Abstract
A higher incidence of cardiovascular disease (CVD) has been observed in several chronic inflammatory diseases. However, data on sarcoidosis are limited. In this study, 345 patients with incident sarcoidosis in 1976–2013 in Olmsted County, Minnesota, were identified based on comprehensive medical record review. 345 sex and age-matched comparators were also identified from the same underlying population. Medical records were individually reviewed for CVD including coronary artery disease, congestive heart failure, atrial fibrillation, cerebrovascular accident, transient ischemic attack, peripheral arterial disease and abdominal aortic aneurysm. Cox proportional hazards models with adjustment for age, sex, calendar year and cardiovascular risk factors were used to compare the rate of development of CVD between cases and comparators. The prevalence of CVD before index date was not significantly different between 2 groups. Adjusting for age, sex and calendar year, the risk of incident CVD after index date was significantly elevated among patients with sarcoidosis with adjusted hazard ratio (HR) of 1.57 (95% CI, 1.15 – 2.16). Adjustment for cardiovascular risk factors yielded adjusted HR of 1.65 (95% CI, 1.08 – 2.53). Significantly increased risk was also observed for several types of CVD including coronary artery disease, congestive heart failure, atrial fibrillation and cerebrovascular accident.
Keywords: Sarcoidosis, Cardiovascular disease, Coronary artery disease, Epidemiology
Introduction
Cardiovascular disease (CVD) is one of the leading causes of morbidity and mortality worldwide. In the United States, it is responsible for 17% of national healthcare expenditure [1]. Traditional risk factors for CVD include diabetes mellitus, obesity, hypertension, dyslipidemia, age, sex, sedentary lifestyle and smoking [2].
Over the past decades, chronic inflammation has been increasingly recognized as a non-traditional risk factor for CVD as several studies have revealed a higher incidence of premature atherosclerosis among patients with chronic inflammatory diseases [3–5]. Oxidative stress and inflammatory cytokines are believed to be the cornerstone of the accelerated atherosclerosis as their deleterious effects on endothelial function have been consistently demonstrated [6, 7].
Sarcoidosis is an inflammatory disorder of unknown etiology that can virtually affect any organ. The prevalence and clinical manifestations of sarcoidosis vary across different populations [8, 9]. Presence of non-caseating granuloma is the histopathological hallmark of the disease. The clinical course of sarcoidosis can range from an acute self-limited process to progressive organ dysfunction with significant morbidity and mortality.
Patients with sarcoidosis might also have a higher risk of CVD. A coding-based study from Sweden has found an increased risk of coronary artery disease among patients with sarcoidosis with standardized incidence ratio of 1.15 (95% confidence interval, 1.09–1.20). Nonetheless, the accuracy of the study was limited by the study design that used an administrative registry to identify the cohort and the outcome of interest without further case/event verification [10].
The current study aimed to better characterize the risk of CVD among patients with sarcoidosis using the previously identified cohort of patients with incident sarcoidosis in Olmsted County, Minnesota (MN), United States (U.S.) from 1976 to 2013. The incidence of CVD among patients with sarcoidosis was compared with the incidence of CVD among sex- and age-matched comparator subjects without sarcoidosis randomly selected from the same population.
Methods
Participants and study design
Through the resources of the Rochester Epidemiology Project (REP), the population of Olmsted County, MN, is well suited for investigation of the epidemiology of sarcoidosis because comprehensive medical records for all residents seeking medical care for over six decades are available. The record linkage system allows ready access to the medical records, both inpatients and outpatient, from all health care providers for the local population, including the Mayo Clinic, the Olmsted Medical Center and their affiliated hospitals, local nursing homes and the few private practitioners. The potential of this data system for use in population-based studies has previously been described [11]. This system ensures virtually complete ascertainment of all clinically recognized cases of sarcoidosis among the residents of Olmsted County, MN. The system also allows complete ascertainment of the outcomes of interest (i.e., CVD) for both cases and comparators.
The sarcoidosis cohort consists of 345 previously identified cases in Olmsted County, MN incident from 1976–2013 [12]. Potential cases were screened for sarcoidosis using diagnosis codes related to sarcoid, sarcoidosis, and contextual noncaseating granuloma, and the medical records of all potential cases were individually reviewed. Inclusion required physician diagnosis supported by histopathology and radiologic features of intrathoracic sarcoidosis, compatible clinical presentation and exclusion of other granulomatous diseases such as tuberculosis. Tissue samples were considered positive if they demonstrated non-caseating granuloma without evidence of acid-fast bacilli or fungi. The only exception to the requirement of histopathological confirmation was stage I pulmonary sarcoidosis that required only radiographic evidence of symmetric bilateral hilar adenopathy in the absence of other identifiable causes. Isolated granulomatous disease of a specific organ except for the skin was also included if there was no better alternative diagnosis [13]. Cases with a diagnosis of sarcoidosis prior to residency in Olmsted County were not included.
For each sarcoidosis patient, one comparator without sarcoidosis at the time of the patient’s sarcoidosis diagnosis was randomly selected and assigned an index date that corresponded to the sarcoidosis incidence date. Matching criteria were similar age (±3 years) and same sex.
The medical records of cases and comparators were then reviewed for cardiovascular events which included coronary artery disease (CAD), congestive heart failure (CHF), atrial fibrillation (AF), cerebrovascular accident (CVA), transient ischemic attack, peripheral arterial disease and abdominal aortic aneurysm. CAD included both nonfatal and fatal myocardial infarction (MI). Classification was based on physician diagnosis from clinical notes supported by coronary angiography. Patients who underwent coronary revascularization procedures (percutaneous transluminal coronary angioplasty and coronary artery bypass grafting) were also classified as CAD. CHF was classified based on Framingham criteria for CHF [14]. AF was classified based on electrocardiogram. CVA was defined as ischemic stroke, hemorrhagic stroke, subarachnoid hemorrhage or death from CVA. Classification required physician diagnosis verified by imaging studies or cerebrospinal fluid analysis. Transient ischemic attack was classified based on physician diagnosis and negative brain imaging study. Peripheral arterial disease was classified based on resting ankle-brachial systolic pressure index (ABI) of less than or equal to 0.9 [15]. Abdominal aortic aneurysm was classified based on imaging showing diameter of abdominal aorta of >1.5 times normal diameter [16]. Data on baseline cardiovascular risk factors, including smoking status, body mass index (BMI), diabetes mellitus, hypertension and dyslipidemia, were also collected. The 10 year general Framingham risk score for CVD was calculated and the office-based 10 year Framingham risk score, which does not include laboratory values, was used when lipid values were unavailable [17]. Data on treatment with disease modifying anti-rheumatic agents (DMARDs), biologic agents and glucocorticoids were also collected. Follow-up was continued until death, migration, or January 1, 2015.
Statistical analysis
Descriptive statistics (percentages, mean, etc.) were used to summarize the characteristics of each cohort. Comparisons between cohorts were performed using Chi-square and rank sum tests. Comparisons of prior CVD overall and by type were compared between cohorts using Fisher’s Exact tests to examine whether there were any increases in prevalence of CVD prior to development of sarcoidosis. The cumulative incidence of CVD overall and by type adjusted for the competing risk of death was estimated [18]. These methods are similar to the Kaplan-Meier method with censoring of patients who are still alive at last follow-up. However, patients who die before experiencing CVD events are appropriately accounted for to avoid overestimation of the rate of occurrence of CVD events, which can occur if such subjects are simply censored at death. For each type of CVD, patients whose first occurrence was prior to the diagnosis of sarcoidosis, or prior to the index date for subjects in the non-sarcoidosis comparison cohort, were excluded from the analysis of cumulative incidence. For the estimation of the cumulative incidence of CVD overall, the date of occurrence was the earliest date of occurrence of the any of the individual CVD events.
Cox proportional hazards models were used to compare the rate of development of CVD, individually and in combination, between patients with sarcoidosis and the non-sarcoidosis comparison cohort. In addition, Cox proportional hazards models were used to assess the association of risk factors on the development of CVD events among patients with sarcoidosis. Risk factors of interest included those described above. Only events affecting an adequate number of patients (n ≥ 20) were examined in these risk factor analyses. Additional analyses to investigate the association between treatment (DMARDs, biologic agents or glucocorticoids) and CVD were also performed among patients with sarcoidosis. Treatment was modeled as a time-dependent covariate. A p-value of less than 0.05 was considered statistically significant for all analyses. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of cases and comparators are described in table 1. Mean length of follow-up was 15.1 years and 16.8 years for cases and comparators, respectively, corresponding to 5,210 and 5,863 total person-years of observation. There were more African-Americans among cases while there were more current smokers among comparators. Mean BMI of cases was higher than comparators but there was no difference in prevalence of other cardiovascular risk factors.
Table 1.
Characteristics of sarcoidosis cases and comparators at sarcoidosis incidence/index date
| Cases (N = 345) | Comparators (N = 345) | P value | |
|---|---|---|---|
|
| |||
| Mean age in years (±SD) | 45.6 (±13.6) | 45.4 (±13.7) | 0.87 |
|
| |||
| Female sex | 174 (50%) | 174 (50%) | 1.0 |
|
| |||
| Ethnicity | <0.001 | ||
| Caucasian | 301 (90%) | 327 (95%) | |
| African-American | 18 (5%) | 2 (1%) | |
| Asian | 6 (2%) | 0 (0%) | |
| Native American | 2 (1%) | 0 (0%) | |
| Other | 7 (2%) | 14 (4%) | |
| Unknown | 11 | ||
|
| |||
| Mean length of follow-up in years (±SD) | 15.1 (±10.5) | 16.8 (±10.8) | -- |
|
| |||
| Smoking status | <0.001 | ||
| Never | 198 (60%) | 132 (42%) | |
| Ex-smoker | 71 (21%) | 70 (22%) | |
| Current smoker | 63 (19%) | 115 (36%) | |
| Unknown | 13 | 28 | |
|
| |||
| Mean BMI in kg/m2 (±SD) | 29.8 (±7.3) | 27.5 (±5.6) | <0.001 |
|
| |||
| Hypertension | 75 (22%) | 75 (22%) | 1.0 |
|
| |||
| Dyslipidemia | 50 (14%) | 57 (17%) | 0.46 |
|
| |||
| Diabetes mellitus | 31 (9%) | 26 (8%) | 0.49 |
|
| |||
| Mean Framingham risk score (±SD) | 8.5 (±9.0) | 9.3 (±11.2) | 0.78 |
SD indicates standard deviation; BMI, body mass index
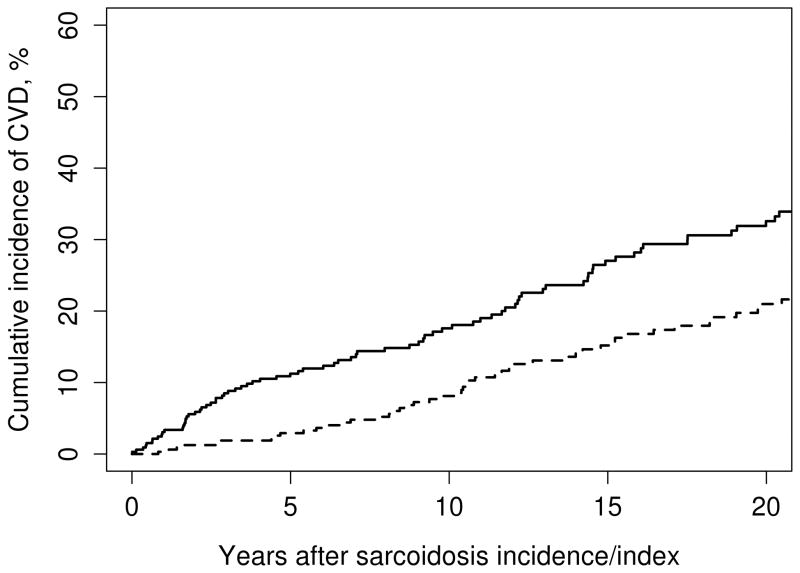
The prevalence of overall CVD prior to date of diagnosis/index date was not significantly different between patients with sarcoidosis and non-sarcoidosis comparison cohort (3.8% and 6.1%, respectively; p = 0.22). Cumulative incidence of CVD after diagnosis/index date for cases and comparators is shown in figure 1. The risk of incident CVD adjusted for age, sex and calendar year was significantly higher among patients with sarcoidosis with HR of 1.57 (95% CI, 1.15 – 2.16). Significantly elevated risk was observed in several subtypes of CVD including CAD, CHF, AF and CVA. Further adjustment for current smoker, obesity, diabetes mellitus, hypertension and dyslipidemia slightly increased HR of overall CVD to 1.65 (95% CI, 1.08 – 2.53).
Figure 1.
Cumulative incidence of overall cardiovascular disease among patients with sarcoidosis (solid line) and comparators without sarcoidosis (dashed line).
To reduce the likelihood of surveillance bias, sensitivity analysis including only CVD events that occurred at least 6 months after date of diagnosis/index date was performed. This resulted in exclusion of 5 overall CVD events among the cases and no events among the comparators. The HR for overall CVD from this sensitivity analysis adjusted for age, sex and calendar year was 1.50 (95% CI, 1.09 – 2.06). Significantly elevated risk was observed in CAD, CHF, AF and CVA, similar to the complete analysis. The numbers of events, cumulative incidence at 10 years and HR of individual CVD comparing patients with sarcoidosis with subjects without sarcoidosis adjusted for demographics and demographics plus baseline cardiovascular risk factors are demonstrated in table 2.
Table 2.
Numbers of events and hazard ratio of individual CVD comparing patients with sarcoidosis with subjects without sarcoidosis
| Subtype of cardiovascular disease | Number of events after index date for case/comparator | Cumulative incidence at 10 years for cases (95% CI) | Cumulative incidence at 10 years for comparators (95% CI) | HR (95% CI) for all events after index date, adjusting for age, sex and calendar year | HR (95% CI) for all events after index date, adjusting for age, sex, calendar year, current smoking, DM, HTN, DLP and obesity |
|---|---|---|---|---|---|
| CAD | 54/38 | 8.3 (5.0, 11.5) | 5.3 (2.6, 7.9) | 1.55 (1.02 – 2.35) | 1.58 (0.89 – 2.80) |
| CHF | 45/24 | 7.6 (4.5, 10.7) | 1.8 (0.2, 3.4) | 2.06 (1.25 – 3.38) | 2.72 (1.46 – 5.06) |
| AF | 33/18 | 4.6 (2.0, 7.1) | 2.2 (0.4, 4.0) | 1.93 (1.08 – 3.43) | 1.90 (0.97 – 3.93) |
| CVA | 32/14 | 5.2 (2.6, 7.7) | 0.6 (0.0, 1.4) | 2.51 (1.34 – 4.71) | 3.32 (1.53 – 7.18) |
| TIA | 6/4 | 1.0 (0.0, 2.2) | 0.4 (0.0, 1.1) | 1.68 (0.47 – 5.96) | 2.12 (0.33 –13.62) |
| PAD | 13/9 | 2.0 (0.4, 3.6) | 0.6 (0.0, 1.4) | 1.55 (0.66 – 3.63) | 2.09 (0.68 – 6.42) |
| AAA | 4/4 | 1.0 (0.0, 2.1) | 0.6 (0.0, 1.5) | 1.13 (0.28 – 4.53) | 1.83 (0.26 –12.60) |
| Any CVD | 96/65 | 17.6 (12.9, 22.0) | 8.1 (4.8, 11.3) | 1.57 (1.15– 2.16) | 1.65 (1.08 –2.53) |
AAA, abdominal aortic aneurysm; AF, atrial fibrillation; CAD indicates coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; HR, hazard ratio; PAD, peripheral arterial disease; TIA, transient ischemic attack; DM, diabetes mellitus; HTN, hypertension; DLP, dyslipidemia; CI, confidence interval
Additional analyses to compare the risk of CVD, individually and in combination, between patients with sarcoidosis who had lower and higher inflammatory burden were performed. The requirement to use immunosuppressive therapy (DMARDs, biologic agents or glucocorticoids) was used as the surrogate for higher inflammatory burden. Treatment was modeled as a time-dependent covariate. 151 patients (44%) required immunosuppressive therapy at some point during follow-up. The HR for overall CVD between those who required and did not require immunosuppressive therapy adjusted for age, sex and calendar year was 1.25 (95% CI, 0.83 – 1.90). HRs of individual CVD were also not statistically significant as demonstrated in table 3.
Table 3.
Hazard ratios for association between treatment (any DMARDs, biologic and/or glucocorticoid) and individual CVD in 345 patients with sarcoidosis in 1976–2013.
| Subtype of cardiovascular disease | HR (95% CI), adjusting for age, sex calendar year of sarcoidosis incidence/index date | p-value |
|---|---|---|
| CAD | 1.25 (0.72, 2.18) | 0.43 |
| CHF | 1.58 (0.86, 2.90) | 0.14 |
| AF | 0.76 (0.37, 1.56) | 0.46 |
| CVA | 0.92 (0.45, 1.89) | 0.83 |
| TIA | 0.27 (0.03, 2.38) | 0.24 |
| PAD | 2.22 (0.71, 6.93) | 0.17 |
| AAA | 0.51 (0.05, 5.49) | 0.58 |
| Any CVD | 1.25 (0.83, 1.90) | 0.29 |
AAA, abdominal aortic aneurysm; AF, atrial fibrillation; CAD indicates coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; HR, hazard ratio; PAD, peripheral arterial disease; TIA, transient ischemic attack; DM, diabetes mellitus; HTN, hypertension; DLP, dyslipidemia; CI, confidence interval; DMARDs, disease modifying agent anti-rheumatic drugs
Discussion
Over the past few decades, mortality from organ damage and other complications directly related to disease pathology has been declining in patients with immune-mediated diseases because of advance in therapy attributable to the better understanding of disease pathogenesis. As a result, those patients live longer and CVD has emerged as a major cause of morbidity and mortality [19].
The current study is the first population-based study with comprehensive medical record review to demonstrate the increased incidence of CVD among patients with sarcoidosis. Increased risk was observed after adjusting for baseline cardiovascular risk factors and after excluding events that occurred within 6 months after date of diagnosis/index date to reduce the likelihood of surveillance bias.
Premature atherosclerosis associated with inflammation is probably the key driver behind this increased risk [6, 7]. While not specifically studied in sarcoidosis, it is likely that several inflammatory cytokines, such as interleukin-1 and tumor necrosis factor, contribute to atherogenesis in this disease as well as other systemic inflammatory diseases through a number of mechanisms including vascular adhesion molecule-1, leukocyte and matrix metalloproteinase activation [20, 21]. Furthermore, chronic inflammation is known to promote initiation and propagation of coagulation cascade, downregulate anti-coagulant pathway and inhibit fibrin removal [22, 23]. Nonetheless, the additional analyses comparing patients with higher and lower disease activity using the requirement of immunosuppressive therapy as the surrogate failed to show a significantly different CVD risk between the two groups which might suggest that other underlying mechanisms, in addition to the increased inflammatory burden, are also responsible for the elevated CVD risk.
It is conceivable that there is a shared genetic predisposition to both immune-mediated inflammatory disease and CVD. This shared genetic predisposition theory is well recognized in rheumatoid arthritis (RA) as its major risk gene, HLA-DRB1, is also associated with increased risk of CAD [24, 25]. HLA-DRB1 appears to predispose to development of RA by promoting the selection and survival of autoreactive CD4+ T cells which can be found in both synovial tissue and atherosclerotic plaque [26]. Interestingly, a large case-control study investigating possible etiologies of sarcoidosis found that DRB1*1101 was significantly associated with increased risk of sarcoidosis in both African-Americans and Caucasians [27]. The data are preliminary and further investigations would be required to evaluate a possible shared genetic predisposition between sarcoidosis and CVD.
Glucocorticoids, the most commonly used medication in management of sarcoidosis, may also be a contributing factor to the increased risk of CVD as their long-term use is associated with several traditional CVD risk factors such as diabetes mellitus, hypertension, and dyslipidemia [28].
The major strengths of this study are that it is a population-based study using a comprehensive record-linkage system that allows capture of nearly all the clinically recognized cases of sarcoidosis in the community. This approach minimizes referral bias and allows capture of the complete spectrum of the disease. The diagnosis of sarcoidosis was verified by medical record and histopathology report review which minimized the likelihood of misclassification, a common concern in coding-based studies. The cohorts also had a long length of follow-up with the mean duration of follow-up of over 15 years. An adequate duration of follow-up is of particular important for CVD study as the events of interest might not occur until several years after index date/date of diagnosis.
The major limitations are those inherent in the retrospective nature of the study. Clinical information of the cohorts was not systematically obtained and, thus, all the pertinent clinical data might not be recorded. Cardiac involvement of sarcoidosis could mimic signs and symptoms of some events of interest including CAD, CHF and AF. It was not possible to retrospectively interrogate a putative relationship of these events to cardiac sarcoidosis. Therefore, it is possible that there were misclassifications which could lead to falsely elevated incidence rates. The analyses did not adjust for several potential confounders such as depression, healthcare access and socioeconomic status. Surveillance bias might play a role as patients with sarcoidosis, because of their illness, might undergo more medical examinations and laboratory investigations. However, sensitivity analysis that included only cardiovascular events that occurred at least 6 months after index date did not yield significantly different results. The results of this study might not be generalizable to other populations as clinical phenotype and epidemiology of sarcoidosis varied among different racial groups [8] but the population of Olmsted County is predominately of Northern European ancestry. Moreover, there is a higher proportion of health-care workers and correspondingly higher education level in this population which might affect pattern of healthcare utilization and disease detection.
Conclusion
Increased incidence of CVD among patients with sarcoidosis was demonstrated in this population-based cohort, even after controlling for baseline traditional atherosclerotic risk factors.
Take home message.
Patients with sarcoidosis have a higher risk of cardiovascular diseases
Acknowledgments
Funding: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement for all authors: We do not have any financial or non-financial potential conflicts of interest.
COMPETING INTEREST
All authors have disclosed no conflict of interest.
CONTRIBUTION
All authors have role in conducting the research and writing the manuscript. The final version of this manuscript is approved by all authors.
ETHIC APPROVAL
This study is approved by Institutional Review Board of Mayo Clinic and Olmsted Medical Center. The need for informed consent was waived.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 4.Bessant R, Hingorani A, Patel L, et al. Risk of coronary heart disease and stroke in a large British cohort of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2004;43:924–929. doi: 10.1093/rheumatology/keh213. [DOI] [PubMed] [Google Scholar]
- 5.Ungprasert P, Suksaranjit P, Spanuchart I, et al. Risk of coronary artery disease in patients with idiopathic inflammatory myopathies: a systematic review and meta-analysis of observational studies. Semin Arthritis Rheum. 2014;44:63–67. doi: 10.1016/j.semarthrit.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Nature. 2012;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 7.Montecucco F, Mach F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology (Oxford) 2009;48:11–22. doi: 10.1093/rheumatology/ken395. [DOI] [PubMed] [Google Scholar]
- 8.Rybicki BA, Major M, Popovich J, Jr, et al. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 9.Cozier YC, Berman JS, Palmer JR, et al. Sarcoidosis in black women in the United States: Data from the black women’s health study. Chest. 2011;139:144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zöller B, Li X, Sundquist J, Sundquist K. Risk of subsequent coronary heart disease in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. PLoS One. 2012;7:e33442. doi: 10.1371/journal.pone.0033442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: Half a century of medical records linkage in a U.S. population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ungprasert P, Carmona EM, Utz JP, et al. Epidemiology of sarcoidosis 1946–2013: A population-based study. Mayo Clin Proc. 2016;91:183–188. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costabel U, Hunninghake GW ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735–737. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 14.Ho KLK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 15.Muir RL. Peripheral arterial disease: Pathophysiology, risk factors, diagnosis, and prevention. J Vasc Nurs. 2009;27:26–30. doi: 10.1016/j.jvn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Moxon JV, Parr A, Emeto TI, et al. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol. 2010;35:512–548. doi: 10.1016/j.cpcardiol.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an update meta-analysis. Lupus. 2016 Jan 24; doi: 10.1177/0961203315627202. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Cybulsky MI, Iiyama K, Li H, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajavashisth TB, Liao JK, Galis ZS, et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30:5–6. 8–9. [PubMed] [Google Scholar]
- 23.Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015;113:1176–1183. doi: 10.1160/TH14-06-0563. [DOI] [PubMed] [Google Scholar]
- 24.Kurko J, Besenyei T, Laki J, et al. Genetics of rheumatoid arthritis – a comprehensive review. Clin Rev Allergy Immunol. 2013;45:170–179. doi: 10.1007/s12016-012-8346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paakkanen R, Lokki ML, Seppanen M, et al. Proinflammatory HLA-DRB1*01-haplotype predisposes to ST-elevation myocardial infarction. Atherosclerosis. 2012;221:461–466. doi: 10.1016/j.atherosclerosis.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Crowson CS, Liao KP, Davis JM, 3rd, et al. Rheumatoid arthritis and cardiovascular disease. Am Heart J. 2013;166:622–628. e1. doi: 10.1016/j.ahj.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossman MD, Thompson B, Federick M, et al. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet. 2003;73:720–735. doi: 10.1086/378097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maradit Kremers H, Reinalda MS, Crowson CS, et al. Glucocorticoids and cardiovascular and cerebrovascular events in polymyalgia rheumatica. Arthritis Rheum. 2007;57:279–286. doi: 10.1002/art.22548. [DOI] [PubMed] [Google Scholar]