Abstract
The association of the cytoskeleton with the cadherin–catenin complex is essential for strong cell-cell adhesion in epithelial cells. In this study, we have investigated the effect of microtubule organization on cell-cell adhesion in differentiating keratinocytes. When microtubules of normal human epidermal keratinocytes (NHEKs) grown in low calcium media (0.05 mM) were disrupted with nocodazole or colcemid, cell-cell adhesion was induced through relocalization of the E-cadherin–catenin–actin complex to the cell periphery. This was accompanied by actin polymerization. Also, it was found that microtubule disruption-induced cell-cell adhesion was significantly reduced in more advanced differentiated keratinocytes. For example, when NHEK cells cultured under high calcium (1.2 mM) for 8 d and then in low calcium for 1 d were treated with nocodazole, there was no induction of cell-cell adhesion. Also long-term treatment of a phorbol ester for 48 h inhibited nocodazole-induced cell-cell adhesion of NHEK. Furthermore, this nocodazole-induced cell-cell adhesion could be observed in squamous cancer cell lines (A431 and SCC-5, -9, and -25) under low calcium condition, but not in the keratinocyte cell lines derived from normal epidermis (HaCaT, RHEK). On the other hand, HaCaT cells continuously cultivated in low calcium media regained a less differentiated phenotype such as decreased expression of cytokeratin 10, and increased K5; these changes were accompanied with inducibility of cell-cell adhesion by nocodazole. Together, our results suggest that microtubule disruption can induce the cell-cell adhesion via activation of endogenous E-cadherin in non- or early differentiating keratinocytes. However, this is no longer possible in advanced terminally differentiating keratinocytes, possibly due to irreversible changes effected by cell envelope barrier formation.
INTRODUCTION
Cell-cell adhesion is critical to many aspects of multicellular existence, including morphogenesis, tissue integrity, cell-cell communication, normal cell growth, and differentiation (Takeichi, 1995; Vleminckx and Kemler, 1999; Gumbiner, 2000). Cadherins are major cell-cell adhesion molecules involved in the development and maintenance of all solid tissues (Takeichi, 1991; Gumbiner, 1996). They carry a large extracellular domain, a short transmembrane domain, and a cytoplasmic tail. The extracellular domain is composed of five cadherin repeats that mediate calcium-dependent homophilic cell-cell adhesion. The cytoplasmic tail domain directly interacts with β-catenin or plakoglobin, which in turn associates with α-catenin. The latter appears to link this cadherin–catenin complex to the actin cytoskeleton directly or indirectly through other cytoskeletal proteins such as α-actinin and vinculin. This link between cadherin and the actin cytoskeleton via catenins is essential for strong and rigid adhesion (Fukata et al., 1999).
Cell-cell adhesion mediated by E-cadherin is closely related with terminal differentiation of the skin by regulation through the extracellular calcium concentration (Yuspa et al., 1989). In a low calcium environment, normal primary keratinocytes isolated from fresh skin are in a hyperproliferative state and there is no apparent adhesion between them. By increasing the calcium concentration of the media, keratinocyte proliferation is retarded and cells become adhesive without any change of E-cadherin expression level. Coincident with adhesion, normal keratinocytes initiate terminal differentiation processes in a high calcium environment, and begin to express a variety of proteins and enzymes required for barrier formation. However, the acquisition of an invasive phenotype in various epithelial cancer cell types has been associated with the loss or alteration of E-cadherin and/or their associated proteins (Takeichi, 1993). Down-regulation of E-cadherin in human carcinomas and in experimental model systems is usually linked to loss of differentiation (Navarro et al., 1991; Gamallo et al., 1993).
Cadherin regulation has been examined in numerous model systems and several different mechanisms have been proposed. Because complex formation between cadherin, catenin, and the cytoskeleton is required for strong cell-cell adhesion, changes in the composition of the complex, phosphorylation of components in the complex, and alterations in the interaction of the complex with the actin cytoskeleton have all been suggested to play a role in regulation of adhesion. In certain cells, epidermal growth factor and scatter factor/hepatocyte growth factor induce decreased cell-cell contact (Weinder et al., 1990; Shibamoto et al., 1994), and tyrosine phosphorylation of β-catenin correlates with inhibition of cadherin-mediated adhesion (Behrens et al., 1993; Shibamoto et al., 1994). However, the underlying molecular regulation for cell-cell adhesion seems to be complicated and diverse. For example, endogenous nonfunctional E-cadherin in Chinese hamster ovary cells can be activated by stimulation of ectopically expressed muscarinic acetylcholine receptor through protein kinase C (PKC) activation (Shafer et al., 1999). On the other hand, adhesive activity of Colo205 tumor cells can be activated with staurosporine, which is a serine kinase inhibitor (Aono et al., 1999).
Recently, several studies have suggested that Rho family GTPases are required for cadherin-mediated cell-cell adhesion. Microinjection of C3 exozyme (an inhibitor of Rho) or Rac1N17 (negative dominant mutant form of Rac1) inhibits the accumulation of E-cadherin at sites of cell-cell contact when keratinocytes are transferred from low calcium medium to standard medium (which restores calcium-dependent cell-cell adhesion) (Braga et al., 1997). In fibroblasts, microtubule disruption strongly induces actin polymerization (stress fiber formation) and focal adhesion (Bershadsky et al., 1996), and these phenomena were inhibited by microinjection with C3 exozyme (Liu et al., 1998), again indicating RhoA mediation.
Considering such phenomena, we postulated that microtubule disruption might induce cell-cell adhesion of keratinocytes. This study demonstrates that microtubule disruption induces cell-cell adhesion through activation of E-cadherin in addition to actin stress fiber formation in normal human epidermal keratinocytes (NHEKs) cultured in low calcium environment. Also, we report that cell-cell adhesion induced by microtubule disruption was influenced by the differentiation status of keratinocytes.
MATERIALS AND METHODS
Cells
NHEKs (Clonetics, San Diego, CA) were propagated on the collagen-coated dishes (0.1 mg/ml, collagen type I; Sigma, St. Louis, MO) with the use of serum-free keratinocyte growth medium supplemented with 0.05 mM CaCl2, bovine pituitary extract, epidermal growth factor, insulin, hydrocortisone, transferrin, epinephrine, and gentamicin (KGM; Clonetics, San Diego, CA) and used for experiments at passage 2 or 3. Primary human keratinocytes isolated from neonatal foreskins in our laboratory were also used for comparison with Clonetics keratinocytes. Foreskin keratinocytes were propagated on uncoated dishes with the use of K-SFM (Life Technologies, Grand Island, NY), which was supplemented with 0.05 mM CaCl2, bovine pituitary extract, and epidermal growth factor. We show only the results of experiments done with NHEK cells since similar results were obtained with both cells.
Several keratinocyte cell lines were used. Among them, A431, SCC-4 (ATCC no. CRL-1624), SCC-9 (CRL-1629), and SCC-25 (CRL-1628) were derived from a squamous cancer of the oral cavity (Rheinwald and Beckett, 1981), whereas HaCaT (Boukemp et al., 1988) and RHEK (Rhim et al., 1985) were derived from normal skin. These cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. All cells were grown under 5% CO2 atmosphere in humidified incubator.
Chemicals and Antibodies
Microtubule-disrupting agents nocodazole and colcemid were used at the final concentration of 33 μM and 1 μg/ml, respectively. For actin disruption, cytochalasin B (25 μM) was used. Phorbol 12-myristate 13-acetate (PMA), a PKC activator, was used at the concentration of 10 nM. GF190203X, a PKC antagonist, was used at 1 μM. All chemicals were purchased from Calbiochem (La Jolla, CA).
Monoclonal antibodies (mAb) against the C-terminal region of E-cadherin, β-catenin, and phosphotyrosine (PY20) were purchased from Transduction Laboratories (San Diego, CA). mAbs against β-tubulin and pan-keratin (AE1/AE3) were from Roche Molecular Biochemicals (Indianapolis, IN). mAbs against tyrosinated tubulin, acetylated tubulin, vinculin, and cytokeratins were from Sigma. Goat antisera against α-catenin was from Santa Cruz Biotechnologies (Santa Cruz, CA). Anti-involucrin antibody was from NeoMarker (Fremont, CA) and antiloricrin was purchased from Babco (Richmond, VA). Species-specific fluorescein isothiocyanate (FITC)- or Texas Red-conjugated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). Peroxidase-conjugated secondary antibody was from Bio-Rad (Hercules, CA). FITC-conjugated phalloidin (Sigma) was used for immunofluorescence staining of actin fibers.
Induction of Cell-Cell Adhesion
NHEKs were grown on coverslips or tissue culture dishes in low calcium KGM until they reached 60–70% confluency and then treated with various chemicals for 1–48 h. Because confluent growth of keratinocytes induced some cell-cell adhesion even in low calcium condition, we did not use cells that had grown >70% confluency. The treated cells were analyzed for E-cadherin accumulation between cell-cell boundaries by immunofluorescence (IF) assay. To analyze the effects of keratinocyte differentiation on the cell-cell adhesion induced by microtubule disruption, NHEK cells were induced to differentiate with KGM containing high calcium (1.2 mM) for up to 8 d and then transferred to low calcium KGM to dissociate adhesive keratinocytes into individual cells. Usually, culture for 24 h in low calcium KGM was sufficient to dissociate cells. After microscopic confirmation of cell dissociation, cell-cell adhesion was induced as mentioned above.
In all other cell lines, cells were grown at 50% confluency, transferred from 10% DMEM containing high calcium to low calcium KGM, and cultured for 24 h to dissociate cell-cell adhesion. Because the cell lines have higher growth rates than NHEKs, we performed experiments with the use of less confluent grown cells than NHEKs. Cell-cell adhesion was induced as above.
IF Assay
For IF staining, cells were plated, grown, and treated with various chemicals. Cells were rinsed with ice-cold phosphate buffered saline (PBS, pH 7.4) and fixed with 4% paraformaldehyde in PBS. In some experiments, cells were pretreated with 0.4% Triton X-100 in PBS for 3 min to remove soluble cytosolic proteins before fixation. For the clear visualization of actin accumulation at the cell-cell adhesion sites, cells were incubated with the cell permeable cross-linking agent dithiobis(succinimidyl propionate) (Pierce, Rockford, IL) before fixation. Briefly, rinsed cells were incubated with 10 μg/ml dithiobis(succinimidyl propionate) in Hanks' balanced salt solution for 15 min in a 37°C incubator and then washed with PBS containing 1% Triton X-100 three times. After fixation, cells were permeabilized by incubation with 0.4% Triton X-100 in PBS. Permeabilized cells were incubated with 5% bovine serum albumin in PBS containing 0.05% Tween 20 for 30 min and then reacted with corresponding primary antibodies diluted in blocking solution. After washing three times with PBS containing 0.05% Tween 20, the binding of the primary antibodies was detected by species-specific fluorochrome-conjugated antibodies (Texas-red or FITC). These stained cells were mounted onto the slide glass with the use of mounting medium (Vectashield; Vector Laboratories) and then observed by fluorescence microscopy (MicrophoT-FXA; Nikon, Tokyo, Japan). For the actin staining, phalloidin conjugated with FITC was used.
Western Blot and Immunoprecipitation Analyses
For Western blot analysis of cytokeratin expression in keratinocytes, Triton X-100 insoluble fractions were used. Briefly, keratinocyte cultures in dishes were rinsed with cold PBS and incubated with 1% Triton X-100 in Tris-buffered saline containing protease inhibitor cocktail (Roche Molecular Biochemicals) by shaking in a cold room for 30 min. Cells collected by scraping were centrifuged at 20,000 × g for 15 min and pellets were used as Triton X-100 insoluble fractions. This contained almost all cytokeratins. For Western blot analysis of modified tubulin, total cell lysates extracted from cultured cells with the use of lysis buffer (6.25 mM Tris [pH 6.8], 2% SDS, 100 μM β-mercaptoethanol) were used. PAGE and transfer to nitrocellulose membranes were performed with the use of the manufacturer's protocols (Novex, San Diego, CA). Blocking, primary, secondary antibody, and chemiluminescence substrate reactions were performed as described previously (Kee et al., 1998).
For immunoprecipitation, Triton X-100 soluble fractions were used. These fractions were precleaned by incubation with 5 μl of normal mouse serum and protein A-agarose beads (Santa Cruz Biotechnologies) for 2 h at 4°C. Anti-E-cadherin antibody (1 μg) was mixed with this precleaned lysate for 1 h in ice and antigen–antibody complexes were precipitated with protein A-agarose beads. After extensive washing of precipitates with Tris-buffered saline containing 1% Triton X-100, bound proteins were eluted with SDS gel sample buffer (Novex) and subjected to Western blot analysis.
RESULTS
Disruption of Microtubules Induces Cell-Cell Adhesion in Normal Epidermal Keratinocytes
We are interested in exploring some of the molecular events that occur during the earliest stages of initiation of terminal differentiation in epidermal keratinocytes. It is now well known that within 1 d of initiation of this process, a wide range of new proteins and enzymes are expressed that are subsequently used for effective barrier formation (Nemes and Steinert, 1999; Steinert, 2000). However, one striking, much earlier event concerns cell adhesion. For example, keratinocytes such as normal mouse or human epidermal keratinocyte cells grown under subconfluent proliferating conditions in low calcium media do not adhere to each other. But within <1 h after transferance to high calcium media, they move together, start to adhere, and form desmosomes and other junctions (Hennings et al., 1980), a process that is complete within ∼12 hr.
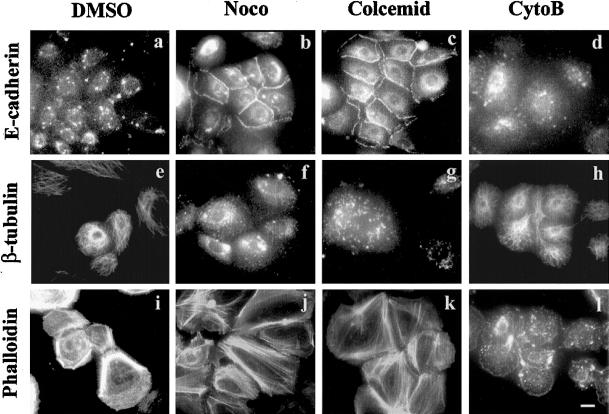
In this study, we have explored the role of microtubule organization on cell-cell adhesion in keratinocytes. As an initial step, we treated subconfluent NHEK cells grown in low calcium media with microtubule disruption agents (nocodazole and colcemid), and an F-actin disruption agent (cytochalasin B), and then assayed the role of E-cadherin in cell-cell adhesion by IF. Within 1 h after treatment with nocodazole or colcemid, cell-cell adhesion between virtually all cells could be observed by phase contrast microscopy (our unpublished results). E-cadherin accumulation at cell-cell junction completely correlated with this adhesion (Figure 1, b and c). However, the dimethyl sulfoxide (DMSO) (vehicle) control and cytochalasin B treatment did not induce cell-cell adhesion at all (Figure 1, a and d). Nocodazole and colcemid collapsed the microtubules almost completely (Figure 1, f and g), whereas these structures were maintained in DMSO and cytochalasin B treatment (Figure 1, e and h). Microtubule disruption in NHEK cells also induced strong actin polymerization within 1 h after treatment (Figure 1, j and k), in comparison to DMSO and cytochalasin B-treated NHEKs (Figure 1, i and l). Similar effects have been reported in fibroblasts (Bershadsky et al., 1996; Liu et al., 1998).
Figure 1.
Microtubule disruption induced cell-cell adhesion in NHEK cells. NHEK cells cultured in low calcium media (KGM) were treated with DMSO (vehicle control) (a, e, and i), nocodazole (b, f, and j), colcemid (c, g, and k), and cytochalasin B (d, h, and l) for 1 h. The treated cells were visualized by IF staining with anti-E-cadherin (a–d), anti-β-tubulin (e–h) antibodies and FITC-conjugated phalloidin for F-actin (i–l). Bar, 20 μm.
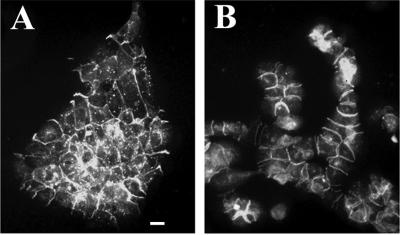
In contrast, when NHEK cells are transferred into high calcium medium (1.2 mM), up to 12 h is required for complete cell adhesion and formation of regularly shaped colonies (Figure 2A), as expected. As a control, NHEK cells grown in low calcium medium with nocodzole for 12 h maintained cell adhesion in colonies, whereas only a small proportion of cells failed to adhere (Figure 2B). It is well known that microtubules play direct or cooperative roles in cellular migration in many cell types (Gotlieb et al., 1983; Liao et al., 1995; Ballestrem et al., 2000). We therefore set out to explore the basis of this very rapid nocodazole-induced cell adhesion phenomenon.
Figure 2.
Comparison of cell morphology between high calcium and nocodazole-induced cell-cell adhesion of NHEK. (A) Cell adhesion induced 1.2 mM calcium in 12 h. (B) Cell adhesion induced by nocodazole in 12 h. Cells were visualized by IF staining with anti-E-cadherin antibody. Bar, 50 μm.
Members of Cadherin–Catenin Protein Complex Accumulate at Cell Junctions in Nocodazole-treated NHEK Cells
E-cadherin accumulation at cell-cell adhesion sites is known to be accompanied with other components of cadherin–catenin complexes. Therefore, we explored whether these complexes were formed in microtubule-disrupted NHEK cells grown in low calcium media. The accumulation of α- and β-catenin could be observed at cell-cell adhesion sites in 1 h after nocodazole treatment (Figure 3A, a, b, and c). Vinculin and tyrosyl phosphorylated proteins were also found at cell-cell adhesion sites in addition to focal adhesion plaques (Figure 3A, d, e, and f). It is known that vinculin plays roles in both cell-cell and cell-matrix adhesion (Gumbiner, 2000) and mediates association of actin with the cadherin–catenin complex (Hazan et al., 1997). Therefore, our results suggest that the nocodazole-induced cell-cell adhesion involving E-cadherin occurred while maintaining an intact cadherin–catenin complex.
Figure 3.
Various components of the cadherin–catenin complex localized at cell junctions in nocodazole-treated NHEK cells. (A) NHEK cells in KGM were treated with DMSO (a and d) and nocodazole (b, c, e, and f) for 1 h, and then examined by IF with: anti-β-catenin (a and b), anti-α-catenin (c), antivinculin (d and e), and anti-PY20 antibodies (f). All these antibodies detected proteins accumulated at cell-cell adhesion sites. (B) Expression levels of E-cadherin were not changed with various chemical treatments. E-cadherin in NHEK cells was immunoprecipitated and analyzed by Western blot assay with anti-E-cadherin mAb. NHEK treated with DMSO (lane 1), nocodazole (lane 2), colcemid (lane 3), and cytochalasin B (lane 4).
Notably, all of these events occurred without any changes in E-cadherin expression levels, as shown by Western blot analysis of treated cells (Figure 3B)
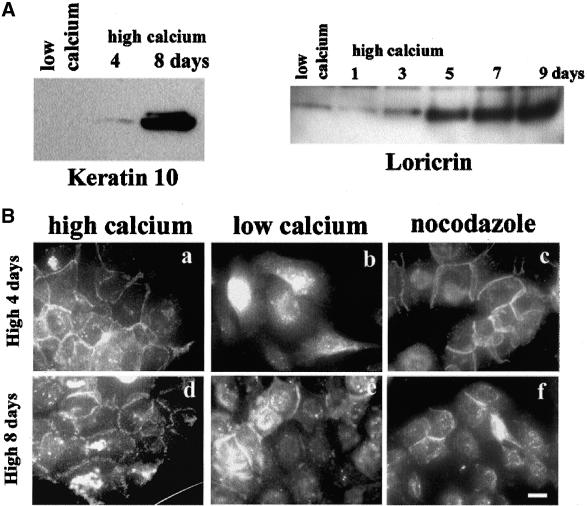
Calcium-induced Terminal Differentiation of NHEK Cells Decreased Nocodazole-induced Cell-Cell Adhesion
Stimulation of primary epidermal keratinocytes to enter a terminal differentiation program can be achieved in several ways, including transference to high calcium medium. Some cells stratify as they terminally differentiate and slough into the medium, whereas most cells retain proliferation potential for many days after induction (Hennings et al., 1980; Yuspa et al., 1989). Therefore, we investigated whether the differentiation status of keratinocytes could influence nocodazole-induced cell-cell adhesion. Western blot analysis showed that cytokeratin 10 (K10) and loricrin expression were up-regulated in NHEK 4 d after induction of differentiation with high calcium (1.2 mM), and achieved a maximal level of expression in ∼8 d (Figure 4A), as expected (Hohl et al., 1991). For the analysis of nocodazole effects on these cells, we first treated cells that had been in high calcium medium for 4 d with low calcium KGM. This enabled removal of terminally differentiated stratified cells, and the remaining proliferating cells that still possessed tight cell-cell adhesions (Figure 4B, a and d) could be almost completely dissociated in low calcium medium (Figure 4B, b). Interestingly, these cells could be induced to adhere by nocodazole treatment (Figure 4B, c). In contrast, more differentiated NHEK (8 d culture in high calcium) could not be dissociated completely (Figure 4B, e) and the proportion of adhesive cells (∼20–30%) did not increase after nocodazole treatment (Figure 4B, f). These results suggest that important molecular changes accompanying keratinocyte differentiation, possibly related to cell envelope barrier formation (Steinert and Marekov, 1999) have an inhibitory effect on nocodazole-induced cell-cell adhesion.
Figure 4.
Long-term culture of NHEK in high calcium media (1.2 mM) decreased potential for cell-cell adhesion with nocodazole. (A) Western blot analyses for K10 and loricrin expression were performed with the use of Triton X-100 insoluble fraction (K10) or whole cell lysates (loricrin). NHEKs were cells cultured in low calcium or high calcium media for indicated time. (B) NHEK cells grown in high calcium media for 4 d (top) or 8 d (bottom) were transferred into low calcium media to dissociate cells and then analyzed for nocodazole effects. NHEK cells grown in high calcium media (a and d), low calcium media with DMSO (b and e), and with nocodazole (c and f) were subjected to IF staining with anti-E-cadherin antibody. Bar, 20 μm.
Protein Kinase C-induced Differentiation Decreased Nocodazole-induced Cell-Cell Adhesion
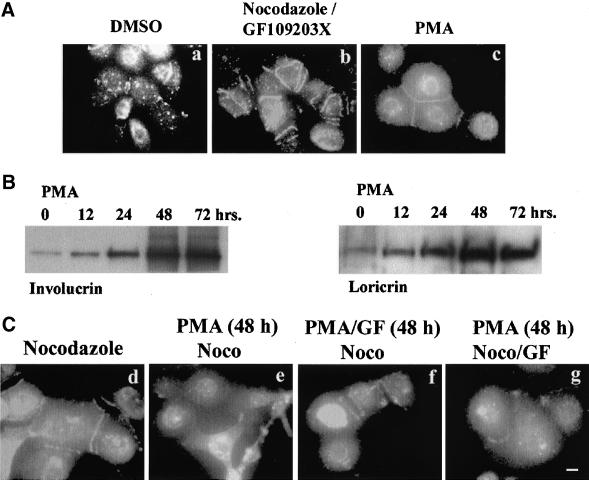
PKC activation is known to play a critical role in keratinocyte differentiation (Dotto, 1999; Mitev and Miteva, 1999). Thus, we asked whether nocodazole-induced cell-cell adhesion occurs in PKC-induced differentiation of keratinocytes. First, we checked the possibility whether intrinsic PKC activity was required for nocodazole-induced cell-cell adhesion. Cotreatment of NHEK in low calcium KGM with nocodazole and GF109203X, a specific PKC inhibitor, did not inhibit cell adhesion at all (Figure 5A, a and b), suggesting that nocodazole-induced cell-cell adhesion did not require PKC activity. But treatment of NHEKs with PMA, a PKC activator, did induce cell-cell adhesion (Figure 5A, c). However, the PMA effect was abolished in a few hours, whereas nocodazole-induced cell-cell adhesion maintained for more than 24 h (our unpublished results), suggesting a different mode of cell-cell adhesion.
Figure 5.
Effects of PKC agonist and antagonists on nocodazole-induced cell-cell adhesion in NHEK cells. (A) NHEK cells were treated with DMSO (a), GF109203X at the concentration of 1 μM (b), and 10 nM PMA (c) for 1 h. PMA itself induced cell-cell adhesion. (B) Western blot analyses for involucrin and loricrin expression after PMA treatment. C, NHEK cells were treated with nocodazole for 48 h (a), PMA for 48 h and then treated with nocodazole for 1 h (b), PMA and GF109203X for 48 h followed by nocodazole treatment for 1 h (c), and PMA for 48 h followed by treatment of GF109203X and nocodazole for 1 h (d). All cells were visualized by IF staining with anti-E-cadherin. GF10903X could not reverse the long-term inhibitory effect of PMA on nocodazole-induced cell-cell adhesion. Bar, 20 μm.
Next, we analyzed the long-term effect of PKC activation on nocodazole-induced cell-cell adhesion. Treatment with PMA induced increased expression of involucrin and loricrin in a time-dependent manner, and reached the maximum level at 48 h after treatment of NHEKs in low calcium (Figure 5B). These PMA-treated NHEK cells for 48 h did not show cell-cell adhesions by nocodazole treatment (Figure 5C, b). However, cell-cell adhesion was maintained when NHEK were cotreated with GF109203X together with PMA (Figure 5C, c), but GF109203X could no longer reverse the inhibition of cell-cell adhesion when GF109203X had been treated after PMA treatment for 48 h (Figure 5C, d). These data suggest that the inhibitory effect of PMA is irreversible. Together, these results suggest that PKC activity itself is not required for nocodazole-induced cell-cell adhesion. However, activation of PKC for 48 h in low calcium renders the NHEK cells unable to adhere to each other after microtubules were disrupted.
Nocodazole-induced Cell-Cell Adhesion Occurs in Keratinocyte Tumor Cell Lines but not in Cell Lines Derived from Normal Epidermis
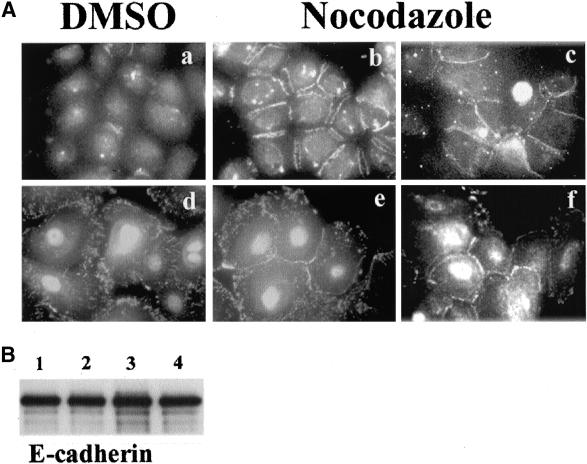
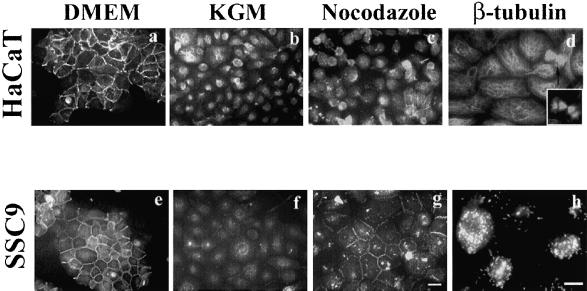
Epithelial carcinongenesis is accompanied by loss of differentiation (Gamallo et al., 1993; Takeichi, 1993). We asked whether there is difference in induciblity of cell-cell adhesion with nocodazole between cancer derived- and normal skin-derived cell lines. To check this, cell lines that were derived from normal epidermis (HaCaT and RHEK) or squamous cancers (A431, SCC-4, SCC-9, and SCC-25) were used. HaCaT and SCC-9 cells that were maintained in conventional media (DMEM containing 10% fetal bovine serum), expressed E-cadherin at cell-cell junctions (Figure 6, a and e), and were almost completely dissociated by cultivation in low calcium KGM for 24 h (Figure 6, b and f). HaCaT cells in KGM showed no significant reduction of viability and growth rates, and mitotic cells could be found frequently in samples stained with β-tubulin antibody (Figure 6d). Microtubules were properly disrupted with nocodazole in SCC-9 cells (Figure 6h). All cell lines showed similar results. Interestingly, however, HaCaT cells did not show any cell-cell adhesion after nocodazole treatment (Figure 6c). Another normal skin-derived cell line, RHEK, displayed the same result (our unpublished results). However, nocodazole-induced cell-cell adhesion did occur in SCC-9 cells (Figure 6g), as well as in the other cancer-derived cell lines A431, SCC-4, and SCC-25 (our unpublished results).
Figure 6.
Nocodazole effects on keratinocyte cell lines. HaCaT and SCC-9 cell lines cultured in DMEM containing 10% fetal bovine serum were transferred into KGM media to dissociate cells and then analyzed for the nocodazole effects. Cells were grown in DMEM (a and e), in KGM (b and c), and in KGM containing nocodazole (c and g) for 1 h and visualized by IF staining with anti-E-cadherin antibody. Nocodazole-induced cell-cell adhesions only in SCC-9 cells. β-Tubulin IF staining of HaCaT cells in KGM (d) and SCC9 cells in KGM containing nocodazole (h). Mitotic cells were frequently found in cells grown in KGM (d, arrow). Bar, 20 μm.
It is generally believed that cancer cells have acquired dedifferentiation phenotypes in comparison to normal cells of origin (Gumbiner, 2000). Our results indicate that nocodazole-induced cell-cell adhesion is correlated to the de- or undifferentiation status of keratinocyte cell lines because only cancer cell lines showed inducibility of cell-cell adhesion by nocodazole.
Continuous Culture of HaCaT Cells in KGM Showed Acquired Nocodazole-induced Cell-Cell Adhesion
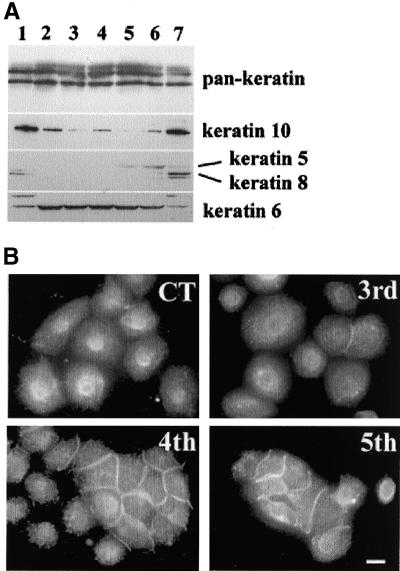
For verification of the above-mentioned hypothesis, we tried to induce dedifferentiation features in HaCaT cells through continuous cultivation in KGM, and analyzed the nocodazole effect. HaCaT cells were grown in low calcium KGM media, and when growth of cells reached 80–90% confluency, they were trypsinized and one-third were regrown. At each passage, we analyzed cytokeratin expression (Figure 7A) and nocodazole effects (Figure 7B). The expression patterns of K8 and K10 were decreased from the first passage but K10 expression was not completely abolished even after the 5th passage. K8 is known to be usually expressed in simple epithelia, and K10 is usually expressed in suprabasal epidermal cells. In the case of K5, which is known to be expressed in basal cells of stratified epithelia, expression began to appear after the 4th passage and expression of K6, which is known to be related with hyperproliferation, increased from the first passage and then slightly decreased. After the 5th passage, the proliferation rate was significantly reduced. Thus, because of the decreased level of K10, and increased expression of K5, HaCaT cells multiply-passaged in low calcium KGM this way mimicked the undifferentiated features of keratinocytes. Interestingly, starting from the 3rd passage, HaCaT cells become inducible for cell-cell adhesion by nocodazole treatment, and >50% cells showed nocodazole-induced cell-cell adhesion in the 5th passage (Figure 7B). These results suggest that dedifferentiated cells acquire the susceptibility for nocodazole-induced cell-cell adhesion.
Figure 7.
(A) Continuous culture of HaCaT in KGM. Triton X-100 insoluble fractions from HaCaT cells after various passages were subjected to Western blot analysis with the use of various cytokeratin antibodies. Lanes 1 and 7, HaCaT cells cultured in DMEM; lane 2, HaCaT at 1st passage in KGM; lane 3, 2nd passage; lane 4, 3rd passage; lane 5, 4th passage; lane 6, 5th passage. (B) Nocodazole-induced cell-cell adhesion of HaCaT cells after various passages. Control was nocodazole-treated HaCaT cells in KGM for 1 d after transferance from DMEM to KGM, 3rd, 4th, and 5th means passage number of HaCaT cells in KGM. Cells were visualized by IF staining with anti-E-cadherin antibody. Bar, 30 μm.
Nocodazole-induced Cell-Cell Adhesion Does Not Correlate with Posttranslational Modification of Microtubules
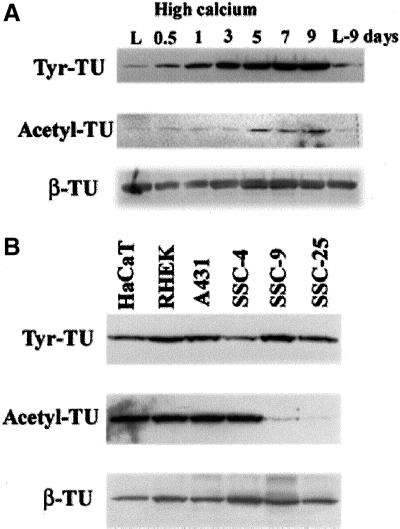
Microtubule structure is profoundly influenced by cell-cell contacts. For example, cell-cell contacts make microtubules stable and less dynamic, which is accompanied by posttranslational modification of tubulin (Kreis, 1987; Nagasaki et al., 1992). On the basis of the hypothesis that posttranslational modifications could affect the interaction of microtubules with different cellular factors that have the potential to alter inducibility of cell-cell adhesion, we asked whether nocodazole-induced cell-cell adhesion was affected by tubulin modification. First, the extent of tubulin modification in relation to differentiation was analyzed with the use of NHEK cells cultured in high calcium KGM. Western blot analyses showed that expression of tyrosinated and acetylated forms of tubulin increased from 12 h and 5 d, respectively, after increasing the calcium concentration in the media although β-tubulin expression was not changed (Figure 8A). In NHEK cells, the acetylation pattern of tubulin was similar to that of K10 expression (Figure 4A), suggesting a correlation with terminal differentiation. However, in the case of keratinocyte cell lines, the extent of tubulin modification was not correlated with inducibility of nocodazole-induced cell-cell adhesion. Western blot analyses with the use of whole cell extracts showed that the extent of tyrosination of tubulin was similar among the six cell lines, including the keratinocyte cell lines (HaCaT and RHEK) (Figure 8B). The extent of acetylation was also similar except for two cancer-derived cell lines (SCC-9 and -25), which showed much less extent of acetylation than other cell lines (Figure 8B). Because all four cancer cell lines have potential for cell-cell adhesion by nocodazole treatment, these results indicate that tubulin modification is unlikely to be directly involved in inducibility of cell-cell adhesion by nocodazole.
Figure 8.
Western blot analyses of modified tubulin with the use of whole cell extracts from keratinocytes. (A) Whole cell extracts from NHEK cells of various differentiation statuses were subjected to Western blot analysis with the use of anti-tyrosinated tubulin, anti-acetylated tubulin, and anti-β-tubulin mAbs. Keratinocyte differentiation was achieved by high calcium (1.2 mM) cultivation according to indicated times after increasing calcium concentration in media. L and L9 denote keratinocytes grown in low calcium media before increasing calcium concentration, and continuous growth in low calcium media for further 9 d, respectively. (B) Expression patterns of modified tubulin in two keratinocytes cell lines from normal skin (HaCaT and RHEK) and four cancer cell lines (A431, SCC-4, -9, and -25). Cell-cell adhesion by microtubule disruption (could not be induced in HaCaT and RHEK cells [Figure 6]). There is no apparent correlation between the extent of tubulin modification and inducibility of cell-cell adhesion by microtubules in the cancer cell lines.
DISCUSSION
Microtubules affect cell shape, cell motility, and cell division (Nogales, 2000). Also microtubules respond to signaling pathways by changing their dynamics and organization in ways that may contribute to the signal transduction pathway. It is known that cell-cell contacts decrease the level of stabilized microtubules and alter the distribution of detyrosinated microtubules (Nagasaki et al., 1992). However, the precise role of microtubules in cell-cell adhesion is not yet clear. As an initial step to investigate the possible role of microtubules in cell adhesion, we studied the relationship between microtubule organization and cell-cell adhesion in keratinocytes. This report shows that in NHEK cells, microtubule disruption can generate the signals for cell-cell adhesion through activation of E-cadherin. Moreover, this was suppressed in terminally differentiating keratinocytes.
Cytoskeleton and Nocodazole-induced Cell-Cell Adhesion
This phenomenon in epidermal keratinocytes could be due to changes in other cytoskeletal components such as actin filaments. Because the various major cytoskeletal elements involved in cellular organization can cross-talk with each other, disruption of microtubules could influence other cytoskeletal components. Actin microfilaments, which are known to play an important role in cell-cell adhesion, coordinate with microtubules in numerous cellular events such as cellular morphogenesis, migration, and focal adhesion contacts (Waterman-Storer and Salmon, 1999). Indeed, our results showed that microtubule disruption in NHEK induced strong actin polymerization in addition to actin accumulation at cell-cell contact sites (Figures 1, j and k, and 3B). Other studies have demonstrated that circumferential cortical actin filaments are a prerequisite for the targeting of the cadherin–catenin complex to the cell surface in kidney epithelial cells and this was not interfered by microtubule or stress fiber disruption (Quinlan and Hyatt, 1999). Generally, it is believed that stress fiber formation is more related to cell-matrix adhesion than cell-cell adhesion (Hall, 1998). Thus, the relationship of stress fiber formation and cell-cell adhesion in nocodazole-treated NHEK is not yet clear.
Another possibility is that microtubules trap E-cadherin in dissociated cells and the release of E-cadherin from microtubules by nocodazole may result in cell-cell adhesion. Recently, it was reported that muscle type cadherin (M-cadherin) and catenin complex interacts with microtubules in skeletal muscle (Kaufmann et al., 1999). This interaction was suggested as an underlying mechanism for the role of M-cadherin in the fusion of mononucleated myoblasts into multinucleated myotubes (Zeschnigk et al., 1995). However, this interaction was muscle-specific because neither M-cadherin nor E-cadherin could be found in a complex with microtubules in epithelial cells ectopically expressing M-cadherin (Kaufmann et al., 1999). In fact, we could not find any evidence for association between E-cadherin and microtubules in keratinocytes (our unpublished results). Thus, the nocodazole-induced cell-cell adhesion phenomenon is not based on simple association or dissociation processes between E-cadherin and microtubules.
A third possibility is that affinity differences between microtubules and associated proteins expressed in keratinocytes could account for the observed phenomenon. It is known that microtubule structure is profoundly influenced by cell-cell contact, morphogenesis, and differentiation through posttranslational modification of tubulin (Bulinski and Gunderson, 1991). There are two kinds of microtubules: dynamic and stable. Stabilized microtubules exhibit a half life on the order of hours rather than the minutes typical of usual dynamic microtubules (Schulze et al., 1987; Webster et al., 1987), and they show increased resistance to microtubule antagonists. Stabilization of microtubules has been known to correlate with accumulation of posttranslationally modified tubulin (Bulinski and Gunderson, 1991). We demonstrate here that NHEK cells showed increased proportions of tyrosinated and acetylated tubulin during differentiation and in a time-dependent manner (Figure 8), suggesting increased microtubule stability. Therefore, it seems that the structural regulation of microtubules and association with microtubule associated proteins could be a plausible cause for nocodazole-induced cell-cell adhesion. In contrast, the proportion of modified tubulin in total tubulin was variable among six keratinocyte cell-lines used in this report. Correlation between the extent of tubulin modification (tyrosinated and acetylated forms) and nocodazole-induced cell-cell adhesion could not be found (Figure 8).
Finally, it is possible that microtubule disruption effects unknown signal pathways for cell-cell adhesion, and that these signals are lost in advanced differentiating keratinocytes. Recent studies have revealed the critical roles of microtubules in the spatial organization of signal transduction (Gunderson and Cook, 1999). Among the signal transducing molecules that are related with microtubules are RhoA family GTPases. It has been reported that microtubule disruption induced stress fiber formation and focal adhesion through RhoA activation in fibroblasts (Bershadsky et al., 1996; Liu et al., 1998). In addition, RhoA is thought to play a role in cell-cell adhesion of epithelial cells (Braga et al., 1997). Stress fiber formation in NHEK cells after nocodazole treatment suggests the possibility that microtubule disruption activates RhoA in keratinocytes. We therefore set out to perform a series of experiments to test this concept. However, we were unable to find any evidence for RhoA involvement in this process. For example, transient expression of the RhoA dominant negative mutant form (N19-RhoA) in NHEK cells did not inhibit nocodazole induced cell-cell adhesion, although stress fiber formation was significantly reduced (our unpublished results). Therefore, at present, our data suggest that RhoA is not directly involved in this process. Nevertheless, we cannot exclude the possibility of a more complex indirect role of RhoA activation in microtubule disrupted NHEK cells, for which additional detailed experiments seem to be warranted.
Loss of Nocodazole-induced Cell-Cell Adhesion in Advanced Differentiating Cells
Our results showed that nocodazole-induced cell-cell adhesion was profoundly influenced by the degree of keratinocyte differentiation. PKC-induced differentiation of NHEK interferred with nocodazole-induced cell-cell adhesion. This role of PKC was different from the previously reported role of PKC in cell-cell adhesion. It has been reported that activation of PKC with the use of PMA could induce cell-cell adhesion in epithelial cells that were defective in cell-cell adhesion even under a high calcium environment (Williams et al., 1993; Shafer et al., 1999). This PMA-induced cell-cell adhesion could be observed in NHEKs grown in a low calcium environment. In comparison with nocodazole-induced cell-cell adhesion, PMA-induced cell-cell adhesion was transitory and PMA could induce cell-cell adhesion in HaCaT cells (our unpublished results), whereas nocodazole could not. In our hands, long-term treatment of PMA in NHEKs showed irreversible inhibitory effects on nocodazole-induced cell-cell adhesion (Figure 5). These results suggest that PKC induced some unknown molecular changes, which in turn inhibited nocodazole-induced cell-cell adhesion. This unknown molecular event must be tightly regulated by the differentiation status of keratinocytes.
In addition, we showed that nocodazole-induced cell-cell adhesion diminished as NHEK cells became more differentiated. Notably, this correlated with increasing expression of advanced terminal differentiation cell envelope products such as loricrin (Figures 4 and 5). Previously, we have demonstrated that after several days in high calcium medium, NHEK cells begin to acquire a cell envelope barrier structure made insoluble by extensive irreversible transglutaminse cross-linking of cell peripheral proteins. In fact, insoluble cell envelopes initially appear as double membrane structures with recognizable desmosomal entities (Steinert and Marekov, 1999). This indicates that a large array of cell peripheral proteins must have become cross-linked, and indeed, proteins such as plakoglobins, desmocolin, desmoglein 3, E-cadherin, and others have been identified as cross-linked products in these structures (Robinson et al., 1997; Steinert and Marekov, unpublished results). Thus, we propose that as differentiation proceeds, the cell periphery changes markedly during cell envelope barrier formation, with the consequent loss of the dynamic interactions between the cytoskeleton and cell periphery. Similar observations are apparent for the HaCaT and RHEK keratinocyte cell lines. However, other epithelial cancer cell lines or cells of simple epithelia do not typically make barrier structures. Thus, the loss of nocodazole-induced cell-cell adhesion in advanced differentiated cells is likely due to the irreversible modification of the cell periphery to make the cell envelope barrier.
NHEK Cells Behave Differently from Newt Lung Epithelial Cells
Recently, breakage of cell-cell adhesion in nocodazole-treated newt lung epithelial cells was reported (Waterman-Storer et al., 2000). It was demonstrated that cell-cell contacts made the microtubule plus end less dynamic, and disruption of the microtubules broke cell-cell contacts. These results suggest that microtubule growth is required for maintenance of cell-cell contacts. These observations are the opposite of our findings described here. The most likely reasons for the differences are origin of species (newt vs. human), organs (lung vs. skin), and culture systems, including temperature for optimal cell growth (20 vs. 37°C). For example, cell-cell adhesion in mammary epithelial cells was not perturbed by microtubule disruption (Quinlan and Hyatt, 1999). In addition, the behavior of F-actin is different between newt and mammary epithelial cells. Treatment of newt lung epithelial cells with nocodazole resulted in an increase of stress fibers within 15 min but stress fibers began to disassemble afterward, and within 1 h, almost all were lost (Waterman-Storer et al., 2000). In contrast, in NHEK, nocodazole-induced stress fibers maintained their structure for several hours. Other reports have shown that stress fibers induced with various stimulations, including microtubule disruption, were maintained for at least 1 h (Bershadsky et al., 1996; Strassheim et al., 1999). Furthermore, culture temperature could be a possible reason for the different observations. Recent studies showed that cell-cell adhesion in mammalian epithelial cells is dynamic and cadherins undergo regulated trafficking to and from surface. This cadherin trafficking was suppressed when culture temperature was shifted from 37 to 18°C, and an increased proportion of cadherin accumulated in the cytoplasm as an internalized pool at 18°C. For example, in Madin-Darby canine kidney cells, up to 80% of surface biotinylated E-cadherin was internalized after 2-h incubation at 18°C (Le et al., 1999). Considering this observation, optimal temperature (<20°C) for growth of newt epithelial cells could perturb the E-cadherin trafficking. Altogether, it seems that newt cells might have unique features in cytoskeletal regulation for cell-cell adhesion compared with usual mammalian cells.
In conclusion, our results document that the differentiation status of keratinocytes influences cell-cell adhesion that can be induced by disruption of microtubules. Furthermore, we suggest that this model can serve as a new tool for studying the mechanisms of keratinocyte cell adhesion.
Abbreviations used:
- DSP
dithiobis(succinimidyl propionate
- IF
indirect immunofluorescence
- mAb
monoclonal antibody
- mACh
NHEK, normal human epidermal keratinocyte
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
REFERENCES
- Aono S, Nakagawa S, Reynolds AB, Takeichi M. P120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol. 1999;145:551–562. doi: 10.1083/jcb.145.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestrem C, Wehrle-Haller B, Hinz B, Imhof B. Actin-dependent lamellipodia formation and microtubule-dependent tail retraction control-directed cell migration. Mol Biol Cell. 2000;11:2999–3012. doi: 10.1091/mbc.11.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Roy FV, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Boukemp PB, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga V, Machesky LM, Hall A, Hotchin NA. The small GTPases rho and rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski JC, Gunderson GG. Stabilization and post-translational modification of microtubules during cellular morphogenesis. Bioassays. 1991;13:285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- Dotto GP. Signal transduction pathways controlling the switch between keratinocytes growth and differentiation. Crit Rev Oral Biol Med. 1999;10:442–457. doi: 10.1177/10454411990100040201. [DOI] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kuroda S, Kaibushi K. Cell adhesion and Rho small GTPases. J Cell Sci. 1999;112:4491–4500. doi: 10.1242/jcs.112.24.4491. [DOI] [PubMed] [Google Scholar]
- Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quitanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast-carcinoma. Am J Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- Gotlieb AI, Subrahmanyan L, Kalnins VI. Microtubule-organizing centers and cell-migration-effect of inhibition of migration and microtubule disruption in endothelial-cells. J Cell Biol. 1983;96:1266–1272. doi: 10.1083/jcb.96.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, M. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–403. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson GG, Cook TA. Microtubule and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPase and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with E-cadherin adhesion complex. J Biol Chem. 1997;272:32448–32453. doi: 10.1074/jbc.272.51.32448. [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert PM, Holbrook KA, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hohl H, Lichti U, Breitkreutz D, Steinert PM, Roop DR. The transcription of loricrin in vitro is induced by calcium and density and suppressed by retinoic acid. J Invest Dermatol. 1991;96:414–418. doi: 10.1111/1523-1747.ep12469779. [DOI] [PubMed] [Google Scholar]
- Kaufmann U, Kirsch J, Irintchev A, Wernig A, Starzinski-Powitz A. The M-cadherin catenin complex interacts with microtubules in skeletal muscle cell: implications for the fusion of myoblasts. J Cell Sci. 1999;112:55–67. doi: 10.1242/jcs.112.1.55. [DOI] [PubMed] [Google Scholar]
- Kee SH, Choi YO, Song YS, Lee HP, Chang WH. Identification of antigenic difference between the phosphorylated and nonphosphorylated forms of the E7 protein of human papillomavirus type16. J Med Virol. 1998;54:129–134. doi: 10.1002/(sici)1096-9071(199802)54:2<129::aid-jmv10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kreis T. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987;6:2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Liao GJ, Nagasaki T, Gunderson GG. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level - implications for the role of dynamic microtubules in cell locomotion. J Cell Sci. 1995;108:3473–3483. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- Liu BP, Chrzanowska-Wodnicka M, Burridgew K. Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via GTP-binding protein Rho. Cell Adhes Commun. 1998;5:249–255. doi: 10.3109/15419069809040295. [DOI] [PubMed] [Google Scholar]
- Mitev V, Miteva L. Signal transduction in keratinocytes. Exp Dermatol. 1999;8:96–108. doi: 10.1111/j.1600-0625.1999.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Chapin CJ, Gunderson GG. Distribution of detyrosinated microtubules in motile NRK fibroblasts is rapidly altered upon cell-cell contact: implications for contact inhibition of locomotion. Cell Motil Cytoskel. 1992;23:45–60. doi: 10.1002/cm.970230106. [DOI] [PubMed] [Google Scholar]
- Navarro P, Gomez M, Pizarro A, Gamallo C, Quitanilla M, Cano A. A role for the E-cadherin cell-cell adhesion molecule during tumor progression of mouse epidermal carcinogensis. J Cell Biol. 1991;115:517–533. doi: 10.1083/jcb.115.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- Quinlan MP, Hyatt JL. Establishment of the circumferential actin filament network is a prerequisite for localization of the cadherin-catenin complex in epithelial cells. Cell. 1999;10:839–854. [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Rhim JS, Jay G, Arnstein P, Price PM, Sanford KK, Aaronson SA. Neoplastic transformation of human epidermal-keratinocytes by AD12-SV40 and kirsten saecoma-viruses. Science. 1985;227:1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- Robinson NA, Lapic S, Welter JF, Eckert RL. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of cornified envelope of cultured human epidermal keratinocytes. J Biol Chem. 1997;272:12035–12046. doi: 10.1074/jbc.272.18.12035. [DOI] [PubMed] [Google Scholar]
- Shafer SH, Puhl HL, Phelps SH, Williams CL. Activation of transfected M1 or M3 Muscarinic acetylcholine receptors induces cell-cell adhesion of Chinese hamster ovary cells expressing endogenous cadherins. Exp Cell Res. 1999;248:148–159. doi: 10.1006/excr.1998.4385. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Steinert PM. The complexity and redundancy of epithelial barrier function. J Cell Biol. 2000;151:F5–F7. doi: 10.1083/jcb.151.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM, Marekov LN. Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol Biol Cell. 1999;10:4247–4261. doi: 10.1091/mbc.10.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassheim D, May LG, Varker KA, Puhl HL, Phelps SH, Porter RA, Aronstam RS, Noti JD, Williams CL. M3 Muscarinic Acetylcholine Receptors regulate cytoplasmic myosin by a process involving RhoA and requiring conventional protein kinase C isoforms. J Biol Chem. 1999;274:18675–18685. doi: 10.1074/jbc.274.26.18675. [DOI] [PubMed] [Google Scholar]
- Schulze E, Asai DJ, Bulinski JC, Kirschner M. Post-translational modifications and microtubule stability. J Cell Biol. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Vleminckx K, Kemler R. Cadherins and tissue formation integration adhesion and signaling. Bioassays. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon E. Positive feedback interaction between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol. 1999;11:61–67. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon WC, Salmon ED. Feedback interaction between cell-cell adherens junction and cytoskeletal dynamics in newt lung epithelial cells. Mol Biol Cell. 2000;11:2471–2483. doi: 10.1091/mbc.11.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Gundersom GG, Bulinski JC. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA. 1987;84:9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinder K, M, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Hayes V, Hummel AM, Tarara JE, Halsey TJ. Rregulation of E-cadherin-mediated adhesion by muscarinic acetylcholine receptors in small cell lung carcinoma. J Cell Biol. 1993;121:643–654. doi: 10.1083/jcb.121.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeschnigk M, Kozian D, Kuch C, Schmoll M, Starzinski-Powitz A. Involvement of M-cadherin in fusion and terminal differentiation of myogenic cells. J Cell Sci. 1995;108:2973–2981. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]