutant, suggesting that the p53 pathway is a functionally relevant (although likely not exclusive) target of miR-126 in the context of this B-ALL model [85]. Similarly, this study demonstrated that primary B-ALL patient cells were dependent on miR-126 to maintain a proliferative state when serially transplanted into immunocompromised mice [85].
By contrast, tumor samples from gastric DLBCL patients have shown downregulated levels of miR-34 [91]. Expression of this miRNA may be repressed by c-Myc as knockdown of c-Myc in two different DLBCL lines resulted in increased miR-34 expression [91]. In addition, introduction of miR-34 in DLBCL cell lines induced mRNA and protein expression of the transcription factor FoxP1, which seems to be a direct target of miR-34 based on luciferase reporter assays [91]. Expression of miR-34, or knock down of FoxP1 in a DLBCL cell line also reduced DLBCL tumor cell proliferation in vitro [91] and led to DLBCL apoptosis in vivo, in a murine xenograft model [92]. Consequently, these findings highlight the therapeutic potential of exploiting the miR-34/FoxP1 axis to treat DCBL[92].
Collectively, the deregulation of miRNA expression has been frequently associated with the development of B cell malignancies and merits further attention, particularly since other, as yet-to-be identified miRNAs might also play important roles in this process (Key Figure, Figure 1). Indeed, impaired expression of various miRNAs associated with B cell neoplasms emphasize the key roles of miRNAs as regulators of normal B cell development and function.
Concluding Remarks
This review aimed to underscore recent advances in our understanding of the role of individual miRNAs in orchestrating B cell lineage commitment, development and lymphomagenesis. Over the past decade, our knowledge on the functional contribution of various miRNAs at different stages of mammalian B cell development has greatly improved, but the full extent of these contributions in B cell hematopoiesis remains to be elucidated. Recent studies have used cell-specific gene targeting to hone in on the role of individual miRNAs and miRNA families in this process, and we have come a long way from the initial observation that Dicer-dependent miRNAs are essential for B cell development. However, our knowledge remains fragmentary.
Some important limitations include the fact that miRNAs can exert pleiotropic effects, targeting multiple genes [10] and signaling networks in any one cell type. In addition, miRNA-target relationships depend on availability and accessibility of target UTRs, as well as on the levels of miRNA expression. Dissecting such miRNA-target relationships is further complicated by the redundancy that often exists between related clusters of miRNAs (see Outstanding Questions and Box # 1). Current efforts to define relevant targets of miRNAs rely on bioinformatic approaches, coupled with high-throughput RNA sequencing to expose genes whose expression is perturbed upon miRNA ablation or over-expression. However, to pinpoint the full spectrum of bona fide miRNA targets, an integrated approach that examines both transriptomics and proteomics will be necessary; indeed, miRNAs can influence both transcript stability and translational repression. Furthermore, defining direct targets of any one given miRNA will require specific mutagenesis assays, including mutations of binding sites within individual mRNA UTRs in a cell type-specific manner, a herculean effort that is rarely undertaken.
Outstanding Questions Box.
What are the most relevant targets of the individual miRNAs that regulate B cell development and function?
How is the expression of miRNAs regulated at specific stages of B cell development – is there redundancy?
What are the upstream signals that control specific miRNA expression through B cell development?
How does antigen receptor signaling contribute to the regulation of miRNAs at discrete stages of B cell development – especially during positive and negative selection?
How do miRNAs affect human B cell development and function (most of the data to date come from mouse models)?
What causes miRNA expression to be deregulated in lymphomas?
How can miRNAs be targeted for the treatment of hematologic malignancies?
Box 1. Clinician’s Corner.
B lymphocytes are essential for an efficient immune response against a variety of pathogens. The development of the diverse repertoire of B cell antigen receptors (antibodies) involves somatic DNA recombination at the immunoglobulin loci and is tightly regulated in the cell. Aberrant recombination can indeed lead to lymphomagenesis.
miRNAs are differentially expressed at different stages of B cell development. They appear to be essential regulators of B cell development and function. Many of the same miRNAs seem to be important at various discrete stages of B cell development. They contribute to the regulation of transcriptional and post-transcriptional programs by targeting the 3′ UTRs of multiple mRNAs.
Some miRNAs important for B cell development are upregulated in B cell lymphomas. Dysregulation of these miRNAs likely contributes to lymphomagenesis. However, further studies are required to fully understand the relevant direct targets of these miRNAs in malignant and pre-malignant cells. Future therapeutic approaches could target miRNAs, but this remains challenging because i) the necessary cell-type specific delivery methods are lacking, ii) short miRNAs are inherently stable and iii) the relevant target of any specific miRNA varies from cell-type to cell-type.
B cell development is a step-wise process initiating from hematopoietic progenitor cells (HPC) in the bone marrow. At the pro-B cell and pre-B cell stages, the immunoglobulin heavy and light chains (respectively) of a B cell receptor (BCR) per cell, undergo V(D)J recombination. From the immature B cell stage onwards, each B cell expresses a unique and functional BCR. Further maturation of B cells occurs in the spleen and gives rise to marginal zone (MZ) or follicular (FO) B cells. Upon stimulation by cognate antigen, B cells enter the germinal center where they proliferate and become plasma cells or memory B cells.
A different subset of B cells, B1 cells, are found in mouse peritoneal and pleural cavities and produce polyreactive “natural” antibodies. These cells are believed to primarily arise from fetal liver precursors.
miRNAs positively or negatively control every step of B cell development. Relevant targets are indicated in parenthesis, when known. miRNAs in red have been found to be overexpressed in B cell malignancies. miRNAs in blue are often downregulated in B cell lymphomas.
Our knowledge of the effect of miRNAs in human B cell development is curtailed by the fact that most studies published so far have used mouse models. Current human studies are generally assessing the levels of miRNAs in samples from patients with either autoimmune diseases such as lupus or rheumatoid arthritis, or B cell malignancies, but frequently, these fail to investigate the mechanisms linking perturbed miRNA levels to disease presentation. Yet, understanding the most relevant miRNA-target relationships at each stage of lymphocyte development and during lymphomagenesis may not only shed light on our basic understanding of lymphocyte biology, but also provide insight regarding critical targets for future candidate therapeutic and diagnostic approaches. A focus on this arena should thus prevail.
Trends Box.
B cells are important players of adaptive immunity and their development requires tight regulation. Most lymphomas and leukemias are of B cell origin.
miRNAs are important gene expression regulators playing essential roles during B cell development, maturation and function.
miRNAs are often deregulated in B cell malignancies. In multiple cases, aberrant expression of these non-coding RNAs, such as the miR-17~92 cluster, can constitute a causal event for these malignancies.
miRNAs appear to be essential for the regulation of the PTEN/PI3K axis in mammalian B lymphocyte development and function.
Fine-tuning via miRNA-dependent gene silencing mechanisms is critical for the maintenance of cell survival and proliferation at all stages of B cell development.
Recent advances in gene targeting technology, including the pioneering use of CRISPR-mediated gene editing tools, will enable investigators to probe the role of miRNA-target interactions by targeting specific miRNA binding sites within the 3′ UTRs of genes of interest.
Acknowledgments
Work in the Koralov laboratory was supported by the grant from The Judith and Stewart Colton Center for Autoimmunity, by NIH R01HL125816, 1R21AI110830 and funding from the Ralph S. French Charitable Trust as well as the Beckman Foundation.
Glossary
- Activation-induced cytidine deaminase (AID)
enzyme that mutates DNA by converting cytosine into uracil. It is responsible for class switch recombination and somatic hypermutation during the germinal center reaction.
- Affinity maturation
a cornerstone process of adaptive immunity where the affinity of the B cell receptor for antigen evolves as a consequence of single base point mutations (somatic hypermutation) that take place within immunoglobulin loci.
- B cell acute lymphoblastic leukemia (B-ALL)
the most common type of cancer in children. It is a malignancy of B cell lineage origin, but the exact nature/stage of the B cell that becomes transformed is unclear.
- B1a cells
a subset of B cells that produce polyreactive antibodies and are found mainly in the peritoneal and pleural cavities in mice. These are viewed as “innate”-like B cells and are responsible for the production of “natural” antibodies.
- Bone marrow (BM) reconstitution experiments
transfer of BM cells from one experimental animal to another via intravenous injection. These experiments allow investigators to define if the donor BM cells are capable of repopulating the hematopoietic compartment of the recipient (host).
- Burkitt’s lymphoma
rapidly growing non-Hodgkin lymphoma of germinal center B cell origin.
- Class switch recombination (CSR)
mechanism by which activated B cells rearrange the constant region of the antibody from one type (isotype) to another. While CSR does not impact antigen specificity, it leads to a change in antibody effector function.
- Cre-Ert2
a tamoxifen inducible version of Cre recombinase – a bacteriophage derived enzyme that carries out site-specific recombination.
- Chronic lymphocytic leukemia (CLL)
this indolent malignancy is the most common type of leukemia in adults and is believed to derive from B1-like B cells.
- Depth of sequencing
refers to how many reads or sequences contribute to the information about a specific nucleotide position. Often, it is the average depth of sequencing that is discussed, as the precise number of reads varies widely for specific sequences in the genome in most genome-wide sequencing approaches.
- Diffuse large B-cell lymphoma (DLBCL)
an aggressive B cell malignancy, predominantly of germinal center origin
- Extrafollicular responses
a response to antigenic challenge driven by B lymphocytes that migrate to the border of the T cell zone and red pulp (outside the GC), primarily characterized by unmutated antibody production of modest/low affinity.
- Follicular (FO) B cells
mature B cell population found in lymphoid follicles of secondary lymphoid organs such as spleen and lymph nodes. FO B cells can recirculate in the blood and require T cell help for activation.
- Germinal center (GC)
Upon activation with their cognate antigens in a T cell-dependent manner, B cells give rise to a specialized secondary structure, called the germinal center within secondary lymphoid organs. In these structures, B cells undergo clonal expansion, somatic hypermutation, and class switch recombination and differentiate into plasma cells or memory B cells.
- Immunoglobulin heavy chain (IgH) and light chain (IgL)
Large and small polypeptide subunits of an antibody. Each antibody comprises two IgH and IgL subunits.
- Lambda-myc (λ-myc) background
the expression of a well characterized oncogene, the mutant human MYC gene isolated from a Burkitt’s lymphoma cell line, is driven by the lambda Ig promoter in these mice.
- Luciferase reporter assay
commonly used technique to evaluate transcriptional activity employing a gene encoding a bioluminescence protein; it can be used to examine the relevance of miRNA binding site(s) within a given 3′ UTR by linking the luciferase gene to the UTR in question.
- Marginal Zone (MZ) B cells
mature B cell population located in the marginal zone of the spleen. These lymphocytes respond rapidly to blood-borne pathogens in a T-cell independent manner. They are non-recirculating and have a long lifespan.
- Memory B cells
long lived high affinity B cells that are fast responding upon secondary antigenic exposure
- Microprocessor complex
protein complex responsible for early stages of miRNA processing in animal cells. Drosha and DGCR8 are the central catalytic components of this complex.
- Natural antibodies
low affinity polyreactive circulating antibodies produced in the absence of detectable foreign antigen exposure. They are primarily produced by B1 cells.
- Non-Hodgkin’s Lymphomas
a group of cancers that develop from lymphocytes, distinct from the very characteristic Hodgkin lymphoma.
- Northern Blotting
gel electrophoresis technique used to evaluate gene expression where RNA is separated based on size and visualized using a sequence-specific hybridization probe.
- PAR-CLIP (photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation)
biochemical technique to identify miRNA-target mRNA relationships in a genome-wide manner. Immunoprecipitation of RNA binding/interacting proteins is followed by crosslinking the RNA that contains photoreactive nucleotides introduced into the nascent transcripts (within living cells).
- Plasma cells
terminally differentiated B lymphocytes secreting high levels of antibodies.
- Plasmablasts
precursors of plasma cells, derived from germinal center B cells.
- RNA silencing
mechanism of negative regulation of gene expression by non-coding RNAs, including miRNAs.
- RNA-induced silencing complex (RISC)
complex of proteins and miRNA that target a mRNA for gene silencing.
- Secondary Lymphoid Organs
organs where an adaptive immune response is often initiated upon exposure of mature lymphocytes to antigen. Examples include lymph nodes and spleen.
- Seed sequence
6–8bp core sequence within a mature miRNA, that through complementarity match, determines the target sequence within the 3′ UTR of the mRNA.
- Somatic hypermutation (SHM)
Mechanism of single base pair mutations at the variable region of a rearranged Ig allele. It can result in a change in affinity or specificity of an antibody for its cognate antigen
- T-cell independent antigen
antigen that has an ability to elicit a B cell response in the absence of T cell help.
- V(D)J recombination
somatic genetic recombination event that occurs at antigen receptor loci of B and T lymphocytes. It results in a diverse repertoire of B and T cell antigen receptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19(6):586–93. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]
- 2.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 3.Lee RC, et al. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 4.Wightman B, et al. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–62. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 5.Lagos-Quintana M, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 6.Lau NC, et al. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294(5543):858–62. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 7.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294(5543):862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 8.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381(6585):751–8. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JK, et al. Related Mechanisms of Antibody Somatic Hypermutation and Class Switch Recombination. Microbiol Spectr. 2015;3(1) doi: 10.1128/microbiolspec.MDNA3-0037-2014. Mdna3-0037-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek D, et al. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso K, et al. Identification of the human mature B cell miRNome. Immunity. 2009;30(5):744–52. doi: 10.1016/j.immuni.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CZ, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 13.Kuchen S, et al. Regulation of microRNA expression and abundance during lymphopoiesis. Immunity. 2010;32(6):828–39. doi: 10.1016/j.immuni.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spierings DC, et al. Ordered progression of stage-specific miRNA profiles in the mouse B2 B-cell lineage. Blood. 2011;117(20):5340–5349. doi: 10.1182/blood-2010-10-316034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood. 2009;113(19):4586–94. doi: 10.1182/blood-2008-09-178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard CC, et al. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13(5):358–69. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svoboda P. A toolbox for miRNA analysis. FEBS Lett. 2015;589(14):1694–701. doi: 10.1016/j.febslet.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26(6):715–25. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Hystad ME, et al. Characterization of early stages of human B cell development by gene expression profiling. J Immunol. 2007;179(6):3662–71. doi: 10.4049/jimmunol.179.6.3662. [DOI] [PubMed] [Google Scholar]
- 21.Nutt SL, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160–71. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 22.Fragoso R, et al. Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1. PLoS Genet. 2012;8(8):e1002855. doi: 10.1371/journal.pgen.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henao-Mejia J, et al. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38(5):984–97. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuyama K, et al. MicroRNA-126-mediated control of cell fate in B-cell myeloid progenitors as a potential alternative to transcriptional factors. Proc Natl Acad Sci U S A. 2013;110(33):13410–5. doi: 10.1073/pnas.1220710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong KY, et al. MIR-23A microRNA cluster inhibits B-cell development. Exp Hematol. 2010;38(8):629–640. e1. doi: 10.1016/j.exphem.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta A, et al. The microRNA-212/132 cluster regulates B cell development by targeting Sox4. J Exp Med. 2015;212(10):1679–92. doi: 10.1084/jem.20150489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koralov SB, et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132(5):860–74. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Coffre M, et al. miRNAs Are Essential for the Regulation of the PI3K/AKT/FOXO Pathway and Receptor Editing during B Cell Maturation. Cell Rep. 2016;17(9):2271–2285. doi: 10.1016/j.celrep.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandl A, et al. The microprocessor component, DGCR8, is essential for early B-cell development in mice. Eur J Immunol. 2016;46(12):2710–2718. doi: 10.1002/eji.201646348. [DOI] [PubMed] [Google Scholar]
- 30.Ventura A, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–86. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai M, et al. Regulation of B-cell development and tolerance by different members of the miR-17 approximately 92 family microRNAs. Nat Commun. 2016;7:12207. doi: 10.1038/ncomms12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao C, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131(1):146–59. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Zhou B, et al. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104(17):7080–5. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao DS, et al. MicroRNA-34a Perturbs B Lymphocyte Development by Repressing the Forkhead Box Transcription Factor Foxp1. Immunity. 2010 doi: 10.1016/j.immuni.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu H, et al. Foxp1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7(8):819–26. doi: 10.1038/ni1358. [DOI] [PubMed] [Google Scholar]
- 36.Pelanda R, Torres RM. Central B-cell tolerance: where selection begins. Cold Spring Harb Perspect Biol. 2012;4(4):a007146. doi: 10.1101/cshperspect.a007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duong BH, et al. Negative selection by IgM superantigen defines a B cell central tolerance compartment and reveals mutations allowing escape. J Immunol. 2011;187(11):5596–605. doi: 10.4049/jimmunol.1102479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werner M, et al. Role of PI3K in the generation and survival of B cells. Immunol Rev. 2010;237(1):55–71. doi: 10.1111/j.1600-065X.2010.00934.x. [DOI] [PubMed] [Google Scholar]
- 39.Chung JB, et al. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24(6):343–9. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 40.Benhamou D, et al. A c-Myc/miR17-92/Pten Axis Controls PI3K-Mediated Positive and Negative Selection in B Cell Development and Reconstitutes CD19 Deficiency. Cell Rep. 2016;16(2):419–31. doi: 10.1016/j.celrep.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 41.Diamant E, et al. CD19 regulates positive selection and maturation in B lymphopoiesis: lack of CD19 imposes developmental arrest of immature B cells and consequential stimulation of receptor editing. Blood. 2005;105(8):3247–54. doi: 10.1182/blood-2004-08-3165. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Martin A, et al. The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nat Immunol. 2016;17(4):433–40. doi: 10.1038/ni.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvador JM, et al. The autoimmune suppressor Gadd45alpha inhibits the T cell alternative p38 activation pathway. Nat Immunol. 2005;6(4):396–402. doi: 10.1038/ni1176. [DOI] [PubMed] [Google Scholar]
- 44.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6(10):728–40. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 45.Deriano L, et al. The RAG2 C terminus suppresses genomic instability and lymphomagenesis. Nature. 2011;471(7336):119–23. doi: 10.1038/nature09755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills KD, et al. The role of DNA breaks in genomic instability and tumorigenesis. Immunol Rev. 2003;194:77–95. doi: 10.1034/j.1600-065x.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 47.Belver L, et al. MicroRNAs prevent the generation of autoreactive antibodies. Immunity. 2010;33(5):713–22. doi: 10.1016/j.immuni.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9(11):767–77. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 49.Tarlinton D, Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science. 2013;341(6151):1205–11. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- 50.Keim C, et al. Regulation of AID, the B-cell genome mutator. Genes Dev. 2013;27(1):1–17. doi: 10.1101/gad.200014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu S, et al. The RNase III enzyme Dicer is essential for germinal center B-cell formation. Blood. 2012;119(3):767–76. doi: 10.1182/blood-2011-05-355412. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez A, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thai TH, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316(5824):604–8. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 54.Vigorito E, et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27(6):847–59. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houston IB, et al. Reduction in PU.1 activity results in a block to B-cell development, abnormal myeloid proliferation, and neonatal lethality. Exp Hematol. 2007;35(7):1056–68. doi: 10.1016/j.exphem.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu D, et al. The miR-155-PU.1 axis acts on Pax5 to enable efficient terminal B cell differentiation. J Exp Med. 2014;211(11):2183–98. doi: 10.1084/jem.20140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alivernini S, et al. MicroRNA-155 influences B-cell function through PU.1 in rheumatoid arthritis. Nat Commun. 2016;7:12970. doi: 10.1038/ncomms12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dorsett Y, et al. MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity. 2008;28(5):630–8. doi: 10.1016/j.immuni.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teng G, et al. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28(5):621–9. doi: 10.1016/j.immuni.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorsett Y, et al. A role for AID in chromosome translocations between c-myc and the IgH variable region. J Exp Med. 2007;204(9):2225–32. doi: 10.1084/jem.20070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Yebenes VG, et al. miR-181b negatively regulates activation-induced cytidine deaminase in B cells. J Exp Med. 2008;205(10):2199–206. doi: 10.1084/jem.20080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu S, et al. Mir-17-92 regulates bone marrow homing of plasma cells and production of immunoglobulin G2c. Nat Commun. 2015;6:6764. doi: 10.1038/ncomms7764. [DOI] [PubMed] [Google Scholar]
- 63.Kabashima K, et al. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. 2006;203(12):2683–90. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minges Wols HA, et al. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169(8):4213–21. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 65.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 66.Griffin DO, et al. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayakawa K, et al. Early generated B1 B cells with restricted BCRs become chronic lymphocytic leukemia with continued c-Myc and low Bmf expression. J Exp Med. 2016;213(13):3007–3024. doi: 10.1084/jem.20160712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kristiansen TA, et al. Cellular Barcoding Links B-1a B Cell Potential to a Fetal Hematopoietic Stem Cell State at the Single-Cell Level. Immunity. 2016;45(2):346–57. doi: 10.1016/j.immuni.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Yuan J, et al. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335(6073):1195–200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Y, et al. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J Exp Med. 2015;212(4):569–80. doi: 10.1084/jem.20141510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt C, et al. Signalling of the BCR is regulated by a lipid rafts-localised transcription factor, Bright. Embo j. 2009;28(6):711–24. doi: 10.1038/emboj.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao C, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9(4):405–14. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anzelon AN, et al. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4(3):287–94. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 74.Rickert RC, et al. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376(6538):352–5. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 75.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 76.Thomson JM, et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20(16):2202–7. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calin GA, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101(32):11755–60. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui B, et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014;124(4):546–554. doi: 10.1182/blood-2014-03-559690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Danielson LS, et al. Limited miR-17-92 overexpression drives hematologic malignancies. Leuk Res. 2015;39(3):335–41. doi: 10.1016/j.leukres.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eis PS, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A. 2005;102(10):3627–32. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin HY, et al. MicroRNA-17~92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathways. The EMBO Journal. 2013;32(17):2377–2391. doi: 10.1038/emboj.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawrie CH, et al. MicroRNA expression in lymphocyte development and malignancy. Leukemia. 2008;22(7):1440–6. doi: 10.1038/sj.leu.2405083. [DOI] [PubMed] [Google Scholar]
- 84.Mu P, et al. Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 2009;23(24):2806–11. doi: 10.1101/gad.1872909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nucera S, et al. miRNA-126 Orchestrates an Oncogenic Program in B Cell Precursor Acute Lymphoblastic Leukemia. Cancer Cell. 2016;29(6):905–21. doi: 10.1016/j.ccell.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Pede V, et al. CLL cells respond to B-Cell receptor stimulation with a microRNA/mRNA signature associated with MYC activation and cell cycle progression. PLoS One. 2013;8(4):e60275. doi: 10.1371/journal.pone.0060275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sandhu SK, et al. B-cell malignancies in microRNA Emu-miR-17~92 transgenic mice. Proc Natl Acad Sci U S A. 2013;110(45):18208–13. doi: 10.1073/pnas.1315365110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tavolaro S, et al. Increased chronic lymphocytic leukemia proliferation upon IgM stimulation is sustained by the upregulation of miR-132 and miR-212. Genes Chromosomes Cancer. 2015;54(4):222–34. doi: 10.1002/gcc.22236. [DOI] [PubMed] [Google Scholar]
- 89.Ota A, et al. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64(9):3087–95. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 90.Adams JM, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318(6046):533–8. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 91.Craig VJ, et al. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood. 2011;117(23):6227–36. doi: 10.1182/blood-2010-10-312231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Craig VJ, et al. Systemic microRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia. 2012;26(11):2421–4. doi: 10.1038/leu.2012.110. [DOI] [PubMed] [Google Scholar]