Abstract
Previous studies have shown that more active older adults have better cognition and brain health based on a variety of structural neuroimaging measures. Nevertheless, the effects of maintaining physical activity over an extended period of time on future changes in older adults’ cognition and brain structure are unknown. Participants were 141 initially well-functioning community-dwelling older adults (aged 70–79 years at baseline; 60% female; 42% black) studied over a 13-year period. Physical activity (self-reported time spent walking) was assessed annually from years 1 to 10. Magnetic resonance imaging with diffusion tensor was performed at years 10 and 13. Time spent walking decreased on average by 8.4% annually from year 1 to year 10. Independent of initial time spent walking, demographics, and APOE e4 status, better maintenance of time spent walking over the decade predicted less reduction in hippocampal volume (p = .03), smaller increases in global gray matter mean diffusivity and white matter axial diffusivity (p < .01), and maintenance of general cognitive performance (p < .01). Maintenance of cognitive performance was associated with smaller increases in white matter axial diffusivity (p < .01). PA at baseline and at year 10, as well as changes in PA over a five-year period were less predictive of future changes in brain structure and cognition. Thus, how physical activity levels change over longer periods of aging may be an important contributor to cognitive and neural protection.
Keywords: Physical Activity, Walking, Neuroimaging, Cognition, Hippocampus
1. INTRODUCTION
Moderate intensity physical activity (PA)—including walking—has been identified as a behavior to mitigate age-related cognitive decline and atrophy in the brain, including in the hippocampus. Cross-sectional and prospective cohort studies have shown that older adults who engage in greater amounts of PA have larger gray matter brain volumes across cortical and subcortical regions (Boyle et al., 2014; Bugg and Head, 2011; Erickson et al., 2010; Makizako et al., 2015), higher microstructural integrity in several white matter tracts (Burzynska et al., 2014; Tian et al., 2014a; Tian et al., 2015), and fewer white matter lesions (Burzynska et al., 2014; Sexton et al., 2016). Intervention studies have shown that structured exercise training—e.g. in the form of walking programs—increases cortical and hippocampal gray matter volume (Colcombe et al., 2006; Erickson et al., 2011; Rosano et al., 2016; Ten Brinke et al., 2014) and that training-induced improvements in aerobic fitness correlate with improved microstructural integrity (Voss et al., 2013) among previously sedentary older adults. PA-related structural changes in the brain have also been associated with improved cognitive health, including memory performance (Erickson et al., 2011) and a lower risk of cognitive impairment (Erickson et al., 2010).
There are unresolved issues regarding the effects of PA on brain structure and cognition in the elderly. First, prospective cohort studies typically use a single assessment of PA to determine the degree to which an individual is physically active. However, previous studies have shown that PA is not especially stable over longer periods of time—e.g., 5 or more years (Fortier et al., 2001; Telama et al., 2005)—which means that individuals identified as highly active might not be habitually highly active over months or years. Thus, the effects of maintaining PA over extended periods of time on cognition and brain structure are largely unknown. Second, most prospective analyses of the association between PA and brain structure have not examined changes in brain structure over time. Finally, previous prospective studies have generally included adults in the seventh or eighth decade of life and have rarely included those in the ninth decade of life and beyond. A previous study suggested that the association between PA and brain structure might vary across age (Bugg and Head, 2011); hence, it is important to examine whether previous findings extend to the very old.
The current study sought to address these unresolved issues using data from a biracial cohort of older women and men followed over 13 years and into their ninth decade of life. Self-reported time spent walking was assessed annually from year 1 to year 10. Brain macro and microstructure were assessed at years 10 and 13 using brain magnetic resonance imaging (MRI) with diffusion tensor. Macrostructural features included gray matter (GM) volume, and white matter hyperintensities (WMH). Four parameters from the diffusion weighted imaging quantified microstructural integrity: mean diffusivity (MD) of normal-appearing GM, and fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD) of normal-appearing white matter (WM). Additionally, general cognitive performance was measured at years 10 and 13, using the modified mini-mental state examination (Teng and Chui, 1987).
The primary analyses focused on the hippocampus, in light of evidence that it is especially sensitive to PA and physical fitness (Bugg and Head, 2011; Erickson et al., 2010; Erickson et al., 2009; Erickson et al., 2011; Rosano et al., 2016; Ten Brinke et al., 2014). We examined both hippocampal volume, which has been the focus of previous studies (Erickson et al., 2010; Erickson et al., 2011; Ten Brinke et al., 2014), and hippocampal MD, which has been examined infrequently (Tian et al., 2014a). Additionally, we conducted global analyses across normal-appearing WM and GM and hypothesized that a slower rate of decline in regular walking would predict smaller decreases in FA and smaller increases in WMH volume over time. We also explored the effects of PA on WM RD and AD to better understand the underpinnings of the associations between PA and WM FA values. With regard to global GM, we expected that a slower rate of decline in regular walking would predict smaller decreases in GM volume and smaller increases in GM MD. Finally, we examined the effects of walking on cognition and whether walking-induced effects on brain structure correlated with changes in cognition.
2. METHODS AND METHODS
2.1 Study Design and Participants
Participants were drawn from the larger Health, Aging, and Body Composition (Health ABC) Study, a prospective cohort study of adults aged 70–79 years at baseline (May 1997 to June 1998). Three hundred twenty-five participants at the Pittsburgh site were recruited to undergo brain MRI at year 10 (2006 to 2008), and of those 325, 162 completed a second MRI at year 13 (2009 to 2010). Diffusion tensor imaging (DTI) was also acquired on a smaller subset of 285 and 141 at years 10 and 13, respectively. Recruitment of this subsample was based on interest and eligibility to undergo brain MRI, as well as the ability to walk 20 meters. The study was approved by the institution review board at the University of Pittsburgh. All participants gave written informed consent.
2.2 Structural MRI Data Acquisition, Processing, and Analysis
Structural MRI data were acquired with a 3T Siemens TIM TRIO scanner at the MR Research Center of the University of Pittsburgh using a 12-channel receiver array head coil at both years 10 and 13. Magnetization-prepared rapid gradient echo T1-weighted images were acquired in the axial plane: TR = 2300 ms; TE = 3.43 ms; TI = 900 ms; Flip angle = 9°; slice thickness = 1 mm; FOV = 256 mm × 224 mm; voxel size=1mm×1mm; matrix size=256×224; and num- ber of slices = 176. Fluid-attenuated inversion recovery (FLAIR) images were acquired in the axial plane: TR = 9160 ms; TE=89ms;TI=2500ms;FA=150°; FOV=256mm×212mm; slice thickness = 3 mm; matrix size = 256 × 240; number of slices = 48 slices; and voxel size = 1 mm × 1 mm. DTI were acquired using single-short spin-echo echo planar imaging sequence with the following parameters: TR = 5300 ms; TE=88ms; TI=2500ms; Flip angle=90°; FOV=256mm× 256 mm; two diffusion values of b = 0 and 1000 s/mm2 ; 12 diffusion directions; four repeats; 40 slices; matrix size = 128 × 128; voxel size = 2 mm × 2 mm; slice thickness = 3 mm; and GRAPPA = 2.
Brain volumes and cerebrospinal fluid was quantified by segmenting the skull-stripped image in native anatomical space using the FAST – FMRIB’s Automated Segmentation Tool (Zhang et al., 2001). FLAIR T2-weighted imaging quantified WMH, using a fuzzy connected algorithm with automated seed selection (Wu et al., 2006b). Diffusion tensor images were preprocessed using the FMRIB’s Diffusion Toolbox to correct eddy current-induced distortions (Smith et al., 2004). Four repeats were concatenated and processed with FMRIB Software Library to generate FA and MD maps. Diffusion maps were registered to their respective templates using the FMRIB’s non-linear image registration tool (Andersson et al., 2007). Using the segmentation of GM, WM, and WMH, these maps were restricted to normal-appearing WM and GM (NAWM and NAGM) (Shimony et al., 2009). Partial volume effects of MD were minimized by masking the aligned images with the GM segmented from the T1 images.
Whole brain measures of GM volume, NAGM MD, NAWM FA, NAWM RD, NAWM AD, and WMH were computed. Regions of interest and white matter tracts were estimated using atlas-based segmentation (Wu et al., 2006a) with the Automated Anatomical Labeling Atlas (Tzourio-Mazoyer et al., 2002) and the Johns Hopkins University White Matter Atlas (Mori et al., 2008; Wakana et al., 2004), respectively. Regions of interest combined both left and right hemispheres. A radiologist verified that MR images were free of pathological findings.
2.3 Assessment of Physical Activity, Cognition, Demographics, and Other Covariates
Self-reported time spent walking (mins/week) was measured annually from years 1 to 10 using a standardized questionnaire developed for the Health ABC study and modeled on a previous questionnaire (Taylor et al., 1978). Participants were first asked whether they had engaged in walking at least 10 times in the past 12 months and then were asked had they engaged in walking at all in the past 7 days. Those answering in the affirmative were queried about the total number of minutes of walking over the past 7 days. For a subset (n = 114) of participants who also completed objective PA measurement at year 13 using a SenseWear Armband (BodyMedia Inc., Pittsburgh, Pennsylvania) over at least 3 days on the left upper arm, self-reported walking time correlated significantly with objective daily active energy expenditure (r = .27, p < .01), physical activity intensity (r = .29, p < .01), and objective step counts (r = .21, p = .02). General cognitive functioning was measured using the modified mini-mental status examination (3MS). The 3MS is a comprehensive test of orientation, attention, calculation, language, and short-term memory (Teng and Chui, 1987), with scores ranging from 0 to 100. Scores lower than 80 are indicative of cognitive impairment.
Demographics included age, sex, race, and educational attainment. Health behavior included self-reported smoking and drinking status (current, former, or never). Prevalent stroke, diabetes, and cardiovascular disease were determined using prevalent disease algorithms designed to mirror the Cardiovascular Health Study (Fried et al., 1991) using self-report of physician diagnoses, medication use, and laboratory data. Body mass index (kg/m2) provided a measure of body composition. Gait speed (m/s) was measured at usual pace over 3, 4, or 6 meters and was included to account for individual differences in physical performance, which might impact PA engagement. Usual gait speed has been shown to be a valid and reliable marker of physical performance in older adults (Guralnik et al., 1995). Apolipoprotein E ε4 carrier status was determined by standard single-nucleotide polymorphism analyses. Intracranial volume was obtained from the MRI scans at years 10 and 13.
2.4 Statistical Analyses
Due to substantial positive skew, walking minutes per week and WMH were log-transformed prior to analysis. The primary analyses used robust linear mixed effects models (LMM) using the statistical package ‘robustlmm’ (version 1.8) in R (Version 3.2.3). Robust LMM applies robustness weights to influential observations to guard against bias related to departures from normality in the residuals. These models used a smoothed Huber function with tuning parameter k = 2.28 and s = 10. An initial LMM was constructed to estimate the individual-level intercepts and slope values for self-reported walking minutes per week over the 10 annual assessments. Based on model fit statistics, a linear slope fit the data as well or better than quadratic, cubic, and piecewise slopes, and was chosen as the most parsimonious model. Next, these intercepts and slope values were standardized (M = 0, SD = 1) and entered into LMMs as predictors of changes in the brain structural variables. Standardized intercept and slope values were used to increase the interpretability of the estimates. In these LMMs, the brain structure of interested was entered as a repeatedly-measured outcome variable; time, walking intercept, walking slope, and the interactions of walking intercept and slope with time were entered as fixed effects. The brain structure intercept was specified as a random effect. Because our primary interest was on the associations between walking and changes in brain structure, we focused on the walking intercept*time and walking slope*time estimates. (The PA intercept and slope did not predict year 10 neuroimaging measures either separately or jointly, with all P > .05; see Supplemental Table 1).
Models were tested first with a minimal set of demographic covariates (age, sex, race, education), APOE ε4 carrier status, and time-varying intra-cranial volume (volumetric and gray matter mean diffusivity outcomes only). For the fully-adjusted model, the following physical health and functioning variables measured at year 10 were added to the previous model: body mass index, gait speed, chronic disease conditions (summed across diabetes, stroke, cardiovascular disease), health behaviors (a sum of smoking and drinking status), systolic blood pressure, and use of hypertension-treating medications. For all covariates, the main effect and interaction with time were included in the regression model. For the primary hippocampal outcomes, we applied a nominal significance threshold of p < .05; for global neuroimaging measures and cognition, we applied the Benjamini-Hochberg false discovery rate (FDR) correction (Benjamini and Hochberg, 1995) to account for multiple testing.
3. RESULTS
3.1 Sample Characteristics and Preliminary Analyses
The current sample consisted of 141 individuals who completed the MRI assessment with DTI at each time point. Supplemental Table 2 compares the study sample to the remaining participants of the Health ABC cohort (n = 2934) and to those participants who completed the baseline MRI but who did not complete the second MRI and/or had incomplete data in other ways (n = 172). Generally, the current study sample was younger, and in comparison to the overall Health ABC sample, had higher baseline cognition, faster gait speed, and a higher walking intercept. Descriptive statistics for the sample are shown in Table 1. Regular walking decreased significantly on an annual basis (slope estimate = −0.09 log mins/wk, 95% confidence interval: −0.10, −0.07, P < .001), which translates into a 8.4% reduction per year in total minutes/week spent walking. The intraclass correlation coefficient across the 10 time points was 0.41, suggesting a fair amount of stability over time (Cicchetti, 1994); however, there was variation in slope scores, with some individuals showing much greater reductions and others showing minimal reduction or even increases in walking over the decade (see Fig. 1A). An individual whose slope score was 1 SD above the mean was estimated to experience a 0.7% annual decrease in walking minutes/week, whereas an individual whose slope score was 1 SD below the mean was estimated to experience a 15.4% annual decrease in walking. Supplemental Table 3 provides the descriptive statistics for the untransformed self-reported walking minutes of walking at each time point. Supplemental Table 4 provides the correlation of the walking intercept and slope scores with the covariates. Generally, these correlations were non-significant; however, the intercept correlated weakly with participant age (r = .17, p = .04) and with APOE ε4 carrier status (Kendall’s τ = −.19, p = .007), and the slope correlated positively with gait speed (r = .21, p = .02). The walking intercept and slope scores were not significantly correlated (r = −.08, p = .35).
Table 1.
Characteristics of the study sample (n = 141).
| Demographics | |
| Age in years at first PA assessment, mean (SD) | 72.5 (2.5) |
| Age in years at first MRI, mean (SD) | 82.5 (2.5) |
| Age in years at second MRI, mean (SD) | 85.8 (2.5) |
| Sex, female, n (%) | 84 (60%) |
| Race, black, n (%) | 59 (42%) |
| Greater than high school education, n (%) | 72 (51%) |
| Body mass index (kg/m2) at first MRI, mean (SD) | 27.9 (4.4) |
| Chronic disease conditions at first MRI | |
| Cardiovascular disease, n (%) | 35 (25%) |
| Stroke, n (%) | 13 (9%) |
| Diabetes, n (%) | 12 (9%) |
| APOE ε4 carrier, n (%) | 25 (18%) |
| Gait speed (m/s) at first MRI, mean (SD) | 1.1 (0.3) |
| Health behavior at first MRI | |
| Current or former smoker, n (%) | 69 (49%) |
| Current or former alcohol drinker, n (%) | 110 (80%) |
| Systolic blood pressure at first MRI (mmHg) | 134.5 (19.3) |
| Medication for hypertension at first MRI | 96 (69%) |
Figure 1.
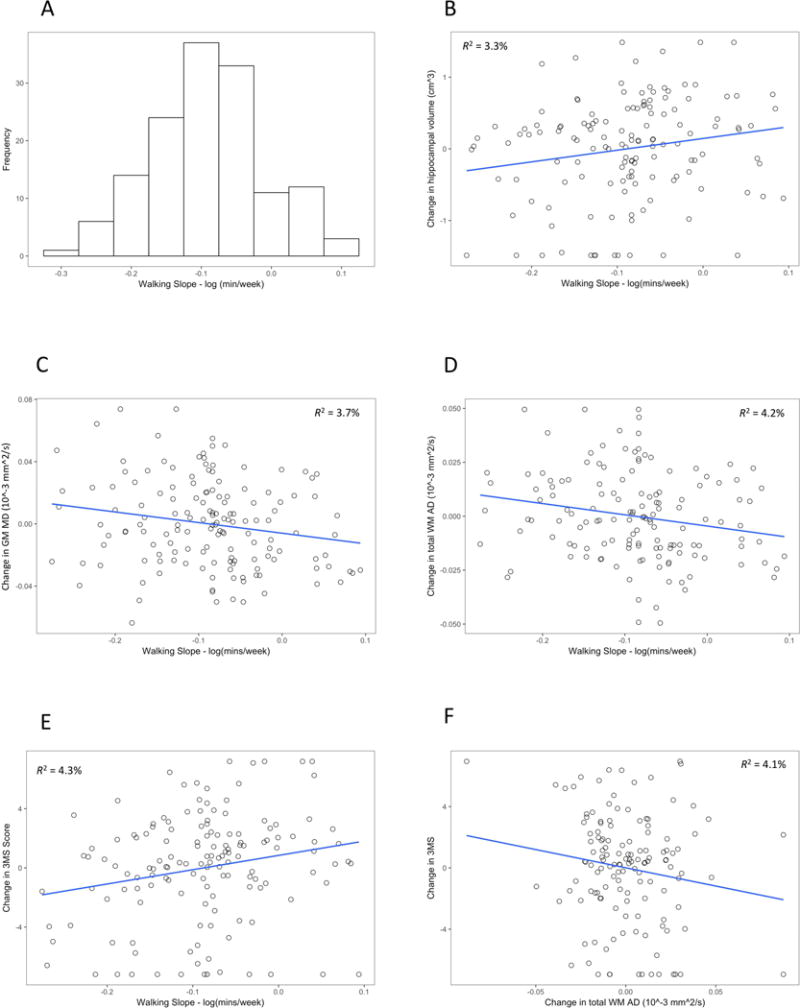
3.2 Changes in Cognition and Brain Structure
Significant change over time was observed in hippocampal volume and mean diffusivity, and in global measures of brain macro- and micro-structure with the exception of NAWM FA, which showed no group-level change over time (Table 2). Cognition, as assessed by the 3MS, decreased slightly over time. Expressed as percent change from year 10 to year 13, the average participant was estimated to experience a 6.2% reduction in hippocampal volume and a 6.0% increase in hippocampal mean diffusivity; a 4.6% decrease in global NAGM volume and a 3.1% increase in global NAGM MD; a 1.0% increase in global NAWM RD and 0.8% increase in global NAWM AD; and finally, a 11.7% increase in WMH.
Table 2.
Year 10 and change values in cognition and neuroimaging measures.
| Outcome | Year 10 Score | Δ Year 10 to Year13 (SE) | |
|---|---|---|---|
| Mean (SD) | Estimate (SE) | P value | |
| Hippocampus | |||
| Volume (cm3) | 9.57 (1.14) | −0.60 (0.08) | <.001 |
| Mean diffusivity (10−3 mm2/s) | 1.33 (0.01) | 0.08 (0.004) | <.001 |
| Global brain measures | |||
| GM volume (cm3) | 532.19 (53.19) | −24.61 (1.86) | <.001 |
| GM mean diffusivity (10−3 mm2/s) | 1.10 (0.52) | 0.03 (0.003) | <.001 |
| WM fractional anisotropy | 0.36 (0.01) | −0.0008 (0.0006) | .142 |
| WM radial diffusivity (10−3 mm2/s) | 0.70 (0.33) | 0.007 (0.002) | <.001 |
| WM axial diffusivity (10−3 mm2/s) | 1.12 (0.03) | 0.009 (0.002) | <.001 |
| WM hyperintensities1 | 5.51 (7.11) | 3.59 (0.47) | <.001 |
| Cognition | |||
| Modified Mini-Mental State | 93.65 (6.38) | −0.85 (0.44) | .054 |
| Examination | |||
Notes. GM = gray matter. SE = standard error of the mean. WM = white matter.
Log-transformed.
3.3 Predictive Effects of Walking on Changes in Cognition and Brain Structure
Table 3 reports on the associations between walking (both the intercept and slope) and changes in 3MS, hippocampal volume, and global micro- and macro-structural variables when adjusting for demographic variables and APOE ε4 carrier status. The walking intercept did not predict changes in cognition or brain structure (all p > .05). In contrast, the walking slope predicted changes in hippocampal volume (Fig. 1B), global NAGM MD (Fig. 1C), NAWM axial diffusivity (Fig. 1D), and 3MS score (Fig. 1E). Further adjustment for physical health and functioning at year 10 attenuated the effects of the walking slope on the diffusivity measures to some degree but had limited impact on cognition or hippocampal volume (Table 4). We then tested whether any of these brain changes were associated with 3MS changes, independently of the walking intercept and slope scores. We observed only that decreases in NAWM AD correlated with increases in 3MS (B = −.20, p = .007; Fig. 1F). Changes in hippocampal volume or NAGM MD were not correlated with changes in cognition (p > .10).
Table 3.
Effects of the time spent walking intercept and slope on changes in cognition, hippocampal volume, and global structural measures.
| Outcome | Predictor | |||
|---|---|---|---|---|
| Walking Intercept | Walking Slope | |||
| Estimate (SE) | P value | Estimate (SE) | P value | |
| Hippocampus | ||||
| Δ Volume (cm3) | 0.10 (0.07) | .176 | 0.16 (0.07) | .032 |
| Δ Mean diffusivity (10−3 mm2/s) | 0.004 (0.008) | .593 | 0.002 (0.008) | .844 |
| Global brain measures | ||||
| Δ GM volume (cm3) | 0.73 (2.05) | .721 | 4.91 (2.05) | .016 |
| Δ GM mean diffusivity | 0.001 (0.003) | .674 | −0.007 (0.003) | .005* |
| Δ WM fractional anisotropy | 0.004 (0.006) | .453 | 0.004 (0.006) | .456 |
| Δ WM radial diffusivity (10−3 mm2/s) | 0.001 (0.002) | .760 | −0.003 (0.002) | .037 |
| Δ WM axial diffusivity (10−3 mm2/s) | 0.003 (0.002) | .112 | −0.006 (0.002) | .004* |
| Δ WM hyperintensities | 0.56 (0.30) | .063 | 0.14 (0.31) | .654 |
| Cognition | ||||
| Δ Modified Mini-Mental State Examination | 0.49 (0.35) | .164 | 0.97 (0.34) | .004* |
Adjusted for intracranial volume (volumetric and mean diffusivity measures only), age, gender, race, education, and APOE ε4 carrier status.
GM = gray matter. WM = white matter.
Statistically-significant when applying false discovery rate correction to global neuroimaging and cognition outcomes.
Table 4.
Predictive effects of the walking slope on brain structure and cognition, adjusting for demographics, genetic risk, and year 10 health factors.
| Outcome | Walking Slope | |
|---|---|---|
| Estimate (SE) | P value | |
| Hippocampus | ||
| Δ Volume (cm3) | 0.17 (0.08) | .048 |
| Global brain measures | ||
| Δ GM mean diffusivity | −0.005 (0.003) | .053 |
| Δ WM axial diffusivity (10−3 mm2/s) | −0.005 (0.002) | .019 |
| Cognition | ||
| Δ Modified Mini-Mental State Examination | 1.01 (0.37) | .007 |
Notes. Adjusted for walking intercept, intracranial volume (volumetric and mean diffusivity measures only), age, gender, race, education, APOE ε4 carrier status, body mass index, gait speed, chronic disease conditions (diabetes, stroke, cardiovascular disease), health behaviours (smoking and drinking status), systolic blood pressure, and use of hypertensive medications. GM = gray matter. WM = white matter.
Next, we used the results from the fully-adjusted regression models in Table 4 to estimate the impact of a 1 standard deviation increase in the walking slope on the percent change in these brain structures relative to the percent change expected of an average individual in the sample. As noted above, the average slope score represented an 8.4% annual reduction in time spent walking, and a one standard deviation increase in the slope score from the mean represented a 0.7% annual reduction in time spent walking. A 1 standard deviation increase in the walking slope predicted a 4.5% decrease in hippocampal volume (average = 6.2% decrease), a 2.4% increase in global NAGM volume (average = 3.1% increase), and a 0.6% increase in global NAWM AD (average = 0.8% increase).
Four supplemental analyses followed. First, we tested whether the above findings were altered when we removed individuals with stroke (see Supplement Table 5); these results suggested similar, if not slightly stronger, effects than the results using the full sample. Second, we examined whether the year 10 time spent walking score was predictive of changes in cognition and brain structure (see Supplemental Table 6). There was no predictive association with changes in the hippocampus (nominal p > .05) or in global neuroimaging measures or cognition (FDR-corrected p > .05). Third, we used walking scores between years 6 to 10 to determine whether changes in walking over the most recent five years predicted subsequent changes in brain and cognition (see Supplemental Table 7). With the exception of NAGM MD, these findings suggested that changes over 5 years were not as predictive as changes over 10 years in regular walking. Fourth, we examined whether 10-year changes in 400-meter walk time, an objective measure of physical performance and cardiorespiratory capacity (Simonsick et al., 2006), predicted subsequent changes in cognition and brain structure. Although the associations were in the expected direction, none reach our statistical significance threshold (see Supplemental Table 8).
3.4 Exploratory Analyses
In light of the effects of the walking slope on global NAGM MD and NAWM AD changes, exploratory analyses were conducted to determine whether the walking slope predicted MD and AD changes in specific regions of interest. Based on previous studies examining the effects of physical activity and fitness on brain structure in older adults (Burzynska et al., 2014; Colcombe et al., 2006; Erickson et al., 2010; Erickson et al., 2014; Marks et al., 2011; Tian et al., 2014b; Tseng et al., 2013), GM regions included the anterior cingulate cortex, dorso-lateral and superior prefrontal cortex, and supplemental motor area; WM tracts included the cingulum (partitioned into dorsal and parahippocampal portions), superior longitudinal fasciculus, inferior longitudinal fasciculus, and uncinate fasciculus. In models adjusting for demographic variables and APOE ε4 carrier status, the walking slope predicted MD changes in the anterior cingulate cortex (B = −0.02, p = .048) and dorso-lateral cortex (B = −0.03, p = .008), and AD changes the parahippocampal (B = −0.01, p = .008) and dorsal regions (B = −0.01, p = .048) of the cingulum, and inferior longitudinal fasciculus (B = −0.01, p = .036). Moreover, independently of the walking intercept and slope, changes in 3MS correlated with MD changes in the anterior cingulate cortex (B = −0.16, p = .02) and AD changes in the parahippocampal (B = −0.16, p = .032) and dorsal (B = −0.17, p = .015) portions of the cingulum, and inferior longitudinal fasciculus (B = −0.18, p = .001).
4. DISCUSSION
This study found that changes in self-reported time spent walking over one decade, independent of initial levels of walking, predicted subsequent changes over 3 years in hippocampal volume, in global microstructural features of NAGM and NAWM, and in general cognitive functioning among older adults. Importantly, changes in NAWM AD correlated with changes in cognition, which suggests that long-term maintenance of regular walking in older age might benefit cognitive health through preservation of WM integrity.
A key finding was that neither the slope intercept—which quantifies initial level of walking at the beginning of the study—nor year 10 walking level was as predictive of subsequent changes in brain and cognition as was the walking slope. Furthermore, changes in walking over a shorter period of time (i.e., from year 6 to year 10) were not as predictive as the longer-term changes from year 1 to year 10. Thus, in order to better understand an older adult’s risk for cognitive and neural decline, clinicians should consider asking older adults on a regular basis (e.g., annually or semi-annually) about their physical activity behavior (including walking) and then tracking changes over time. An average individual in this sample experienced an 8.4% annual reduction in walking minutes/week, an individual with a gradual walking slope score (defined as one standard deviation above the mean) showed a 0.7% annual decrease, and an individual with a steep walking slope score (defined as one standard deviation below the mean) showed a 15.4% annual decrease. To put these values more concretely, for a hypothetical individual who began the study with 100 minutes/week of walking, an average slope score would result in the individual engaging in roughly 45 minutes/week of walking at year 10; a gradual slope score in roughly 94 minutes/week at year 10; and a steep slope score in roughly 22 minutes/week of walking at year 10.
The hippocampus shrinks rapidly starting around age 60, with estimates of ~1% annual reduction for older individuals aged 60 to 90 (Fjell et al., 2013; Raz et al., 2005). Our findings suggest that this rate of hippocampal atrophy might vary as a function of how an individual’s walking behavior changed over the previous decade. Specifically, we found that the average individual in our sample (aged 82.5 years at first MRI) demonstrated a 6.2% reduction in bilateral hippocampal volume over 3 years, whereas individuals who had maintained walking over the previous decade (based on a slope value 1 SD above the mean) demonstrated a 4.5% reduction in bilateral hippocampal volume. Although previous studies have shown that PA— including walking—might mitigate hippocampal atrophy, our study adds to the literature by showing that this mitigation effect is not restricted to individuals under the age of 80 (Bugg and Head, 2011; Erickson et al., 2011; Makizako et al., 2015; Rosano et al., 2016; Ten Brinke et al., 2014). Our results also add to the literature by suggesting that walking effects on hippocampal structure might primarily involve macro- rather than microstructural protection, as there was no evidence that regular walking had an impact on changes in hippocampal MD.
There is substantial interest in the mechanisms underlying the effects of PA on hippocampal structure and function. Rodent studies suggest that voluntary wheel running has various effects on the hippocampus, including promoting angiogenesis and neurogenesis, and modulating synaptic plasticity (Duzel et al., 2016). Upregulation of neurotrophic factors, such as bran-derived neurotrophic factor, insulin-like growth factor, and vascular endothelial growth factor, have been posited to mediate these structural and functional changes (Cotman et al., 2007). In older adults, Erickson and colleagues (2011) demonstrated that six months of progressive walking increased hippocampal volume and that increases in hippocampal volume correlated with concurrent increases in serum brain-derived neurotrophic factor level. Whether long-term changes in routine walking correlate with circulating levels of brain-derived neurotrophic factor is unknown; however, this possibility should be pursued in future research to elucidate the results described herein.
We also observed that long-term changes in walking predicted subsequent changes in global NAGM and NAWM diffusivity. Specifically, gradual slope scores (representing better maintenance of walking over the decade) were associated with smaller increases in NAGM MD and NAWM AD. Previous cross-sectional studies have shown that NAWM AD increases with advanced age (Hsu et al., 2010; Michielse et al., 2010) and can distinguish healthy older adults from those with mild cognitive impairment or dementia (Bosch et al., 2012). Longitudinal studies show that AD increases over relatively brief periods of time (i.e., <5 years) among older adults (Burzynska et al., 2017; Rieckmann et al., 2016; Sexton et al., 2014; Storsve et al., 2016), a finding that was confirmed in the current study. Previous studies that have examined the association between PA and WM integrity in older adults primarily have been cross-sectional and have suggested an inverse correlation between PA and WM diffusivity (Sexton et al., 2016). In the current study, we did not observe strong evidence for this cross-sectional association (Supplemental Table 6). With regard to longitudinal associations, a previous intervention study (Voss et al., 2013) found no effect of one year of walking on changes in WM diffusion indices. In light of our findings, it is possible that this previous study was insufficiently long to observe the associations between walking and WM diffusivity we observed herein. In follow-up exploratory analyses, we observed that the association between changes in walking and changes in NAWM AD were evident in particular regions, including the cingulum (dorsal and parahippocampal portions) and the inferior longitudinal fasciculus. These regions were also identified in a previous cross-sectional study of physical fitness and WM integrity in older adults (Tseng et al., 2013).
With regard to NAWM FA (a second-order statistic derived from the WM AD and RD values), we observed no effects of walking (intercept or slope) or significant changes over time, a finding that stands in contrast to previous longitudinal studies (Rieckmann et al., 2016; Sexton et al., 2014; Storsve et al., 2016). One important discrepancy between our study and these previous ones is that we examined global NAWM FA, rather than specific tracts separately. There appears to be substantial spatial heterogeneity in age-related changes in FA values (Burzynska et al., 2010; Sexton et al., 2014), which might be obscured by our examination of global FA changes. Moreover, WM diffusivity indices (both RD and AD) appear to change more markedly with age than FA values (Storsve et al., 2016), which is consistent with our results.
Less research has examined diffusion of gray matter within the context of aging or physical activity. GM MD is thought to increase as microstructural cellular atrophy occurs and the extracellular space enlarges, which would allow for freer movement of water molecules (Le Bihan, 2003; Whitwell et al., 2010). A recent systematic review suggested that elevated hippocampal and cortical MD might be a more sensitive marker of future cognitive impairment than volume loss (Weston et al., 2015). A previous cross-sectional study, also derived from the Health ABC cohort, observed that active individuals, as compared to their sedentary counterparts, had lower GM MD values in the medial temporal lobe and anterior cingulate cortex (Tian et al., 2014a). Our exploratory analyses extend this latter finding by showing that changes in anterior cingulate cortex MD is also sensitive to previous changes in walking.
Beyond the potential role of neurotrophic factors in explaining the effects of regular walking on brain health described above, prior studies have suggested that walking affects brain health via the amelioration of cerebrovascular risk factors (Burzynska et al., 2014). Indeed, when we adjusted for such risk factors (blood pressure, BMI, chronic diseases, as well as health behaviours) measured at the time of the first MRI (see the fully-adjusted models in Table 4), we observed that the association between the walking slope and changes in GM and WM diffusivity were attenuated to a degree. This could suggest that these associations are partly mediated by these risk factors but could also imply that there are a variety of mechanisms that contribute to the association between regular walking and structural changes to the brain with advanced age.
A critical finding was that better maintenance of walking over time, as indicated by gradual walking slope scores, predicted smaller decline in general cognitive performance, and further, that changes in cognition correlated significantly with changes in global NAWM AD, as well as AD in parahippocampal and dorsal portions of the cingulum, inferior longitudinal fasciculus, and MD in the anterior cingulate cortex. A previous meta-analysis showed that compared to controls, diffusivity in a variety of WM tracts is increased in individuals with Alzheimer’s disease and to a lesser degree, in individuals with mild cognitive impairment (Sexton et al., 2011). Moreover, this previous meta-analysis showed a significant negative correlation between the severity of the cognitive impairment and WM diffusivity, which is supported by the findings of our study.
Although our results provide evidence that decade-long changes in walking predict subsequent changes in various neuroimaging markers and cognition, the results do not imply causality, and thus, it is possible that cognitive impairment and underlying brain atrophy lead to reduced walking. Recent research has suggested that the associations between health behaviour and cognitive function might be explained by the notion of ‘neuroselection’ (better cognitive performance leads to more frequent engagement in positive health behaviours) in addition to the notion of ‘neuroprotection’ (engagement in positive health behaviours leads to better maintenance of cognitive functioning) (Belsky et al., 2015). It is likely that the associations observed in this study reflect a complex, bidirectional relation between time spent walking and brain health.
Another limitation is that PA was measured via a self-report measure of time spent walking. This might increase measurement error from inaccurate recall due to cognitive limitations and social desirability. Previous research suggests that individuals both over-report (Prince et al., 2008) and under-report their engagement in PA(Johnson-Kozlow et al., 2007). Indeed, in the current study, the correlations between the self-report PA measure and objective activity and energy expenditure were modest. Also, because the measure focuses exclusively on walking, it does not capture the full range of activities that older adults might engage in. However, walking is the most common PA (Siegel et al., 1995) and was the one form of PA assessed repeatedly over time in this cohort, which was required for the analyses conducted for the current study. Also, the strongest previous evidence for a link between PA and brain structure come from randomized controlled trials that have used walking as the primary form of PA (Colcombe et al., 2006; Erickson et al., 2011; Ten Brinke et al., 2014). Finally, our study sample is not a random sample of the overall Health ABC study; this selection bias could impact the generalizability of the findings to older adults.
The primary strength of this study is the longitudinal assessment of PA, cognition, and brain structure in a biracial cohort of older women and men. This allowed us to show that earlier changes in PA predict later changes in cognition and brain structure. Our results add to the growing body of evidence that the hippocampus is a gray matter region sensitive to changes in PA. Importantly, our findings also suggest that when examining brain structure at a global level, microstructural features of the brain (i.e., diffusivity) might be especially sensitive to changes in PA. Our examination of brain-cognition associations revealed that the impact of sustained walking over many years on cognition might be attributed in part to gray and white matter microstructural integrity. Another key finding was that initial PA level 10 years earlier, year 10 PA level, and changes in PA over a shorter period of time were all less informative in predicting future changes in brain structure and cognition than long-term changes in PA over a decade. Thus, older adults should be encouraged to maintain regular walking throughout aging to buffer against cognitive and structural brain changes.
Supplementary Material
Highlights.
Change in walking and subsequent changes in brain structure and cognition were assessed.
Changes in walking predicted changes in cognition and hippocampus
Global gray matter and white matter axial diffusivity were affected by walking changes
Initial walking levels were not predictive of cognitive or brain changes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson JL, Jenkinson M, Smith S. Non-linear registration, Aka spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007. [Google Scholar]
- Belsky DW, Caspi A, Israel S, Blumenthal JA, Poulton R, Moffitt TE. Cardiorespiratory fitness and cognitive function in midlife: neuroprotection or neuroselection? Annals of neurology. 2015;77(4):607–617. doi: 10.1002/ana.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Pena-Gomez C, Bargallo N, Molinuevo JL, Bartres-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2012;33(1):61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Boyle CP, Raji CA, Erickson KI, Lopez OL, Becker JT, Gach HM, Longstreth WT, Jr, Teverovskiy L, Kuller LH, Carmichael OT, Thompson PM. Physical activity, body mass index, and brain atrophy in Alzheimer’s disease. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Chaddock-Heyman L, Voss MW, Wong CN, Gothe NP, Olson EA, Knecht A, Lewis A, Monti JM, Cooke GE, Wojcicki TR, Fanning J, Chung HD, Awick E, McAuley E, Kramer AF. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One. 2014;9(9):e107413. doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, Gothe N, Voss MW, McAuley E, Kramer AF. White Matter Integrity Declined Over 6-Months, but Dance Intervention Improved Integrity of the Fornix of Older Adults. Front Aging Neurosci. 2017;9:59. doi: 10.3389/fnagi.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49(3):2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6(4):282–290. [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. Journal of Gerontology Series A: Biological and Medical Sciences. 2006;61A:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain. 2016;139(Pt 3):662–673. doi: 10.1093/brain/awv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Raji C, Lopez O, Becker J, Rosano C, Newman A, Gach H, Thompson P, Ho A, Kuller L. Physical activity predicts gray matter volume in late adulthood The Cardiovascular Health Study. Neurology. 2010;75(16):1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35(Suppl 2):S20–28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB, Alzheimer Disease Neuroimaging, I. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging. 2013;34(10):2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier M, Katzmarzyk PT, Malina RM, Bouchard C. Seven-year stability of physical activity and musculoskeletal fitness in the Canadian population. Medicine & Science in Sports & Exercise. 2001;33(11):1905–1911. doi: 10.1097/00005768-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright PL, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty BM, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New England Journal of Medicine. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JL, Van Hecke W, Bai CH, Lee CH, Tsai YF, Chiu HC, Jaw FS, Hsu CY, Leu JG, Chen WH, Leemans A. Microstructural white matter changes in normal aging: a diffusion tensor imaging study with higher-order polynomial regression models. Neuroimage. 2010;49(1):32–43. doi: 10.1016/j.neuroimage.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenback KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breat cancer. Am J Health Behav. 2007;31(2):193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Makizako H, Liu-Ambrose T, Shimada H, Doi T, Park H, Tsutsumimoto K, Uemura K, Suzuki T. Moderate-Intensity Physical Activity, Hippocampal Volume, and Memory in Older Adults With Mild Cognitive Impairment. J Gerontol A Biol Sci Med Sci. 2015;70(4):480–486. doi: 10.1093/gerona/glu136. [DOI] [PubMed] [Google Scholar]
- Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. Br J Sports Med. 2011;45(15):1208–1215. doi: 10.1136/bjsm.2009.068114. [DOI] [PubMed] [Google Scholar]
- Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. Neuroimage. 2010;52(4):1190–1201. doi: 10.1016/j.neuroimage.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Van Dijk KR, Sperling RA, Johnson KA, Buckner RL, Hedden T. Accelerated decline in white matter integrity in clinically normal individuals at risk for Alzheimer’s disease. Neurobiol Aging. 2016;42:177–188. doi: 10.1016/j.neurobiolaging.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Guralnik J, Pahor M, Glynn NW, Newman AB, Ibrahim T, Erickson K, Cohen R, Shaaban CE, MacCloud RL, Aizenstein HJ. Hippocampal Response to a 24-Month Physical Activity Intervention in Sedentary Older Adults. The American Journal of Geriatric Psychiatry. 2016 doi: 10.1016/j.jagp.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. 2016;131:81–90. doi: 10.1016/j.neuroimage.2015.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2011;32(12):2322–e2325. 2318. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 2014;34(46):15425–15436. doi: 10.1523/JNEUROSCI.0203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TL, Mintun MA, McKinstry RC, Snyder AZ. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66(3):245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel PZ, Brackbill RM, Heath GW. The epidemiology of walking for exercise: implications for promoting activity among sedentary groups. American Journal of Public Health. 1995;85(5):706–710. doi: 10.2105/ajph.85.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54(1):127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Yendiki A, Walhovd KB. Longitudinal Changes in White Matter Tract Integrity across the Adult Lifespan and Its Relation to Cortical Thinning. PLoS One. 2016;11(6):e0156770. doi: 10.1371/journal.pone.0156770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. Journal of Chronic Diseases. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Telama R, Yang X, Viikari J, Valimaki I, Wanne O, Raitakari O. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med. 2005;28(3):267–273. doi: 10.1016/j.amepre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br J Sports Med. 2014 doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. Journal of Clinical Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Tian Q, Erickson KI, Simonsick EM, Aizenstein HJ, Glynn NW, Boudreau RM, Newman AB, Kritchevsky SB, Yaffe K, Harris TB, Rosano C. Physical Activity Predicts Microstructural Integrity in Memory-Related Networks in Very Old Adults. J Gerontol A Biol Sci Med Sci. 2014a doi: 10.1093/gerona/glt287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Glynn NW, Erickson KI, Aizenstein HJ, Simonsick EM, Yaffe K, Harris TB, Kritchevsky SB, Boudreau RM, Newman AB, Lopez OL, Saxton J, Rosano C, Health, A.B.C.s Objective measures of physical activity, white matter integrity and cognitive status in adults over age 80. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Simonsick EM, Erickson KI, Aizenstein HJ, Glynn NW, Boudreau RM, Newman AB, Kritchevsky SB, Yaffe K, Harris T, Rosano C, for the Health, A.B.C.s. Cardiorespiratory fitness and brain diffusion tensor imaging in adults over 80 years of age. Brain research. 2014b doi: 10.1016/j.brainres.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, Zhang R. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wojcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Human brain mapping. 2013;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Weston PS, Simpson IJ, Ryan NS, Ourselin S, Fox NC. Diffusion imaging changes in grey matter in Alzheimer’s disease: a potential marker of early neurodegeneration. Alzheimer’s research & therapy. 2015;7(1):47. doi: 10.1186/s13195-015-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, Petersen RC, Josephs KA, Jack CR., Jr Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74:1279–1287. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Human brain mapping. 2006a;27(9):747–754. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Research: Neuroimaging. 2006b;148(2):133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Medical Imaging, IEEE Transactions on. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


