Abstract
Histamine iontophoresis with laser Doppler monitoring (HILD) is a robust and dynamic surrogate for histamine microvasculature response. We characterized histamine pharmacodynamics in children using HILD. HILD was performed in 54 children with allergic rhinitis. A non-compartmental analysis and non-linear mixed-effects model with a linked effect PK/PD model was used to provide estimates for area under the effect curve (AUEC), maximal response over baseline (EffmaxNT), and time of EffmaxNT (Tmax). Data were placed in sub-groups by visualization of time vs. response relationships. ANOVA and regression analyses were used for sub-group comparisons. Three histamine response phenotypes were identified. One group demonstrated a hyper-responsive phenotype (higher Tmax, EffmaxNt and AUEC, P < .01). AUEC and EffmaxNT were more strongly associated in this group (r2 = 0.86) than the entire cohort (r2 = 0.64). These data demonstrate a hyper-responsive histamine phenotype via HILD. This finding is important to future pharmacologic studies of antihistamines.
Keywords: allergic rhinitis, histamine, biomarkers
Histamine is a central mediator in the allergic and inflammatory cascade and is responsible for initiating and perpetuating the allergic response. Histamine also induces the wheal and flare response via activation of the H1-receptor which results in release of nitric oxide by the vascular endothelium and ultimately, increased vasodilatation and vascular permeability. The epicutaneous (i.e., skin prick) histamine (EH) test is the current “gold standard” method of assessing the microvasculature response to histamine for clinical allergy testing. It has also been used to assess the pharmacodynamic response to antihistamines in children and adults. Despite its wide use, the EH test has several limitations that limit its utility which include an inability of providing a continuous measurement, inter-operator variability, lack of finite dosing of histamine, and inherent variability relative to devices/procedures for measurement of response.
We have previously demonstrated that in adults, histamine iontophoresis with laser Doppler monitoring (HILD) is capable of characterizing the microvasculature response to histamine in a continuous, robust fashion thereby rendering it a surrogate endpoint for evaluating histamine response which is superior to EH.1 In this previous investigation, we observed a variable response to histamine within the study cohort; a finding interpreted to be consistent with the inter-individual variability in antihistamine response in patients with allergic disease and asthma.2,3 This observation led us to our current study to further evaluate varying phenotypes of histamine response using HILD in a cohort of children with an established diagnosis of allergic rhinitis. The results from this study are reported herein.
Methods
Subjects
After approval by the Children’s Mercy Hospital Pediatric Institutional Review Board, children with allergic rhinitis age 7–18 years were recruited from the Allergy/Asthma/Immunology clinics at the institution by informed parental permission and as appropriate (i.e., age ≥ 7 years), by patient assent. Allergic rhinitis was defined by a pediatric allergy specialist using currently accepted clinical criteria. Patients who had evidence of any of the following exclusion criteria were not enrolled into the study: (1) previous history or laboratory evidence of McCune Albright syndrome, immunodeficiency, or mastocytosis; (2) receipt of immunomodulatory treatment; (3) chronic conditions associated with abnormalities of the integument (including active atopic dermatitis of the volar surface of the forearm); (4) hepatic or renal compromise; (5) neoplastic disease; (6) movement or neurologic disorder; (7) uncontrolled attention deficit hyperactivity disorder; (8) recent history of anaphylactic or anaphylactoid reaction; (9) evidence of pregnancy (by urinary hCG) or lactation; (10) receipt of drugs (within a specified time period) of agents capable of altering the response to histamine provocation (e.g., antihistamines, systemic corticosteroids, tricyclic antidepressants; (11) perceived (by the principal investigator) inability to adhere to and/or tolerate required study procedures. At enrollment study participants were asked to temporarily discontinue the use of antihistamines, systemic corticosteroids and/or antidepressants for 10, 30, and 30 days, respectively, prior to HILD. Clinical information in addition to that required to confirm the diagnosis of allergic rhinitis was obtained by history which included: diagnosis of asthma, asthma severity (defined by current step therapy according to the 2007 Guidelines for the Diagnosis and Management of Asthma)4 and history of hospitalization for asthma.
HILD
HILD was performed in the 54 study participants in an identical fashion. Specifically, a solid-state, single-frequency laser probe was inserted into the center of an iontophoresis chamber attached to a laser Doppler blood flow monitor (DRT4, Moor Instruments Ltd., Wilmington, DE) and placed on a visibly nonvascular area of the skin on the volar surface of the forearm midway between the wrist and crease of the elbow. A second laser Doppler probe to serve as a control was placed at a distance of at least 1 cm from the iontophoresis site. Two hundred microliters of histamine dihydrochloride solution (1%; Sigma Chemical Ltd., Dorset, UK), dissolved in a 2% methylcellulose gel (Sigma Chemical Co, St Louis, MI), was then placed in the reservoir of the iontophoresis chamber. Probe temperatures were maintained at 32°C during the duration of the study and the ambient temperature was maintained within a range of 25–28°C. A platinum electrode in the iontophoresis chamber was connected to the positive terminal of a constant current source, and a reference electrode was fixed approximately 7 cm proximal to the active laser Doppler flow probe, which served as a cathode. For iontophoresis, constant anodal current (50 μA) was applied for 10 seconds. Values for small vessel blood flow at each of the probe sites were simultaneously calculated using a proprietary software package provided by the manufacturer (Moor Instruments Ltd., Devon, UK) and were expressed as in perfusion units (flux). Flux is defined by the photocurrent produced by the scattering of light by moving red blood cells. Baseline small vessel blood flow was assessed for 2 minutes before histamine iontophoresis. After histamine iontophoresis, blood flow was continuously assessed until blood flow measurements returned to baseline or to a maximum duration of 2 hours.
Data Analysis
Data were sampled utilizing an averaging algorithm and data delays were removed to normalize initial parameters. Real-time traces of microvascular blood flow velocity were collected at a sampling rate of 40 Hz. A sampling frequency of 30 values per minute was chosen as this was the least frequent rate that provided a good fit of the blood flow versus time curve based on our previous experience with the technique.1 Initially a non-compartmental analysis was undertaken using Phoenix® WinNonlin version 6.2 (Pharsight, Mountain View, CA), providing parameter estimates for area under the effect curve (AUEC) a function of flux and time, time to “peak” effect (Tmax) and apparent maximum “peak” effect (Emax). Data were further analyzed using a simple pharmacodynamic (PD) Emax model (Phoenix v6.2), which provided estimates for the relative maximal response over baseline (EffmaxNt) and time of EffmaxNt (Tmax) in minutes. A non-linear mixed-effects model utilized a linked effect PK/PD model (NONMEM, version 7.2, ICON Dev. Soln., Ellicott City, MD). Visual inspection of response vs. time relationships demonstrated the potential for apparent subgroups within the cohort.
Evaluation of the simple PD Emax model reviewed effect data and were initially evaluated by visualization of time versus response (dependent variable, DV) relationships. For the PD model, standard error, CV%, and confidence intervals were assessed. For the EffmaxNt results, only one subject had a 95% CI that contained a zero.
For the linked effect models, the HILD data were assessed for %RSE (percent relative standard error, 100% × SE/EST) and 95% CI and objective function value (OFV). Inter-individual variability and residual variability was assessed, as well as diagnostic plots such as DV versus predicted (PRED).
Differences in model parameters between groups were determined using ANOVA and Tukey’s HSD post hoc analysis. Linear regression was used to explore associations between parameters to validate apparent sub-group differences in PD. Demographic and clinical variables were compared between group classifications via ANOVA and Tukey’s HSD post hoc analysis, and Fisher’s Exact test. The level of significance set for all statistical analyses was α = 0.05.
Results
Fifty-four children were enrolled in the study and underwent HILD. Complete and evaluable data were available for 44 children. Data from seven participants were not evaluable due to technical malfunction with the iontophoresis/laser Doppler system. An additional two participants were lost to follow-up after enrollment and did not complete the study. One subject’s data were not pharmacokinetic in nature (i.e., ascending and descending response pattern over time) and thus, could not be reliably modeled to determine PD parameters.
Observation of histamine flux versus time curves revealed visually demonstrable differences in histamine response patterns with apparent predominant patterns present (Supplementary Figure 1). When observed histamine flux data were plotted against predicted parameters, three distinct groups of response was identified (Figure 1 Panel A; corresponding flux vs. time curves are shown in Panel B). Forty-three of 44 of the subjects were able to be visually assigned into these three groups and were included in final data analysis. Demographic information for the 43 participants is summarized in Table 1.
Figure 1.
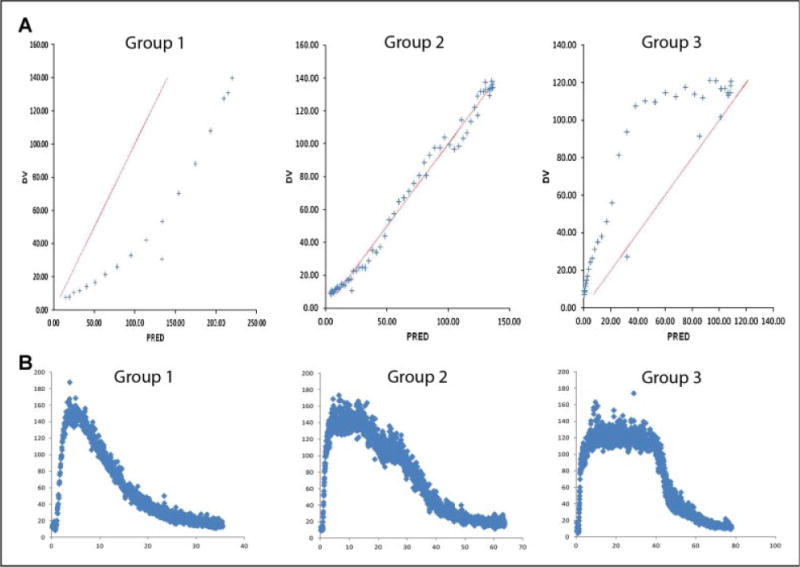
Predicted versus actual flux. Panel A: Representative predicted (PD) on the x-axis versus actual (DV) on the y-axis flux (perfusion units provided by the manufacturer) plots which suggest distinct histamine response types. Group 1, hypo-responsive group; Group 2, neutro-responsive group; Group 3, hyper-responsive group. Panel B: Representative actual flux vs. time curves which correspond to above histamine response types. Group 1, hypo-responsive group; Group 2, neutro-responsive group; Group 3, hyper-responsive group.
Table 1.
Demographic characteristics of all participants classified into histamine groups.
| Characteristics | N (%) or Mean ± SD |
|---|---|
| Age (years) | 12.5 (2.9) |
| Male | 28 (65) |
| Female | 15(35) |
| White (non-hispanic) | 24 (56) |
| Black (non-hispanic) | 14 (32) |
| Hispanic | 5 (12) |
| Weight (kg) | 59 ± 20 |
| Height (cm) | 156 ± 17 |
| BMI | 24 ± 6 |
| History of asthma | 32 (74) |
| History of hospitalization for asthma | 17 (53) |
BMI, body mass index.
Demographic characteristics expressed in number (N) and percentages (%) or mean ± standard deviation (SD).
A variable response to histamine was observed whereby: 27 (63%) subjects exhibited observed PD parameters that were less than predicted and were therefore classified as “hypo-responsive” (Group 1); 9 (21%) subjects exhibited close agreement between observed and predicted values and were classified as “normo-responsive” to histamine (Group 2); and 7 subjects (16%) exhibited parameters that were higher than predicted and were classified as “hyper-responsive” (Group 3). Significant differences in demographic or clinical characteristics were not observed between the response groups (Table 2). Potential trends were observed whereby African Americans and Hispanics were not represented in Group 2 and Group 3, respectively (P = .05).
Table 2.
Demographic Characteristics by Histamine Response Classification.
| Group 1 (N = 27) | Group 2 (N = 9) | Group 3 (N = 7) | |
|---|---|---|---|
| Demographic characteristics by group | |||
| Age (years, mean ± SD) | 13 ± 3 | 13 ± 3 | 11 ± 4 |
| Male N (%) | 18 (67) | 6 (67) | 4 (57) |
| White non-hispanic N (%) | 14 (52) | 6 (67) | 4 (57) |
| African American N (%) | 11 (41) | 0(0) | 3 (43) |
| Hispanic N (%) | 2 (7) | 3 (33) | 0 (0) |
| Weight (mean ± SD) | 57 ± 22 | 71 ± 8 | 51 ± 19 |
| Height (mean ± SD) | 156 ± 3 | 159 ± 8 | 152 ± 7 |
| BMI (mean ± SD) | 23 ± 7 | 27 ± 2 | 21 ± 3 |
| History of Asthma N (%) | 22 (81) | 6 (67) | 4 (57) |
| History of hospitalization for asthma N (%) | 13(59) | 2 (33) | 2 (50) |
BMI, body mass index.
Demographic characteristics expressed in mean ± standard deviation (SD) or numbers (N) and percentages (%) by group classification (Group 1 hyporesponsive; Group 2 neutro-responsive group; and Group 3 hyper-responsive).
No difference was observed for age, gender, race, weight, height, BMI, history of asthma, or asthma hospitalization between groups, P > .05.
Mean values (±SD) for AUEC, EffmaxNt, and Tmax for the entire cohort were 5,225 ± 3,114 flux units × minutes, 140 ± 54 flux units, and 11 ± 7 minutes, respectively. Differences were observed for mean values of AUEC, EffmaxNt, and Tmax for each of the phenotype groups (Table 3). The greatest differences were observed for the hyper-responsive group (Group 3) relative to the other groups, which demonstrated consistently higher values for all three parameters measured relative to the other two groups (P < .05 for all comparisons; Figure 2). The hypo-responsive group (Group 1) demonstrated a lower EffmaxNt relative to the other two groups however, differences were only statistically significant for comparison with the hyper-responsive group (Group 3). There was no difference between Group 2 (normo-responsive) and Group 1 (hypo-responsive) for Tmax or AUEC.
Table 3.
Histamine Pharmacodynamic Parameters by Group.
| Group 1 | Group 2 | Group3* | |
|---|---|---|---|
| AUEC | 4,654 ± 2,997 | 4,233 ± 1,819 | 8,702 ± 2,776* |
| EffmaxNt | 127 ± 52 | 136 ± 33 | 198 ± 53* |
| Tmax | 9±6 | 10 ± 6 | 18± 7* |
Comparisons of area under the effect curve (AUEC) in flux units × minutes, maximal effect over baseline (EffmaxNt) in flux, and time to maximal effect over baseline (Tmax) in minutes between Groups 1, 2, and 3.
Mean ± standard deviation values for each response group,
P < .01.
Figure 2.
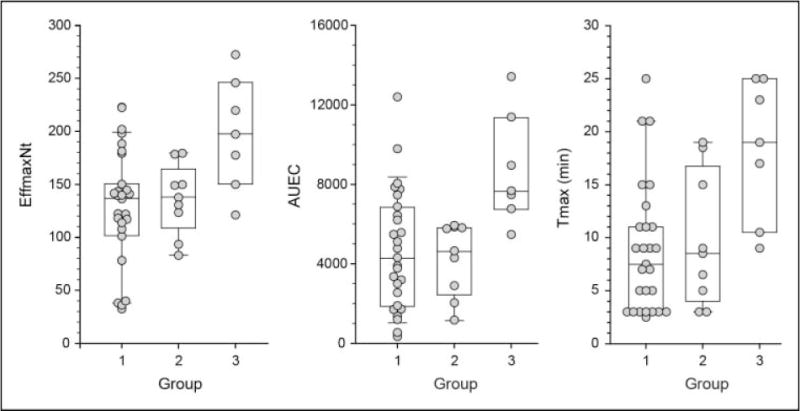
Observed parameter differences relative to histamine response phenotype. Comparisons of AUEC (flux × minutes), EffmaxNt (flux), and Tmax (minutes) between Groups 1, 2, 3.
Identification of the histamine response phenotypes were further investigated and validated by the observation of stronger association between AUEC and EffmaxNt (r2 = 0.86, P = .002) in the hyper-responsive group (Group 3) when compared to the entire cohort (r2 = 0.64, P < .001; Figure 3). EffmaxNt and AUEC was moderately associated in the hypo-responsive group (Group 1; r2 = 0.56, P < .001). Significant associations were not observed between EffmaxNt and AUEC within the normo-responsive group (Group 2).
Figure 3.
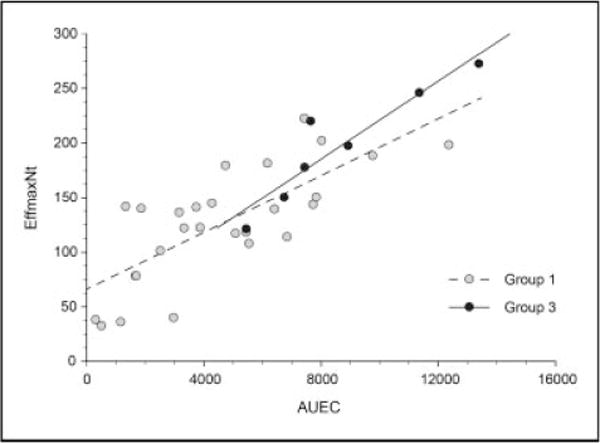
Association between AUEC (flux × minutes) and EffmaxNt (flux) in Hypo-responsive group and Hyper-responsive group. Group 1, r2 = 0.64, P < .001 in the hypo-responsive group. *Group 3, r2 = 0.86, P = .002 in the hyper-responsive group.
Discussion
We have characterized the microvasculature response to histamine in children with allergic rhinitis and identified phenotypically different patterns of histamine response. Both intra- and inter-individual variability has previously been identified among subjects undergoing EH testing which has been attributed to limitations of the method (e.g., operator error, variability in histamine dosing, effect of skin color on laser Doppler technique).5 In our current study we are able to control for many of these factors through the use of HILD. The discontinuous nature of the EH test (i.e., discrete, time-dependent measurements of the wheal and flare response) also does not lend itself to identification of discerning more subtle differences that were revealed by the continuous assessment of histamine response by HILD. In our study we identified three distinct phenotypes associated with histamine response. The hyper-responsive group exhibited a statistically higher response to histamine among all measured PD parameters (AUEC, EffmaxNT, and Tmax) relative to the other response groups. This distinctive phenotype was further validated by observations of stronger association between observed PD parameters which are expected to be associated (AUEC and EffmaxNt) within the hyper-responsive group relative to the entire cohort. We also indentified a potential distinction between the hypo-responsive group and the other two groups (i.e., hyper- and normo-responsive) whereby this group exhibited a lower maximal histamine response (EffmaxNt). Observation of statistically significant differences among the other response phenotypes may have been precluded in our current study by the relatively small sample size.
Despite this potential limitation, our data clearly identified one group of children with increased response to histamine relative to other children with allergic disease. It is therefore plausible that a child with a higher baseline response to histamine may exhibit differences in the pathophysiology of histamine-mediated diseases and differences in response to antihistamine treatment. We did not identify a difference in response pattern relative to reported asthma diagnosis or severity (hospitalization history). However, other objective measures of severity such as Asthma Control Test® or spirometry were not done given that these evaluations were not related to the primary aims of the current study. Also, the size of the study cohort would not have provided sufficient power to detect differences related to asthma or allergic rhinitis severity given the disease heterogeneity. We also did not observe differences in histamine response type associated with demographic characteristics (e.g., age, sex, race, and body size) of our study cohort.
Histamine modulates activity in various tissues including the dermis, small intestine, stomach, lung, and brain and is responsible for the classical symptoms associated with the allergic response.6 Histamine is also involved in actions in the lung such as mucous production, bronchoconstriction, and airway remodeling which are associated with pathophysiology of asthma.6–8 As histamine is a central mediator in the allergic response, it is important to identify potential differences in histamine response patterns that may influence disease expression or response to treatment.
It is also well known that antihistamines exhibit variability in pharmacologic and clinical response among different chemical entities.9,10 Previous studies have suggested that antihistamines may be beneficial in certain subsets of patients with asthma. For example, antihistamines administered to children with dust mite allergy may prevent the onset of asthma.11 Antihistamine use in infants considered high-risk for developing asthma also appear to prevent the onset of asthma when compared with placebo.12 In contrast, general consensus suggests that antihistamines are not generally effective in the treatment of asthma; a perception that may be associated with the heterogeneous response to this drug class among the general population of patients with asthma.7 Our findings demonstrate that variability exists among individuals in the response to histamine and importantly, response may be grouped into observable patterns or phenotypes. Identification of such groups of response to histamine may certainly be important in discerning the variability in antihistamine response. To our knowledge, our study is the first to demonstrate the existence of discrete, definable histamine response phenotypes.
Biological reasoning behind the differences in histamine response observed in our study may be related to differences within the histamine pathway. The histamine pathway involves enzymes responsible for the synthesis (histidine decarboxylase) and degradation (histamine-N-methyltransferase and diamine oxidase) of the amine. The action of histamine is mediated via four known receptors (H1,H2,H3,H4). These enzymes and receptors exhibit genetic variation which is capable of modulating their expression and/or function.13 For example, histamine-N-methyltransferase is the major enzyme responsible for the biotransformation of histamine in the body. A single nucleotide polymorphyism (SNP) C314T in the HNMT gene is associated with altered folding of the enzyme and resultant decreased function.14 Studies have also found that SNPs in the histamine−4 receptor gene are associated with presence of atopic dermatitis and expression of the receptor is associated with the diagnosis of asthma.15,16 The differences in response to histamine observed in our study cohort may indeed have a genomic basis. This is suggested by our observation that no African American participants in our study had a normo-responsive histamine PD profile. While the sample size of our study cohort precluded proper evaluation of genotype-phenotype relationships, future studies in larger numbers of subjects are warranted.
As denoted above, HILD is associated with important advantages over the traditional EH test in assessing histamine PD. Despite the advantages of HILD, there may be local (i.e., at the site of transcutaneous histamine administration and laser Doppler monitoring) physiologic factors that could possibly contribute to some of the variability associated with measurement of microvascular blood flow. These include differences in dermal thickness, skin hydration and fat content, skin color; all of which would be extremely difficult to control for in a study involving diverse pediatric study participants with wide age ranges. Finally, another potential limitation to our study is that we did not utilize a “true control” (e.g., saline control) as has been done in previous studies of histamine pharmacodynamics.17 We did adjust our data for baseline levels of blood flow to account for non-histamine induced differences in microvasculature blood flow among subjects.
Techniques to more readily assess the pharmacodynamic response to antihistamines and predict clinical response to these medications is needed. The use of antihistamines in children and adults is limited due to the significant side-effect profile related to sedation and other central nervous system effects. The HILD technique may allow identification of children who would most benefit from use of antihistamines thereby eliminating antihistamine exposure and side-effect risk in children who are unlikely to derive clinical benefit. HILD may also be useful to assess the difference in pharmacodynamic response and resultant clinical response between sedating and non-sedating antihistamines. Such studies may provide useful information regarding the clinical benefit of sedating antihistamines versus non-sedating preparations for diseases such as allergic rhinitis, atopic dermatitis, and even rhinitis symptoms related to the common cold.
In conclusion, our data demonstrate the apparent presence of distinct histamine response phenotypes. The ability to discern and characterize these histamine response phenotypes may have implications on the evaluation of antihistamine clinical pharmacology and potentially, in the prediction of treatment response and characterization of disease. Future investigations designed to elucidate the biological mechanisms behind these distinct phenotypes are warranted including, exploration of relevant genotype-phenotype relationships. HILD, now validated in both adult and pediatric populations, will enable these studies to be conducted in patients across the spectrum of age and histamine-associated disease. Histamine pharmacodynamic response has previously been used as a tool to characterize antihistamine pharmacodynamics. However, our data suggests that more important clues may be gleaned from how one responds to the amine. Histamine pharmacodynamic response may be associated with clinical response to antihistamines as well as underlying pathophysiology related to allergic/inflammatory disease states. Identification of histamine response patterns may provide better classification of disease phenotypes and thus allow identification of phenotypes which benefit most from antihistamine treatment. With further validation this technique offers an important tool as a non-invasive surrogate endpoint that may be utilized in the diagnosis and treatment of allergic and inflammatory disease states.
Supplementary Material
Figure S1 Variability of histamine pharmacodynamic response in children with allergic rhinitis.
Acknowledgments
We would like to acknowledge the efforts of the pediatric clinical pharmacology research coordinators at Children’s Mercy Hospital and Clinics and specifically the efforts of Cassandra Martinez BPS CCRC.
Grant sponsor: Katherine B. Richardson Foundation; Grant number: # 1 U10; Grant sponsor: Pediatric Pharmacology Research Unit Network, Eunice Kennedy; Grant number: HD31313-16S1.
Funding
These studies were supported in part by the Katherine B. Richardson Foundation, grant# 1 U10. HD31313-16S1 (G.L.K. and B.L.J.) Pediatric Pharmacology Research Unit Network, Eunice Kennedy. Shriver National Institute of Child Health and Human Development, and grant# 5K23HL105783 (B.L.J.) from the National Heart Lung and Blood Institute.
Footnotes
Supporting Information
Additional supporting Information may be found in the online version of this article at the publisher’s web-site.
Declaration of Conflicting Interests
The authors report no known conflicts of interest associated with the preparation of this manuscript.
References
- 1.Jones BL, Abdel-Rahman SM, Simon SD, Kearns GL, Neville KA. Assessment of histamine pharmacodynamics by microvasculature response of histamine using histamine iontophoresis laser Doppler flowimetry. J Clin Pharmacol. 2009;49(5):600–605. doi: 10.1177/0091270009332247. [DOI] [PubMed] [Google Scholar]
- 2.Berger W. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233–250. doi: 10.2165/00148581-200406040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Grant JA, Nicodemus CF, Findlay SR, et al. Cetirizine in patients with seasonal rhinitis and concomitant asthma: prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol. 1995;95(5 Pt 1):923–932. doi: 10.1016/s0091-6749(95)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Spiewak R. Inter-individual and intra-individual variability of skin reactivity to histamine at prick testing. Dermatol Online J. 1995;1(4) [Google Scholar]
- 6.Golightly L, Greos L. Second-generation antihistamines: actions and efficacy in the management of allergic disorders. Drugs. 2005;65:341–384. doi: 10.2165/00003495-200565030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kunzmann S, Schmidt-Weber C, Zingg JM, et al. Connective tissue growth factor expression is regulated by histamine in lung fibroblasts: potential role of histamine in airway remodeling. J Allergy Clin Immunol. 2007;119(6):1398–1407. doi: 10.1016/j.jaci.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Marone G, Granata F, Spadaro G, Genovese A, Triggiani M. The histamine-cytokine network in allergic inflammation. J Aller Clin Immunol. 2003;112:S83–S88. doi: 10.1016/s0091-6749(03)01881-5. [DOI] [PubMed] [Google Scholar]
- 9.de Benedictis FM, de Benedictis D, Canonica GW. New oral H1 antihistamines in children: facts and unmeet needs. Allergy. 2008;63(10):1395–1404. doi: 10.1111/j.1398-9995.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Ganse E, Kaufman L, Derde MP, Yernault JC, Delaunois L, Vincken W. Effects of antihistamines in adult asthma: a meta-analysis of clinical trials. Eur Respir J. 1997;10(10):2216–2224. doi: 10.1183/09031936.97.10102216. [DOI] [PubMed] [Google Scholar]
- 11.Warner JO. A double-blinded, randomized, placebo-controlled trial of cetirizine in preventing the onset of asthma in children with atopic dermatitis: 18 months’ treatment and 18 months’ posttreatment follow-up. J Allergy Clin Immunol. 2001;108(6):929–937. doi: 10.1067/mai.2001.120015. [DOI] [PubMed] [Google Scholar]
- 12.Bustos GJ, Bustos D, Romero O. Prevention of asthma with ketotifen in preasthmatic children: a three-year follow-up study. Clin Exp Allergy. 1995;25(6):568–573. doi: 10.1111/j.1365-2222.1995.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones BL, Kearns GL. Histamine: new thoughts about a familiar mediator. Clin Pharmacol Ther. 2011;89(2):189–197. doi: 10.1038/clpt.2010.256. [DOI] [PubMed] [Google Scholar]
- 14.Preuss C, Wood T, Szumlanksi C, et al. Human histamine N-methyltransferase pharmacogenetics: common genetic polymorphisms that alter activity. Mol Pharm Exp Ther. 1998;53:708–717. doi: 10.1124/mol.53.4.708. [DOI] [PubMed] [Google Scholar]
- 15.Yu B, Shao Y, Zhang J, et al. Polymorphisms in human histamine receptor H4 gene are associated with atopic dermatitis. Br J Dermatol. 2010;162(5):1038–1043. doi: 10.1111/j.1365-2133.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones BL, Rosenwasser LJ, Riffel AK, Graham BK, Vyhlidal CA. Buccal mucosal sampling is a reliable method to determine differences in gene expression patterns between subjects with asthma compared to healthy controls. J Allergy Clin Immunol. 2011;127(2):AB213. [Google Scholar]
- 17.Olsson P, Hammarlund A, Pipkorn U. Wheal-and-flare reactions induced by allergen and histamine: evaluation of blood flow with laser Doppler flowmetry. J Allergy Clin Immunol. 1988;82(2):291–296. doi: 10.1016/0091-6749(88)91014-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Variability of histamine pharmacodynamic response in children with allergic rhinitis.


