Abstract
Adenocarcinomas exhibiting gastric differentiation represent a recently described and uncommon subtype of non-human papillomavirus (HPV) related cervical adenocarcinoma. They comprise a spectrum from a well differentiated variant (adenoma malignum/mucinous variant of minimal deviation adenocarcinoma) to a more poorly overtly malignant form, generally referred to as gastric-type adenocarcinoma. Rarely, such tumours have also been described as primary vaginal neoplasms. Gastric-type adenocarcinomas exhibit considerable morphological overlap with adenocarcinomas originating outside the female genital tract, especially mucinous adenocarcinomas arising in the pancreas and biliary tract. Moreover they often metastasize to unusual sites, such as the ovary and peritoneum/omentum, where they can be mistaken for metastatic adenocarcinomas from other, non-gynaecological sites. There is little information regarding the immunophenotype of gastric-type adenocarcinomas and knowledge of this is important to aid in the distinction from other adenocarcinomas. In this study, we undertook a detailed immunohistochemical analysis of a large series of cervical (n=45) and vaginal (n=2) gastric-type adenocarcinomas. Markers included were CK7, CK20, CDX2, CEA, CA125, CA19.9, p16, ER, PR, MUC6, PAX8, PAX2, p53, hepatocyte nuclear factor 1 beta (HNF1β), carbonic anhydrase IX (CAIX), HER2 and MMR proteins. All markers were classified as negative, focal (<50% of tumour cells positive) or diffuse (≥50% tumour cells positive) except for p53 (classified as “wild-type” or “mutation-type”), HER2 (scored using the College of American Pathologists guidelines for gastric carcinomas) and MMR proteins (categorised as retained or lost). There was positive staining with CK7 (47/47 – 45 diffuse, 2 focal), MUC6 (17/21 – 6 diffuse, 11 focal), CEA (25/31 – 12 diffuse, 13 focal), CAIX (20/24 – 8 diffuse, 12 focal), PAX8 (32/47 – 20 diffuse, 12 focal), CA125 (36/45 – 5 diffuse, 31 focal), CA19.9 (11/11 – 8 diffuse, 3 focal), HNF1β (13/14 – 12 diffuse, 1 focal), CDX2 (24/47 – 4 diffuse, 20 focal), CK20 (23/47 – 6 diffuse, 17 focal) and p16 (18/47 – 4 diffuse, 14 focal). Most cases were negative with ER (29/31), PR (10/11), PAX2 (18/19) and HER2 (25/26). p53 showed “wild-type” and ”mutation-type” staining in 27/46 and 19/46 cases respectively. MMR protein expression was retained in 19/20 cases with loss of MSH6 staining in one patient with Lynch syndrome. Molecular studies for HPV were undertaken in two tumours which exhibited diffuse” block-type” immunoreactivity with p16 and both were negative. This is the first detailed immunohistochemical study of a large series of gastric-type adenocarcinomas of the lower female genital tract. Our results indicate immunophenotypic overlap with pancreaticobiliary adenocarcinomas but suggest that PAX8 immunoreactivity may be especially useful in distinguishing gastric-type adenocarcinomas from pancreaticobiliary and other non-gynaecological adenocarcinomas which are usually negative. Diffuse “block-type”p16 immunoreactivity in a cervical adenocarcinoma is not necessarily indicative of a high risk HPV-associated tumour.
Keywords: cervix, vagina, adenocarcinoma, gastric-type, immunohistochemistry, p16, PAX8
INTRODUCTION
So-called gastric-type adenocarcinoma is an uncommon variant of non-human papillomavirus (HPV) related cervical adenocarcinoma. The spectrum of these neoplasms has expanded in recent years and gastric-type adenocarcinoma is included in the 2014 World Health Organization (WHO) Classification of cervical adenocarcinomas (1–3). These form a continuum from adenoma malignum (mucinous variant of minimal deviation adenocarcinoma), which is now regarded as the well differentiated end of the spectrum, to gastric-type adenocarcinoma which represents the more poorly differentiated variant. Adenoma malignum and gastric-type adenocarcinoma form part of a spectrum with overlapping morphologies (4); indeed, it is well known that more atypical foci may be present in adenoma malignum and the identification of such areas is one of the most useful features in distinguishing adenoma malignum from various benign endocervical glandular lesions. Therefore, there seems no point in separating adenoma malignum from gastric-type adenocarcinoma and it is recommended that the term gastric-type adenocarcinoma is used to encompass this spectrum of neoplasia (5). Rarely, such tumours have also been described as primary vaginal neoplasms (6).
As discussed, these neoplasms are not associated with HPV infection and immunohistochemically they are typically negative or only focally positive with p16, unlike usual HPV-related cervical adenocarcinomas which are diffusely positive (1,7–10). Gastric-type adenocarcinomas of the cervix or vagina may be confused with other adenocarcinomas; for example, there is significant morphological overlap with pancreaticobiliary adenocarcinomas and often the diagnosis is one of exclusion. These neoplasms exhibit an aggressive behaviour and often have spread beyond the cervix at diagnosis with a particular tendency to metastasise to the ovary, peritoneum and omentum (1,11,12). Apart from typically being negative or focally positive with p16, little is known of the immunophenotype of cervical (and vaginal) gastric-type adenocarcinomas, although there have been several studies in the older literature which investigated the immunophenotype of adenoma malignum using a limited panel of markers (13–15). The purpose of this study is to undertake a detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Knowledge of the immunophenotype of these uncommon tumours is important to aid in their distinction from other neoplasms.
MATERIALS AND METHODS
47 cases were included in the study. 21 were derived from Department of Pathology, Belfast Health and Social Care Trust, Belfast and 26 from Department of Pathology, Memorial Sloan Kettering Cancer Center, New York. The material included both in-house (20 cases) and referral cases (27 cases). They comprised 45 primary cervical neoplasms (3 of which morphologically were in keeping with adenoma malignum) and 2 primary vaginal gastric-type adenocarcinomas. In occasional cases, the metastatic tumour rather than the primary neoplasm was stained with the various markers. Figure 1 shows examples of the tumours included in the study.
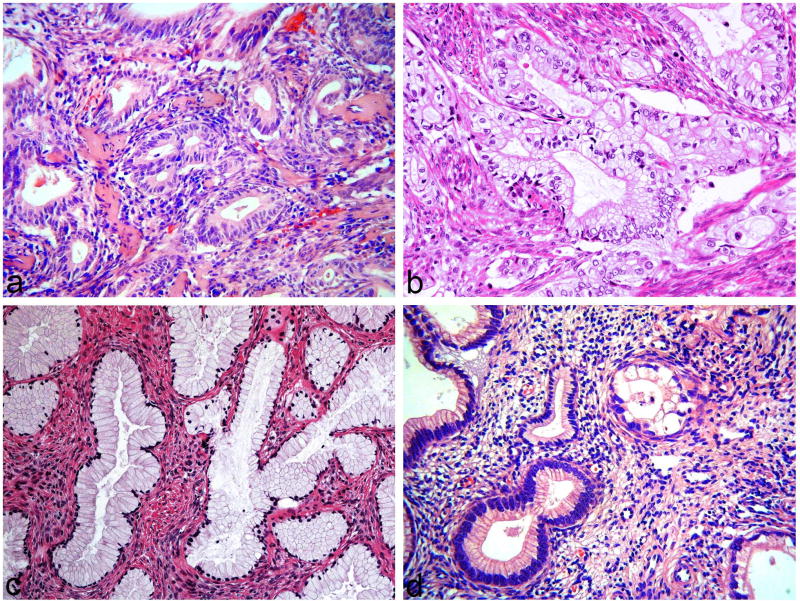
Figure 1.
Examples of gastric-type adenocarcinomas included in study. Tumour composed of crowded atypical mucinous glands (a), tumour composed of cells with abundant clear cytoplasm (b), tumour composed of bland mucinous glands, in keeping with adenoma malignum (c) and tumour composed of atypical and bland mucinous glands (d).
The haematoxylin and eosin stained sections and immunohistochemical slides were reviewed. Most of the immunohistochemistry had been performed at the referring laboratories or in the laboratories of the authors at the time of initial reporting using a variety of antibodies and methods. The markers included are shown in table 1. For all markers except p53, mismatch repair (MMR) proteins and Human epidermal receptor 2 (HER2), staining was classified as negative, focally positive (<50% cells staining) or diffusely positive (≥50% cells staining). For oestrogen receptor (ER), progesterone receptor (PR), PAX8, PAX2, CDX2, p53, MMR proteins and hepatocyte nuclear factor-1 beta (HNF1β) only nuclear staining was considered positive. For CK7, CK20, CA125, CA19.9, carcinoembryonic antigen (CEA), MUC6 and carbonic anhydrase IX (CAIX), cytoplasmic staining was interpreted as positive. For p16, nuclear and cytoplasmic staining and for HER2 membrane staining was considered positive. p53 staining was classified as “mutation-type” (totally negative “null” pattern with positive internal control in the form of weak nuclear staining of a proportion of stromal cells or diffuse strong immunoreactivity of >80% of tumour cell nuclei) or “wild-type” (heterogenous staining of <80% of tumour cell nuclei). MMR immunohistochemistry was performed for the MMR proteins MLH1, MSH2, MSH6 and PMS2. This was classified as retained (positive staining in tumour cell nuclei with all 4 proteins) or lost for one or more of the proteins (no nuclear staining in tumour cell nuclei with retention of staining in peritumoural lymphocytes). HER2 staining was classified using the College of American Pathologists (CAP) guidelines for gastric carcinomas as positive (complete intense membrane staining in >30% of tumour cells), equivocal (incomplete and/or weak/moderate staining in >10% of tumour cells or complete intense staining in ≤10% of tumour cells) or negative (no staining or faint/barely perceptible membrane staining).
Table 1.
Immunohistochemical Staining Results
| Antibody | Result | Percentage Positive (%) |
|---|---|---|
| CK7 | 2f, 45d | 100 |
| CK20 | 24n, 17f, 6d | 49 |
| CDX2 | 23n, 20f, 4d | 51 |
| CEA | 6n, 13f, 12d | 81 |
| CA125 | 9n, 31f, 5d | 80 |
| PAX8 | 15n, 12f, 20d | 68 |
| PAX2 | 18n, 1f | 5 |
| ER | 29n, 2f | 6 |
| PR | 10n, 1f | 9 |
| MUC6 | 4n, 11f, 6d | 81 |
| CAIX | 4n, 12f, 8d | 83 |
| CA19.9 | 3f, 8d | 100 |
| HNF1β | 1n, 1f, 12d | 93 |
| p16 | 29n, 14f, 4d | 38 |
| p53 | 27wt, 11mtd, 8mtn | 41 (mutation type); 59 (wild type) |
| HER2 | 21n, 4eq, 1p | 4 |
| MMR | 19r, 1l (MSH6) | 95 |
n-negative; f-focal; d-diffuse, wt-wild type, mtd- mutation type-diffuse, mtn-mutation type-null, r-retained, l-lost, eq-equivocal, p-positive.
Linear array HPV genotyping (Roche Molecular Diagnostics, Pleasanton, California, USA) was performed on paraffin blocks of the two cervical cases which exhibited diffuse “block-type” immunoreactivity with p16.
RESULTS
Immunohistochemistry
The immunohistochemical results are summarized in Table 1.
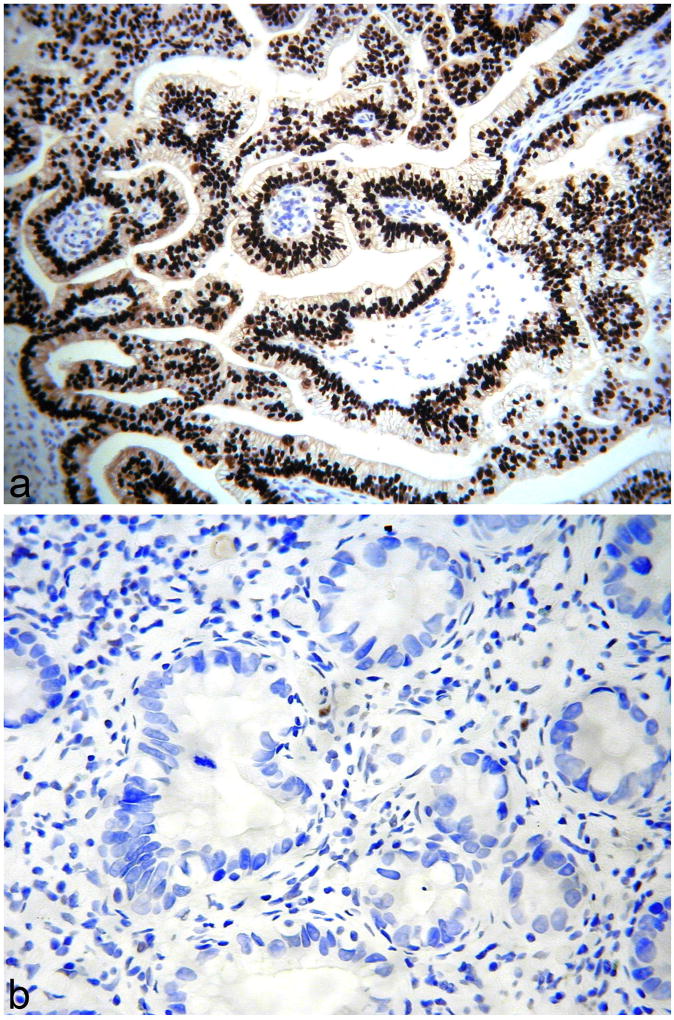
CK7, CK20, PAX8, p16 and CDX2 staining was performed in all 47 cases and was positive in 47 (100%), 23 (49%), 32 (68%), 18 (38%) and 24 (51%) cases respectively. Staining was typically diffuse with CK7 and PAX8 and focal with CK20 and CDX2, although there were exceptions with all markers. With p16, 14 cases showed focal positivity and 4 diffuse immunoreactivity; in 2 of the diffusely positive cases, there was “block-type” immunoreactivity involving essentially 100% of the tumour cells.
MUC6 was performed in 21 cases and positivity was observed in 17 (81%) (6 diffuse, 11 focal). 31 cases had CEA performed of which 25 were positive (81%) (13 focal, 12 diffuse) and CAIX was positive in 20 out of 24 cases (83%) (12 focal, 8 diffuse). CA125 was positive in 36 out of 45 cases (80%) (31 focal, 5 diffuse) and CA19.9 in 11 of 11 cases (100%). Of the 14 cases stained with HNF1β, 13 (93%) were positive and although the intensity of staining was weak in many of the cases it was nearly always diffuse (1 focal, 12 diffuse).
ER and PR staining was performed in 31 and 11 cases respectively. ER was focally positive in 2 cases (6%) and PR focally positive in 1 (9%). In all of the cases which were positive with hormone receptors, only occasional nuclei stained. PAX2 exhibited focal positivity in 1 of 19 cases (5%). HER2 was positive in 1 of 26 cases (4%), equivocal in 4 and negative in 21.
p53 was performed in 46 cases. 27 of these (59%) showed “wild type” staining while 19 (41%) showed “mutation type” staining which was diffuse in 11 cases and “null type” in 8.
MMR was retained in 19/20 (95%) cases. One case showed loss of staining with MSH6. This was in a patient who was known to have Lynch syndrome with a germline mutation in MSH6.
Figures 2–6 are representative images of the immunohistochemistry.
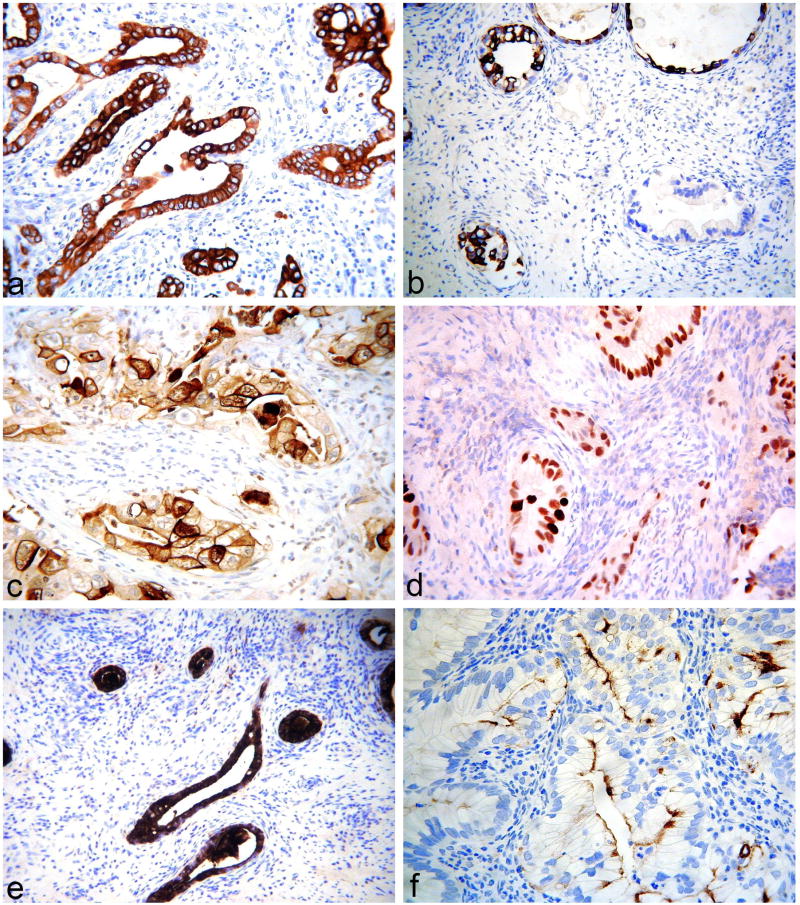
Figure 2.
Gastric-type adenocarcinomas exhibiting diffuse positivity with CK7 (a), focal positivity with CK20 (b), diffuse positivity with CEA (c), diffuse positivity with CDX2 (d), diffuse positivity with CA19.9 (e) and focal positivity with CA125 (f).
Figure 6.
Gastric-type adenocarcinomas exhibiting negative (a) and diffuse “block-type” immunoreactivity (b) with p16.
HPV Studies
Linear array HPV genotyping was negative in the two cases where this was performed.
DISCUSSION
Adenocarcinomas exhibiting gastric differentiation represent an uncommon, but probably under-recognised, subtype of non-HPV related cervical adenocarcinoma (1–4). As discussed, they show a spectrum of differentiation including adenoma malignum as the well differentiated variant and gastric-type adenocarcinoma as the more poorly differentiated form (1–4). They are characterised morphologically by tumour cells with abundant clear or pale eosinophilic cytoplasm, sometimes with prominent cell membranes; in many cases, the tumour cells contain obvious intracytoplasmic mucin. One study reported two examples of primary vaginal gastric-type adenocarcinomas (6). The term gastric-type adenocarcinoma was introduced because of the morphological features and the expression of gastric markers such as HIK1083 and MUC6 (1–4,16). However, the morphological appearances often more closely resembles a pancreaticobiliary mucinous adenocarcinoma rather than one of gastric origin and this is often the main differential diagnosis, especially when there is spread to the adnexa, omentum or peritoneum, as is sometimes the case with these neoplasms (1,11,12); this represents an unusual pattern of spread for usual HPV-related cervical adenocarcinomas, although it occasionally occurs (17,18). Even when clinically confined to the cervix, a metastasis may be considered by the pathologist given the significantly different morphological appearances from usual HPV-related cervical adenocarcinomas. Given the potential for confusion with other adenocarcinomas, especially of mucinous type, knowledge of the immunophenotype of cervical gastric-type adenocarcinomas is important. Despite this, other than documentation of staining with gastric markers and p16, there has been very limited investigation of this in the literature. Older studies which examined the immunophenotype of adenoma malignum found lack of expression of ER and PR and decreased expression of CA125 compared to other cervical adenocarcinomas; CEA was generally positive (13–15). More recently, Park et al studied the immunophenotype of a series of unusual endocervical adenocarcinomas including 11 gastric-type adenocarcinomas (7). They found that gastric-type adenocarcinomas were generally positive for HIK1083 and CEA whilst p16 was either negative or showed focal/patchy positivity (7). ER and PR were negative (7).
As already discussed, positive staining with gastric markers, such as MUC6 and HIK1083, was one of the main reasons for designating these neoplasms as gastric-type (1,2,5). The MUC6 antibody recognises pyloric gland mucin. Recently CAIX, another gastric marker, has been shown to be positive in conventional HPV-associated cervical glandular lesions (adenocarcinoma in situ (AIS) and adenocarcinoma) and both gastric-type adenocarcinoma and lobular endocervical glandular hyperplasia (LEGH), a benign cervical glandular lesion exhibiting gastric differentiation (19,20). In our study, MUC6 was positive in 81% of cases, although positive staining was more often focal than diffuse and some cases were negative. CAIX was positive in 83% of cases but again more often focally than diffuse. HIK1083 staining was not performed in this study.
Most usual HPV-related cervical adenocarcinomas are positive with CK7 and negative with CK20 and the intestinal transcription factor CDX2, although the latter two markers can be positive in those neoplasms which exhibit intestinal differentiation (21). In our study, all gastric-type adenocarcinomas were CK7 and CA19.9 positive, almost always with diffuse immunoreactivity. 49% and 51% of cases tested were positive with CK20 and CDX2 respectively, usually with focal immunoreactivity. 100% of cases tested were positive with CA19.9 (most diffuse), 81% with CEA (approximately half focal and half diffuse) and 80% with CA125 (usually focal); expression of these markers has not previously been investigated in cervical gastric-type adenocarcinomas, except for adenoma malignum. This immunophenotype (CK7, CEA, CA19.9, CA125 usually positive; CK20, CDX2 positive or negative) is characteristic of that expected with a primary pancreatic or biliary adenocarcinoma and highlights that markers commonly used when dealing with a mucinous adenocarcinoma of unknown primary may not distinguish a cervical gastric-type adenocarcinoma from one of pancreaticobiliary origin. .
PAX8 (paired box protein 8) is a paired-box gene crucial in embryogenesis of the thyroid gland, kidney and Mullerian system (22). PAX8 is positive in normal thyroid, renal and Mullerian epithelia and in most carcinomas arising in these organs (22). In the female genital tract, most adenocarcinomas of the ovary, fallopian tube, endometrium and cervix are positive. However, primary mucinous adenocarcinomas of the ovary are usually negative (or at the most exhibit focal weak immunoreactivity) and cervical adenocarcinomas are less likely to be positive than endometrial adenocarcinomas and non-mucinous ovarian adenocarcinomas (23). PAX8 is negative in the vast majority of gastrointestinal tumours, including pancreaticobiliary carcinomas (22). Therefore, in contrast to CK7, CK20, CA19.9, CEA and CDX2, PAX8 may be a useful marker in distinguishing cervical gastric-type adenocarcinoma (usually, but not always, positive) from other mucinous adenocarcinomas which are generally negative. In this scenario, although positive staining is useful in suggesting a cervical gastric-type adenocarcinoma, negative staining is of no value in determining the site of primary tumour.
PAX2 is another member of the PAX family of transcription factors and plays a major role in orchestrating the development of the Mullerian duct, kidneys and Wolffian duct during embryogenesis (24). Several studies have shown that while PAX2 is generally expressed in non-neoplastic epithelia in the female genital tract, there is loss of expression in a number of gynaecological adenocarcinomas and precursor lesions, such as serous tubal intraepithelial carcinoma (STIC), atypical hyperplasia/endometrial intraepithelial neoplasia, most endometrial and ovarian adenocarcinomas, AIS of the cervix and usual HPV-related cervical adenocarcinomas (24–26). In one study, PAX2 was positive in all cases of LEGH (5/5) and negative in all cases of adenoma malignum (0/5), suggesting that this marker may be useful in distinguishing between these two lesions, one benign and one malignant, exhibiting gastric differentiation (26). There have been no other studies examining PAX2 expression in cervical gastric-type adenocarcinomas. In our study, only 1 of 19 (5%) cases was PAX2 positive, suggesting that this transcription factor is also lost during the development of cervical gastric-type adenocarcinomas.
p16 is a cyclin-dependent kinase-4 inhibitor which becomes overexpressed in the presence of integrated oncogenic HPV (9). Diffuse “block-type”, as opposed to focal/patchy, expression of p16 has been considered a surrogate marker of the presence of high-risk HPV in the cervix and most cervical adenocarcinomas of usual type are diffusely positive with this marker (10,27). As discussed, it has been established that cervical gastric-type adenocarcinomas are not associated with HPV infection (1–4); in keeping with this, most cases in our study were either completely negative or focally positive with p16. Most usual HPV-related cervical adenocarcinomas look morphologically quite different from gastric-type adenocarcinoma in that they are not overtly mucinous and they exhibit much more conspicuous apoptotic activity. However, in problematic cases, p16 may be useful in distinguishing a gastric-type adenocarcinoma (negative or focally positive) from an HPV related adenocarcinoma (diffuse “block-type” immunoreactivity). In our study, 4 cases of gastric-type adenocarcinoma were diffusely p16 positive. In 2 of these, there was diffuse “block-type” immunoreactivity, as is typical of an HPV-related neoplasm; HPV testing was performed on these two tumours and both were HPV negative. Houghton et al found that 11 of 20 cervical adenocarcinomas of unusual morphological type were positive with p16, including 6 cases which exhibited diffuse positivity (defined as >50% cells positive) (10). All of these were negative for HPV except one case of serous carcinoma (10). The authors concluded that diffuse p16 expression in a cervical adenocarcinoma is not necessarily related to the presence of oncogenic HPV (10). Furthermore, there is a recent case report of adenoma malignum and gastric-type adenocarcinoma occurring together in a patient with Peutz-Jeghers syndrome where there was diffuse p16 positivity in both components of the tumour (28).
There is a spectrum of benign, premalignant and malignant cervical glandular lesions exhibiting gastric differentiation and all these lesions are characteristically totally negative (“flat negative”) with hormone receptors ER and PR (1–5). The results of our study confirm this with almost all cases of cervical gastric-type adenocarcinoma tested being completely negative with these markers; in those rare cases that were positive only very occasional nuclei stained.
As discussed, gastric-type adenocarcinomas are characterized by abundant clear or pale eosinophilic cytoplasm, sometimes with prominent cell membranes. As such, clear cell carcinoma (which in the cervix is also mostly unrelated to HPV infection) may enter into the differential diagnosis, although gastric-type adenocarcinomas lack the admixture of architectural patterns characteristic of clear cell carcinoma. HNF1β is considered a sensitive marker of ovarian and endometrial clear cell carcinoma, although it is not specific (29, 30). In a prior study, HNF1β exhibited patchy nuclear positivity in less than a third of cases of cervical gastric-type adenocarcinoma (7). In the current study, HNF1β was positive in 13 of 14 (93%) tumours tested and although immunoreactivity was weak in a number of cases, it was almost always diffuse. In another study, 42 of 56 (75%) cervical adenocarcinomas exhibited positive nuclear staining with HNF1 β but the morphological subtypes were not detailed (31). Given that some clear cell carcinomas are only weakly positive with HNF1β, this marker is of no value in distinguishing gastric-type adenocarcinoma from clear cell carcinoma. Napsin A has recently been suggested as a useful marker of gynaecological clear cell carcinomas with greater specificity than HNF1β (32); we did not stain our cases with Napsin A.
Mutation of the tumour suppressor gene p53 is one of the most frequent molecular alterations in human cancer and p53 mutations frequently occur in gynaecological malignancies, particularly ovarian and endometrial adenocarcinomas (34). In cervical cancer, the frequency of p53 mutations is low, both in HPV and non-HPV related cervical carcinomas (35, 36). In our series, we found mutation-type p53 staining, either diffuse or “null-type” (37), in 41% of the cases tested. This suggests that p53 mutation is involved in the pathogenesis of a significant percentage of cervical gastric-type adenocarcinomas.
HER2 is a proto-oncogene belonging to the epidermal growth factor receptor (EGFR) family. In cervical carcinomas, both squamous cell carcinoma and adenocarcinoma, the significance of HER2 amplification has yielded conflicting results (38) and HER2 testing is not routinely undertaken. However, the clinical significance of HER2 amplification in gastric carcinomas is well-established; HER2 is amplified in approximately 20% of gastric adenocarcinomas and predicts response to HER2 inhibitors such as trastuzumab (39). To date, there have been no studies which have investigated HER2 expression in cervical gastric-type adenocarcinomas and given that these neoplasms exhibit gastric differentiation we explored this. Only one of 26 cases tested was positive suggesting that it is unlikely that trastuzumab will be effective in the management of cervical gastric-type adenocarcinomas.
There is a single case report in the literature of a cervical gastric-type adenocarcinoma occurring in a patient with Lynch syndrome; this patient had a germline mutation in the MSH6 gene and represents one of the cases in our study (40). Lynch syndrome is associated with mutations in genes encoding proteins in the DNA mismatch repair pathway, particularly MLH1, MSH2, MSH6 and PMS2. Patients with Lynch syndrome have a predisposition to develop colorectal carcinoma and gynaecological tumours, including both endometrial and ovarian carcinomas, but there is no known association with cervical cancer (41–44). There are only occasional reports in the literature of cervical carcinomas occurring in the setting of Lynch syndrome (41–45). To investigate a possible association between cervical gastric-type adenocarcinomas and MMR abnormalities, we performed MMR immunohistochemistry in a number of our cases. The case reported previously, and included in our study (40), was the only one which exhibited abnormal staining in the form of loss of immunoreactivity with MSH6. In all the other cases, staining with MMR proteins was retained. This suggests that the MMR pathway is not commonly involved in the pathogenesis of cervical gastric-type adenocarcinomas.
In summary, this study provides novel and important information regarding the immunohistochemical profile of cervical and vaginal gastric-type adenocarcinomas. With many of the markers, there is significant immunophenotypic overlap with pancreatic and biliary adenocarcinomas. In this regard, PAX8 staining may be especially useful in distinguishing cervical gastric-type adenocarcinoma (usually, but not always positive) from pancreatic, biliary and other mucinous adenocarcinomas which are usually negative. Gastric-type adenocarcinomas are commonly positive with HNF1β and this marker is of no value in distinguishing these neoplasms from a clear cell carcinoma which may be in the differential diagnosis. Most gastric-type adenocarcinomas are negative or only focally positive with p16 and this marker is of value in distinction of these neoplasms from usual HPV-related cervical adenocarcinomas which are diffusely p16 positive; however, occasional gastric-type cervical adenocarcinomas exhibit diffuse “block-type” immunoreactivity with p16 and HPV testing may be necessary to distinguish these from an HPV-related cervical adenocarcinoma. Whilst the MMR pathway is unlikely to be involved in the pathogenesis of gastric-type adenocarcinomas, mutation of p53 may play a role in a significant percentage of cases. Occasional cervical gastric-type adenocarcinomas exhibit diffuse “block-type” immunoreactivity with p16 which can result in confusion with a usual HPV-related cervical adenocarcinoma; HPV studies may be necessary in these uncommon cases.
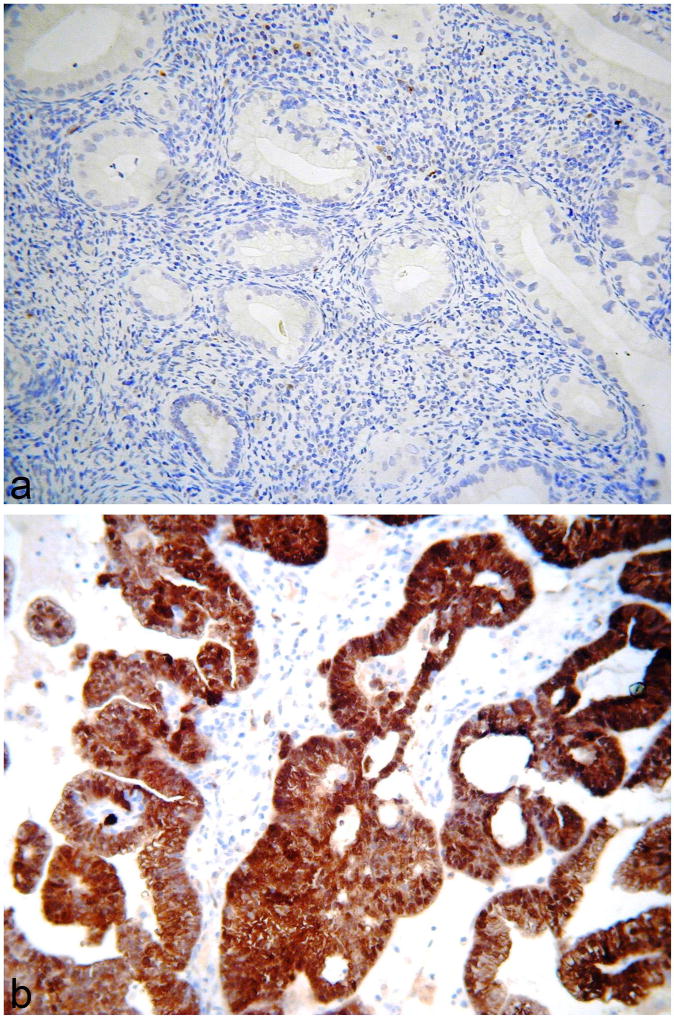
Figure 3.
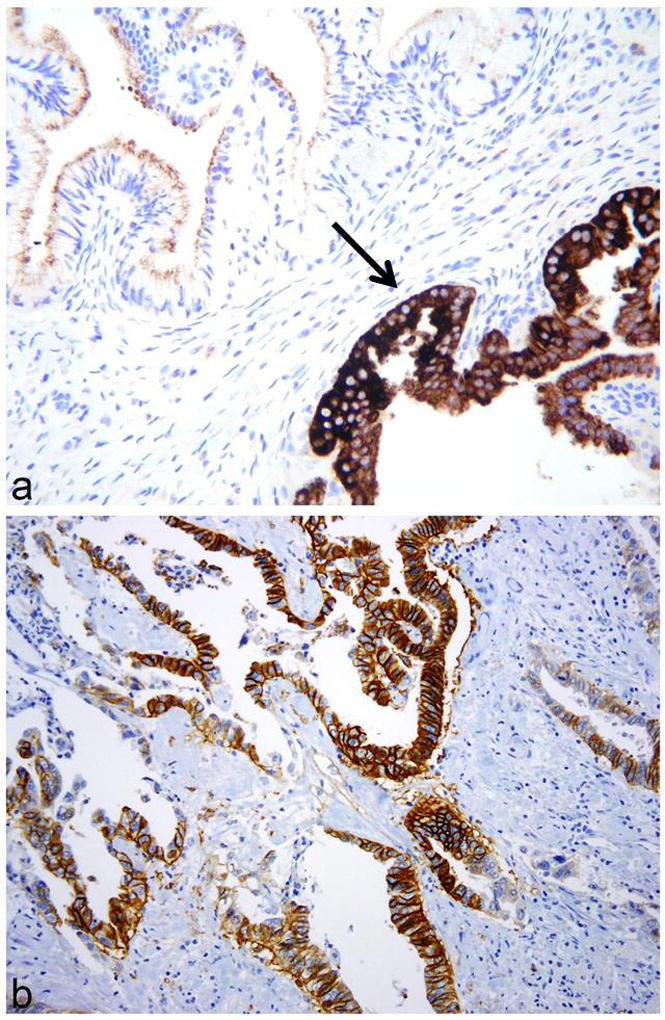
Gastric-type adenocarcinomas exhibiting focal cytoplasmic positivity with MUC6 (arrow) (a) and diffuse positivity with CAIX (b).
Figure 4.
Gastric-type adenocarcinomas exhibiting diffuse nuclear positivity with PAX8 (a) and HNF1β (b).
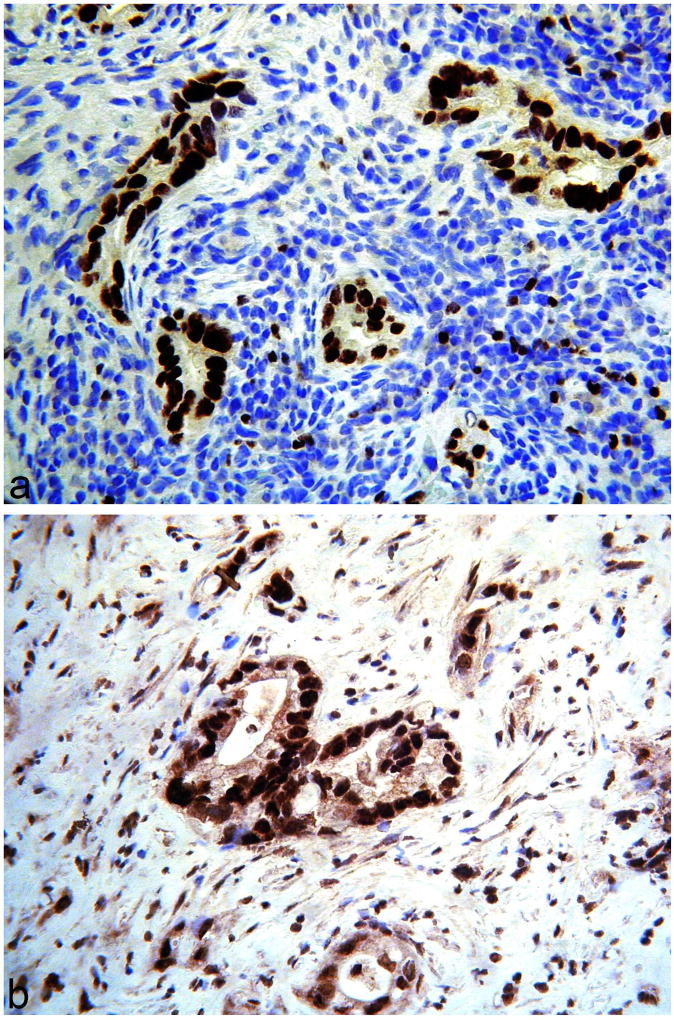
Figure 5.
Some gastric-type adenocarcinomas exhibit “mutation-type” staining, either of diffuse (a) or “null” type (b), with p53.
References
- 1.Kojima A, Mikami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–672. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- 2.Mikami Y, McCluggage WG. Endocervical glandular lesions exhibiting gastric differentiation: an emerging spectrum of benign, premalignant and malignant lesions. Adv Anat Pathol. 2013;20:227–237. doi: 10.1097/PAP.0b013e31829c2d66. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. International Agency for Research on Cancer; Lyon: 2014. [Google Scholar]
- 4.McCluggage WG, Harley I, Houghton JP, Geyer FC, McKay A, Reis-Filho JS. Composite cervical adenocarcinoma composed of adenoma malignum and gastric type adenocarcinoma (dedifferentiated adenoma malignum) in patient with Peutz-Jeghers syndrome. J Clin Pathol. 2010;63:935–941. doi: 10.1136/jcp.2010.080150. [DOI] [PubMed] [Google Scholar]
- 5.McCluggage WG. Recent developments in non-HPV related adenocarcinomas of the lower female genital tract and their precursors. Adv Anat Pathol. doi: 10.1097/PAP.0000000000000095. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Talia KL, Scurry J, Manolitsas T, McCluggage WG. Primary vaginal mucinous adenocarcinoma of gastric type arising in adenosis: a report of 2 cases, 1 associated with uterus didelphys. Int J Gynecol Pathol. 2012;31:184–191. doi: 10.1097/PGP.0b013e31822c8036. [DOI] [PubMed] [Google Scholar]
- 7.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 8.Kusanagi Y, Kojima A, Mikami Y, et al. Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–2175. doi: 10.2353/ajpath.2010.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill CJ, McCluggage WG. p16 expression in the female genital tract and its value in diagnosis. Adv Anat Pathol. 2006;13:8–15. doi: 10.1097/01.pap.0000201828.92719.f3. [DOI] [PubMed] [Google Scholar]
- 10.Houghton O, Jamison J, Wilson R, Carson J, McCluggage WG. p16 immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology. 2010;57:342–350. doi: 10.1111/j.1365-2559.2010.03632.x. [DOI] [PubMed] [Google Scholar]
- 11.Chou YY, Lin MC, Huang LW. Human papillomavirus-unrelated gastric type of cervical adenocarcinoma presenting with a metastatic ovarian tumor: report of a case. J Low Genit Tract Dis. 2013;17:218–222. doi: 10.1097/LGT.0b013e3182652625. [DOI] [PubMed] [Google Scholar]
- 12.Karamurzin YS, Kiyokawa T, Parkash V, et al. Gastric-type endocervical adenocarcinoma. An aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol. doi: 10.1097/PAS.0000000000000532. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toki T, Shiozawa T, Hosaka N, Ishii K, Nikaido T, Fujii S. Minimal deviation adenocarcinoma of the uterine cervix has abnormal expression of sex steroid receptors, CA125, and gastric mucin. Int J Gynecol Pathol. 1997;16:111–116. doi: 10.1097/00004347-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Gilks CB, Young RH, Aguirre P, et al. Adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix. A clinicopathological and immunohistochemical analysis of 26 cases. Am J Surg Pathol. 1989;13:717–729. doi: 10.1097/00000478-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ishii K, Hosaka N, Toki T, et al. A new view of the so-called adenoma malignum of the uterine cervix. Virchows Arch. 1998;432:315–322. doi: 10.1007/s004280050172. [DOI] [PubMed] [Google Scholar]
- 16.Mikami Y, Kiyokawa T, Hata S, et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum’. Mod Pathol. 2004;17:962–972. doi: 10.1038/modpathol.3800148. [DOI] [PubMed] [Google Scholar]
- 17.Ronnett BM, Yemelyanova AV, Vang R, Gilks CB, Miller D, Gravitt PE, Kurman RJ. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumors and the ability of the metastases to simulate primary ovarian neoplasms. Am J Surg Pathol. 2008;32:1835–1853. doi: 10.1097/PAS.0b013e3181758831. [DOI] [PubMed] [Google Scholar]
- 18.Chang MC, Nevadunsky NS, Viswanathan AN, Crum CP, Feltmate CM. Endocervical adenocarcinoma in situ with ovarian metastases: a unique variant with potential for long-term survival. Int J Gynecol Pathol. 2010;29:88–92. doi: 10.1097/PGP.0b013e3181acefbf. [DOI] [PubMed] [Google Scholar]
- 19.Mikami Y, Minamiguchi S, Teramoto N, et al. Carbonic anhydrase type IX expression in lobular endocervical glandular hyperplasia and gastric-type adenocarcinoma of the uterine cervix. Pathol Res Pract. 2013;209:173–178. doi: 10.1016/j.prp.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Liao SY, Rodgers WH, Kauderer J, et al. Endocervical glandular neoplasia associated with lobular endocervical glandular hyperplasia is HPV-independent and correlates with carbonic anhydrase-IX expression: a Gynaecological Oncology Group Study. Br J Cancer. 2013;108:613–620. doi: 10.1038/bjc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCluggage WG, Shah R, Connolly LE, McBride HA. Intestinal-type cervical adenocarcinoma in situ and adenocarcinoma exhibit a partial enteric immunophenotype with consistent expression of CDX2. Int J Gynecol Pathol. 2008;27:92–100. doi: 10.1097/pgp.0b013e31815698e7. [DOI] [PubMed] [Google Scholar]
- 22.Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 23.Yemelyanova A, Gown AM, Wu LS, Holmes BJ, Ronnett BM, Vang R. PAX8 expression in uterine adenocarcinomas and mesonephric proliferations. Int J Gynecol Pathol. 2014;33:492–499. doi: 10.1097/PGP.0b013e3182a54afa. [DOI] [PubMed] [Google Scholar]
- 24.Ordóñez NG. Value of PAX2 Immunostaining in Tumor Diagnosis: A Review and Update. Adv Anat Pathol. 2012;19:401–409. doi: 10.1097/PAP.0b013e318271a382. [DOI] [PubMed] [Google Scholar]
- 25.Shukla A, Thomas D, Roh MH. PAX8 and PAX2 expression in endocervical adenocarcinoma in situ and high-grade squamous dysplasia. Int J Gynecol Pathol. 2013;32:116–121. doi: 10.1097/PGP.0b013e318257df46. [DOI] [PubMed] [Google Scholar]
- 26.Rabban JT, McAlhany S, Lerwill MF, et al. PAX2 distinguishes benign mesonephric and mullerian glandular lesions of the cervix from endocervical adenocarcinoma, including minimal deviation adenocarcinoma. Am J Surg Pathol. 2010;34:137–146. doi: 10.1097/PAS.0b013e3181c89c98. [DOI] [PubMed] [Google Scholar]
- 27.McCluggage WG, Jenkins D. p16 immunoreactivity may assist in the distinction between endometrial and endocervical adenocarcinoma. Int J Gynecol Pathol. 2003;22:231–235. doi: 10.1097/01.PGP.0000055172.04957.2F. [DOI] [PubMed] [Google Scholar]
- 28.Peng WX, Kure S, Ishino K, et al. P16-positive continuous minimal deviation adenocarcinoma and gastric type adenocarcinoma in a patient with Peutz-Jeghers syndrome. Int J Clin Exp Pathol. 2015;8:5877–5882. [PMC free article] [PubMed] [Google Scholar]
- 29.Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2006;19:83–89. doi: 10.1038/modpathol.3800492. [DOI] [PubMed] [Google Scholar]
- 30.Fadare O, Zhao C, Khabele D, et al. Comparative analysis of Napsin A, alpha-methylacyl-coenzyme A racemase (AMACR, P504S), and hepatocyte nuclear factor 1 beta as diagnostic markers of ovarian clear cell carcinoma: an immunohistochemical study of 279 ovarian tumours. Pathology. 2015;47:105–111. doi: 10.1097/PAT.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 31.Němejcová K, Cibula D, Dundr P. Expression of HNF-1β in cervical carcinomas: an immunohistochemical study of 155 cases. Diagn Pathol. 2015 Mar 25;10:8. doi: 10.1186/s13000-015-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim D, Ip PP, Cheung AN, Kiyokawa T, Oliva E. Immunohistochemical Comparison of Ovarian and Uterine Endometrioid Carcinoma, Endometrioid Carcinoma With Clear Cell Change, and Clear Cell Carcinoma. Am J Surg Pathol. 2015;39:1061–1069. doi: 10.1097/PAS.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 33.Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc 4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21:391–400. doi: 10.1097/00004347-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed AA, Etemadmoghadam D, Temple J, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinomas of the ovary. J Pathol. 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berchuck A, Kohler MF, Marks JR, Wiseman R, Boyd J, Bast RC., Jr The p53 tumor suppressor gene frequently is altered in gynaecologic cancers. Am J Obstet Gynecol. 1994;170:246–252. doi: 10.1016/s0002-9378(94)70414-7. [DOI] [PubMed] [Google Scholar]
- 36.Park DJ, Wilczynski SP, Paquette RL, Miller CW, Koeffler HP. p53 mutations in HPV-negative cervical carcinoma. Oncogene. 1994;9:205–210. [PubMed] [Google Scholar]
- 37.McCluggage WG, Soslow RA, Gilks CB. Patterns of p53 immunoreactivity in endometrial carcinomas: 'all or nothing' staining is of importance. Histopathology. 2011;59:786–788. doi: 10.1111/j.1365-2559.2011.03907.x. [DOI] [PubMed] [Google Scholar]
- 38.Kihana T, Tsuda H, Teshima S, et al. Prognostic significance of the overexpression of c-erbB-2 protein in adenocarcinoma of the uterine cervix. Cancer. 1994;73:148–153. doi: 10.1002/1097-0142(19940101)73:1<148::aid-cncr2820730125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 39.Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 40.Moat M, O’Donnell RL, McCluggage WG, Ralte A, Edmondson RJ. Gastric-type adenocarcinoma of the cervix in a patient with Lynch syndrome: A case report. Gynecol Oncol Rep. 2014;10:41–43. doi: 10.1016/j.gynor.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills AM, Liou S, Kong CS, Longacre TA. Are women with endocervical adenocarcinoma at risk for lynch syndrome? Evaluation of 101 cases including unusual subtypes and lower uterine segment tumors. Int J Gynecol Pathol. 2012;31:463–469. doi: 10.1097/PGP.0b013e31824a1dad. [DOI] [PubMed] [Google Scholar]
- 42.Barrow E, Hill J, Evans DG. Cancer risk in Lynch syndrome. Fam Cancer. 2013;12:229–240. doi: 10.1007/s10689-013-9615-1. [DOI] [PubMed] [Google Scholar]
- 43.Mongiat-Artus P, Miquel C, Fléjou JF, et al. Spectrum of molecular alterations in colorectal, upper urinary tract, endocervical, and renal carcinomas arising in a patient with hereditary non-polyposis colorectal cancer. Virchows Arch. 2006;449:238–243. doi: 10.1007/s00428-006-0182-9. [DOI] [PubMed] [Google Scholar]
- 44.Nair N, Curtin JP, Mittal K, Hiotis KL. Cervical adenocarcinoma in a patient with Lynch syndrome, Muir-Torre variant. J Clin Oncol. 2012;30:E5–E6. doi: 10.1200/JCO.2011.36.3325. [DOI] [PubMed] [Google Scholar]
- 45.Downes MR, Allo G, McCluggage WG, et al. Review of findings in prophylactic gynaecological specimens in Lynch syndrome with literature review and recommendations for grossing. Histopathology. 2014;65:228–239. doi: 10.1111/his.12386. [DOI] [PubMed] [Google Scholar]