Abstract
Background
Age-related peripheral nervous system (PNS) impairments are highly prevalent in older adults. Although sensorimotor and cardiovascular autonomic function have been shown to be related in persons with diabetes, the nature of the relationship in general community-dwelling older adult populations is unknown.
Methods
Health, Aging and Body Composition participants (n=2,399, age=76.5±2.9 years, 52% women, 38% black) underwent peripheral nerve testing at the 2000/01 clinic visit. Nerve conduction amplitude and velocity were measured at the peroneal motor nerve. Sensory nerve function was assessed with vibration detection threshold and monofilament (1.4-g/10-g) testing at the big toe. Symptoms of lower-extremity peripheral neuropathy were collected by self-report. Cardiovascular autonomic function indicators included postural hypotension, resting heart rate (HR), as well as HR response to and recovery from submaximal exercise testing (400m walk). Multivariable modeling adjusted for demographic/lifestyle factors, medication use and comorbid conditions.
Results
In fully adjusted models, poor motor nerve conduction velocity (<40 m/sec) was associated with greater odds of postural hypotension, (OR=1.6, 95% CI: 1.0–2.5), while poor motor amplitude (<1 mV) was associated with 2.3 beats/min (p=0.003) higher resting HR. No associations were observed between sensory nerve function or symptoms of peripheral neuropathy and indicators of cardiovascular autonomic function.
Conclusions
Motor nerve function and indicators cardiovascular autonomic function remained significantly related even after considering many potentially shared risk factors. Future studies should investigate common underlying processes for developing multiple PNS impairments in older adults.
Keywords: autonomic nervous system, peripheral nerves, older adults
Introduction
The peripheral nervous system (PNS) consists of two distinct divisions: sensorimotor and autonomic. Both play integral roles in providing information to the central nervous system, appropriately responding to stimuli, and controlling automatic functions of the body, but these divisions are typically examined as separate entities. PNS aging is characterized by a phenomenon known as “selective vulnerability” in which locally specific structural and functional changes can vastly affect some groups of neurons while leaving others relatively intact.1 In particular, long, myelinated axons (like those innervating the lower limbs) are vulnerable to damage. PNS impairments are often present with diabetes or specific neurological conditions, nevertheless, age-related impairments and declines are prevalent even in the absence of any pathologic condition.2
Recent work from the Health, Aging and Body Composition Study (Health ABC) indicated that over half of mobility-intact older adults in this cohort have lower extremity sensorimotor peripheral nerve (PN) impairments.3 These impairments are associated with worse physical performance,4 walking endurance5, lower extremity muscle power6 and strength,7,8 as well as increased risk of falls9 and incident mobility limitation.3 Cardiovascular autonomic impairments inhibit the ability of the cardiovascular system to appropriately respond to stimuli and maintain homeostasis. Impairments, such as postural hypotension, increase with age affecting upwards of 20% of persons aged 80 and over,10 and are associated with falls, cardiovascular events and death.11–19
Damage to nerve fibers in diabetes has largely been attributed to hyperglycemia and poor metabolic control;20–22 yet oxidative stress,23 advanced glycation end products24,25 and cardiovascular risk factors26–28 may jointly contribute to PNS impairments. Work in populations with diabetes indicates that worse sensorimotor function is associated with an increased risk of cardiovascular events.29,30 Those with cardiovascular autonomic neuropathy were more likely to also have worse motor and sensory nerve function,31 diabetic peripheral neuropathy,28 and were more likely to develop diabetic foot ulcers.32 Additionally, increasing severity of cardiovascular autonomic neuropathy has been linked to a higher prevalence of peripheral neuropathy.33,34 However, the relationship between sensorimotor and cardiovascular autonomic function is largely unexplored in general populations of older adults, and examining this relationship was the aim of our study. Because of the common underlying risk factors associated with sensorimotor and autonomic peripheral nerve impairments (particularly cardiovascular risk factors), it is plausible that those with worse sensorimotor function may also have worse cardiovascular autonomic function. We hypothesize that systemic factors may potentially drive late life changes in peripheral nervous system function and may contribute to joint declines in sensorimotor and cardiovascular autonomic function.
2.0 Methods
2.1 Participants
Participants were from the Health, Aging and Body Composition Study (Health ABC) 2000/01 clinic visit. Health ABC is a longitudinal cohort study of black and white, initially well-functioning, community-dwelling older men and women from Pittsburgh, PA and Memphis, TN, which investigated body composition and factors related to functional limitations and disability (n=3075; age 70–79; 48.4% male; 41.6% black at baseline in 1997/98).35 Participants were recruited via mailings to a random sample of white Medicare beneficiaries and to all age-eligible black Medicare beneficiaries and community residents. Eligibility criteria included: no self-reported difficulty in walking ¼ mile, climbing 10 steps, or performing any basic activity of daily living; no life-threatening cancers; and plans to remain in the study area for at least three years. Of the 2,404 participants completing the 2000/01 clinic visit, 2,399 underwent PN testing and were considered for this analysis (Figure 1). All participants provided written informed consent before participating. Protocols were approved by institutional review boards at the University of Pittsburgh and University of Tennessee Health Science Center. All study measures outlined in this paper occurred at the 2000/01 clinic visit on the same day unless stated otherwise. The 2000/01 clinic visit began in the morning because participants came in fasting for blood draws.
Figure 1.
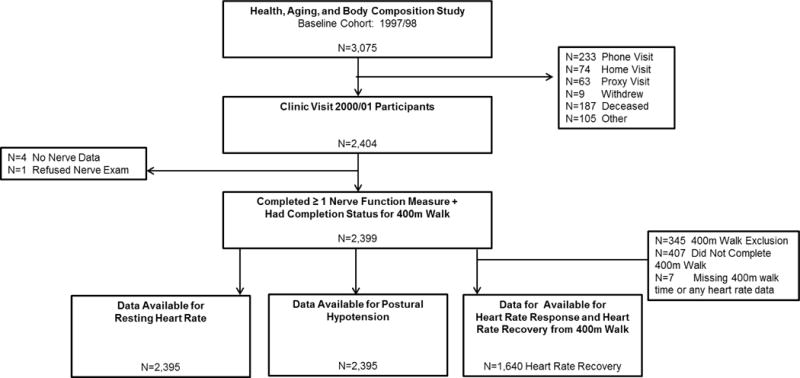
Participant Flow Chart for the 2000/01 Health ABC Clinic Visit.
Note: Participants were considered for inclusion if they completed at least one sensorimotor peripheral nerve function measure (N=2,399), though the sample size varies for each of these different measures: N=2,356 completed monofilament testing; N=2,314 for vibration threshold testing; N=1,865 completed motor amplitude testing, with N=1,757 with motor nerve conduction available, as motor nerve conduction velocity could only be determined in participants with detectable motor amplitude >0 mV, N=1,820.
2.2 Sensorimotor Nerve Function Exam
After warming the participant’s right foot (unless contraindicated, in which case testing was performed on the left side) to 30°C, testing was performed by trained examiners at both study sites. Peroneal motor nerve conduction responses were obtained at the extensor digitorum brevis muscle with stimulation at the popliteal fossa using the NeuroMax 8 (XLTEK, Oakville, Ontario, Canada). Amplitude (millivolts) and motor nerve conduction velocity (meters/second) were recorded, with oversight of certain readings by a board certified neurologist as previously described.36 Vibration detection threshold (micrometers) was measured at the bottom of the great toe with a VSA-3000 Vibratory Sensory Analyzer (Medoc, Durham, North Carolina). Touch sensitivity was assessed using a standard 10-g and light touch 1.4-g monofilament (insensitivity defined as the inability to detect at least 3 of 4 touches; insensitivity to 1.4g or 10g was combined in analyses) at the dorsum of the great toe. The entire clinic exam was performed in the same order for all participants. Self-report symptoms of peripheral neuropathy included: numbness, “asleep feeling,” prickly feeling or tingling; sudden stabbing, burning, or deep aches; or an open persistent sore or gangrene on either the foot or leg in the past 12 months. Poor motor nerve amplitude and poor conduction velocity were defined as <1mV and <40m/sec, respectively37. Poor vibration threshold was defined as >130 micrometers, indicating that the participant was unable to detect the vibration sensation. These measures have been previously described in this cohort as being associated with worse longitudinal quadriceps strength7 and incident mobility limitation.3 In addition to these cut-points, continuous versions were also included in models to determine whether a continuous relationship existed in addition to a threshold effect. Participants (N=2,399; Figure 1) were considered for inclusion in analyses if they completed ≥1 sensorimotor PN test (N=2,356 completed monofilament testing; N=2,314 for vibration threshold testing; N=1,865 completed motor amplitude testing, with N=1,757 with motor nerve conduction available, as motor nerve conduction velocity could only be determined in participants with detectable motor amplitude >0 mV, N=1,820).
2.3 Cardiovascular Autonomic Function
Blood pressure measurements were taken first while the participant was in a seated, resting position. The participant then stood from the seated position, and blood pressure was then taken one minute after transitioning to a standing position. Postural hypotension was defined as either lowering ≥20 mmHg of systolic or ≥10mmHg diastolic blood pressure between seated and standing measurements (n=2,395; Figure 1). This definition is similar to that set by the American Academy of Neurology38, though our standing measure was after one minute rather than three minutes in the consensus statement. Diastolic postural hypotension assessed in this manner was previously found to predict falls in this cohort.39 Resting heart rate (HR) was measured during a resting electrocardiogram (n=2,395; Figure 1) and analyzed as a continuous measure.
HR responses to a fast-paced submaximal 400m walking test (the Long Distance Corridor Walk) were also assessed,40 with only the subset of participants completing the full 400m included in analyses (n=1,640; Figure 1). HR response was defined as the difference between resting HR and HR at the end of the 400m walk. HR recovery was defined as the difference between HR at the end of the 400m walk and HR 2-minutes post-test. These methods have been used previously in assessing the association between 400m walk performance and incident cardiovascular disease, mortality, and mobility limitation.41 HR parameters from submaximal exercise are influenced by exertion; thus, models were adjusted for 400m walk completion time, as appropriate.
2.4 Covariates
Age, sex, race, and clinical site were included as demographic characteristics. Factors which may potentially influence sensorimotor or cardiovascular function were considered as possible covariates, and were measured at the 2000/01 clinic visit unless otherwise stated. Lifestyle factors assessed via self-report included: smoking history (never/former/current; in 1999/2000), current drinking frequency (drinks/week; at baseline), and physical activity (kilocalories/week walking and stair climbing). Weight and height were measured using a standard physician’s balance scale and stadiometer, respectively. Ankle brachial index <0.9 indicated peripheral arterial disease and ≥1.3 arterial stiffening.42 Hypertension was defined by self-report, medication use, or measured systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Diabetes was defined as self-reported physician diagnosis, hypoglycemic medication use, or fasting glucose ≥126 mg/dL, while impaired fasting glucose was defined as fasting glucose level of >100 to ≤126 mg/dL.43 Total cholesterol, Interleukin-6 (IL-6), and C-reactive protein (CRP) were obtained at the 2000/01 clinic visit as described for the study baseline visit.44 Poor vitamin B12 was defined as <260 pmol/L45 and insufficient renal function was defined as Cystatin-C >1 mg/dL.46 Blood samples were collected by venipuncture after an overnight fast.
Other conditions assessed at baseline (1997/98) included cardiovascular disease (bypass/coronary artery bypass graft, carotid endarterectomy, myocardial infarction, angina, or congestive heart failure), Parkinson’s disease, or cerebrovascular disease (transient ischemic attack or stroke). Depressive symptoms were collected using the Center for Epidemiologic Studies Depression Scale.47 Medications from the detailed medication inventory collected in 1999/2000 included: beta-blockers, anti-hypertensive medications (based on the Iowa Drug Information Service database), calcium-channel blockers, tricyclic antidepressants and statins.
2.5 Statistical Methods
Descriptive statistics were calculated and stratified by diabetes since it is a major risk factor for PN impairments. Means and standard deviations were compared using t-tests for normally distributed descriptive factors, while medians and interquartile range were reported and nonparametric tests used where appropriate. Proportions and chi-squared tests (or Fisher’s exact test) compared categorical variables. Spearman correlations between sensorimotor and cardiovascular autonomic function were examined, with partial correlations adjusting for diabetes status in all comparisons, and for 400m walk completion time in correlations involving HR response and HR recovery. Further analyses were stratified if evidence existed that the relationship between peripheral nerve function and indicators of cardiovascular autonomic function varied by diabetes. Logistic regression modeled the association between sensorimotor function and odds of postural hypotension. Linear regression modeled associations between sensorimotor function with resting HR, HR response and HR recovery from the 400m walk. Minimally adjusted models for age, sex, race, site, and diabetes were used to build final adjusted models by progressively adding covariates using manual stepwise techniques. Only factors reaching significance of p<0.05 were included in the final, parsimonious model; however age, sex, race, site, and diabetes was retained in final models regardless of significance. Sensorimotor function measures are weakly correlated (e.g., r=0.32, p<0.001, between motor amplitude and conduction velocity), therefore separate models were built for each. Sensitivity analyses removing 1) participants with diabetes from all models and 2) participants taking any medication potentially influencing heart rate (beta-blocker, calcium-channel blocker, and tricyclic antidepressants) in models of resting HR and HR response were performed. Analyses were run using SAS version 9.3 (SAS Institute, Cary, NC).
3.0 Results
Participants with diabetes were more likely to be male and black, have higher BMI, report more chronic health conditions and depressive symptoms, have higher values of IL-6, lower cholesterol, were less likely to complete the 400m walk and were more likely to take statins and anti-hypertensive medications (Table 1). Participants with diabetes generally had worse sensorimotor and cardiovascular autonomic function (Table 2). Several measures of poor motor and sensory PN function were significantly, albeit weakly, correlated with poor cardiovascular autonomic function, with the exception of monofilament insensitivity and reported symptoms of peripheral neuropathy (Table 3).
Table 1.
Participant Characteristics Stratified by Diabetes Status
| Overall | No Diabetes | Diabetes | P-Value | |
|---|---|---|---|---|
| Participant Characteristics | N=2,399 | N=1,878 | N=521 | |
| Pittsburgh Site, N (%) | 1,224 (51.0) | 962 (51.2) | 262 (50.3) | 0.73 |
| Age, Mean ± SD | 76.5 ± 2.9 | 76.5 ± 2.9 | 76.5 ± 2.8 | 0.86 |
| Women | 1,240 (51.7) | 1,011 (53.8) | 229 (44.10 | <0.001 |
| Black Race | 917 (38.2) | 646 (34.4) | 271 (52.0) | <0.001 |
| Health Fair or Poor | 346 (14.4) | 234 (12.5) | 112 (21.5) | <0.001 |
| Body Mass Index | 27.3 ± 4.8 | 26.8 ± 4.6 | 29.1 ± 4.8 | <0.001 |
| Lifestyle Factors | ||||
| Current Smoker | 160 (7.0) | 130 (7.2) | 30 (6.1) | 0.33 |
| Drink ≥1 Drink/Week | 709 (30.1) | 601 (32.6) | 108 (21.2) | <0.001 |
| Physical Activity (kcal/kg/week) | 2.2 (0.2–7.0) | 2.5 (0.3–7.3) | 1.4 (0.1–5.1) | <0.001 |
| Prevalent Diseases and Conditions | ||||
| Hypertension | 1,912 (79.7) | 1,465 (78.0) | 447 (85.8) | <0.001 |
| Parkinson’s Disease | 14 (0.6) | 11 (0.6) | 3 (0.6) | 0.99 |
| Coronary Heart Disease | 395 (16.8) | 283 (15.3) | 112 (22.0) | <0.001 |
| Cerebrovascular Disease | 158 (6.8) | 118 (6.5) | 40 (7.9) | 0.26 |
| Peripheral Arterial Disease | 372 (16.2) | 255 (14.1) | 117 (23.7) | <0.001 |
| Arterial Stiffening | 118 (5.1) | 78 (4.3) | 40 (8.1) | |
| Impaired Fasting Glucose | 385 (16.1) | 385 (20.5) | — | NA |
| Insufficient Renal Function* | 655 (28.0) | 481 (26.3) | 174 (34.7) | <0.001 |
| Poor Vitamin B12 (<260pmol/L) | 391 (17.1) | 307 (17.1) | 84 (17.2) | 0.93 |
| CES-D Score**, Median (IQR) | 5 (2–9) | 4 (1–9) | 5 (2–10) | <0.001 |
| Laboratory Measures | ||||
| CRP, mg/L | 2.2 (1.0–5.2) | 2.1 (0.9–4.9) | 2.6 (1.1–6.1) | 0.006 |
| IL-6, pg/mL | 2.4 (1.5–4.2) | 2.3 (1.5–4.0) | 2.9 (1.9–4.9) | <0.001 |
| Total Fasting Cholesterol, mg/dL | 191.7 ± 37.3 | 193.2 ± 37.0 | 186.2 ± 38.2 | <0.001 |
| Medications | ||||
| Statins | 461 (19.7) | 337 (18.4) | 124 (24.6) | 0.002 |
| Beta-Blockers | 407 (17.2) | 305 (16.4) | 102 (19.9) | 0.06 |
| Calcium-Channel Blockers | 556 (23.3) | 418 (22.4) | 138 (26.9) | 0.03 |
| Anti-hypertensive Medications | 1,402 (59.0) | 1,030 (55.3) | 372 (72.7) | <0.001 |
| Tricyclic Antidepressants | 55 (2.3) | 42 (2.3) | 13 (2.5) | 0.70 |
Note: Values are mean ± SD, median (interquartile range), n (%). Percentages were calculated using the number of participants with complete values for each covariate rather than the entire sample. Missing covariate data was <5%.
Cystatin-C >1mg/dL,
Centers for Epidemiologic Studies Depression Scale.
Table 2.
Sensorimotor and Cardiovascular Autonomic Function by Diabetes Status
| Overall | No Diabetes | Diabetes | P-Value | |
|---|---|---|---|---|
| N=2,399 | N=1,878 | N=521 | ||
| Motor Nerve Function | ||||
| Completed Amplitude Testing (N) | 1,865 | 1490 | 375 | |
| Amplitude, mV, mean ± SD | 3.3 ± 2.0 | 3.4 ± 2.0 | 3.0 ± 1.9 | <0.001 |
| Poor amplitude (<1mV), n(%) | 210 (11.3) | 153 (10.3) | 57 (15.2) | 0.007 |
| Completed Nerve Conduction Testing | 1,757 | 1,414 | 343 | |
| Conduction velocity, m/sec | 43.6 ± 5.4 | 43.9 ± 5.3 | 42.7 ± 5.4 | <0.001 |
| Poor conduction velocity (<40m/sec) | 392 (22.3) | 296 (21.0) | 96 (28.0) | 0.005 |
| Sensory Nerve Function | ||||
| Completed Vibration Threshold Testing | 2,314 | 1,816 | 498 | |
| Vibration threshold, micometers | 51.6 ± 35.6 | 49.5 ± 34.4 | 59.3 ± 38.8 | <0.001 |
| Poor vibration threshold (>130 micrometers) | 136 (5.9) | 93 (5.1) | 43 (8.6) | 0.005 |
| Completed Monofilament Testing | 2,356 | 1,846 | 510 | |
| 1.4-g monofilament insensitivity | 868 (36.8) | 666 (36.1) | 202 (39.6) | <0.001 |
| 10-g monofilament insensitivity | 209 (8.9) | 139 (7.5) | 70 (13.7) | |
| Symptoms of Peripheral Neuropathy | ||||
| Numbness | 683 (28.6) | 487 (26.1) | 196 (37.8) | <0.001 |
| Pain/Aching | 403 (16.9) | 288 (15.4) | 115 (22.1) | <0.001 |
| Open Sores | 44 (1.8) | 27 (1.4) | 17 (3.3) | 0.006 |
| Cardiovascular Autonomic Function | ||||
| Postural Hypotension1 | 159 (6.6) | 125 (6.7) | 34 (6.6) | 0.92 |
| Resting HR, BPM | 63.2 ± 10.5 | 62.6 ± 10.0 | 65.4 ± 11.9 | <0.001 |
| Completed 400m Walking Test | 1,640 | 1,340 | 300 | |
| Heart Rate Range2 | 44.1 ± 14.0 | 44.6 ± 14.2 | 41.7 ± 12.8 | 0.002 |
| Heart Rate Recovery3 | 18.3 ± 10.2 | 18.5 ± 10.5 | 17.4 ± 9.1 | 0.07 |
Note: Values are mean ± SD, n (%), or as otherwise indicated. Percentages were calculated using the number of participants who completed the test rather than the entire sample. Missing data <5% unless sample size otherwise indicated. Participants were included in the study sample if they completed ≥1 peripheral nerve function test.
Postural hypotension defined as lowering ≥20 mmHg of systolic or ≥10mmHg diastolic blood pressure between seated and standing measurements.
HR Response: Difference between resting HR and HR at end of the 400m walk. (Only reported for participants who completed full 400m walk test).
HR Recovery: Difference between HR at end of the 400m walk and after 2 minutes recovery. (Only reported for participants who completed full 400m walk test.)
Table 3.
Correlation1 Matrix of Continuous Sensorimotor and Autonomic Function
| Cardiovascular Autonomic Function Indicators | ||||
|---|---|---|---|---|
| Sensorimotor Function | Postural Hypotension | Resting HR | HR Response | HR Recovery |
| Motor Amplitude, per standard deviation | 0.01 (N=1,863) |
−0.05 (N= 1,863) |
0.03 (N=1,328) |
0.03 (N=1,325) |
| Motor Conduction Velocity, per standard deviation | −0.09*** (N=1,756) |
0.05 (N=1,755) |
0.10*** (N=1,267) |
0.08** (N=1,264) |
| Vibration Threshold, per unit higher | 0.05* (N=2,312) |
−0.01 (N=2,311) |
−0.04 (N=1,589) |
−0.04 (N=1,586) |
| Any Monofilament Insensitivity (1.4/10g insensitivity vs. no) | 0.001 (N=2,355) |
−0.03 (N=2,354) |
−0.04 (N=1,621) |
0.01 (N=1,617) |
| Presence of Peripheral Neuropathy Symptoms (Any symptoms vs. none) | 0.005 (N=2,381) |
0.03 (N=2,381) |
0.03 (N=1,634) |
0.03 (N=1,631) |
Spearman partial correlations adjusting for diabetes status. Partial correlation coefficients shown for HR Response and HR Recovery further adjusted for 400m walk completion time.
P<0.05,
P<0.01,
P<0.001
3.1 Postural Hypotension
Poor conduction velocity (< 40m/sec) was associated with greater odds of postural hypotension, (OR=1.6, 95% CI: 1.0–2.5) after adjustment for age, sex, race, site, diabetes, and IL-6, with no additional covariates reaching model inclusion criteria (n=1,692 in the final model). Each standard deviation of slower motor nerve conduction velocity was associated with higher odds of postural hypotension (OR=1.4, 95% CI: 1.1–1.7). (Model details included in Table 4.) Diabetes did not attenuate the relationship between nerve conduction velocity and postural hypotension. No evidence of an interaction between conduction velocity and diabetes was found and diabetes was not associated with hypotension in the final models.
Table 4.
Fully Adjusted Logistic Regression Models Estimating the Effects of Motor Nerve Conduction Velocity on Postural Hypotension (n=1,692)
| Poor Motor Nerve Conduction Velocity | Continuous Motor Nerve Conduction Velocity, Effect per Standard Deviation Slower | ||||
|---|---|---|---|---|---|
| Independent Variable | Odds Ratio | 95% Confidence Interval | Independent Variable | Odds Ratio | 95% Confidence Interval |
| Poor Motor Nerve Conduction Velocity <40 m/s | 1.6 | 1.0–2.5 | Motor Nerve Conduction Velocity, Per SD Slower2 | 1.4 | 1.1–1.7 |
| Age1, Per Year Older | 0.9 | 0.9–1.0 | Age1, Per Year Older | 0.9 | 0.9–1.0 |
| Women | 1.0 | 0.7–1.6 | Women | 1.1 | 0.7–1.7 |
| Black Race | 1.0 | 0.6–1.6 | Black Race | 1.0 | 0.7–1.5 |
| Pittsburgh Site | 0.4 | 0.3–0.7 | Pittsburgh Site | 0.5 | 0.3–0.7 |
| Diabetes | 1.0 | 0.6–1.6 | Diabetes | 0.9 | 0.6–1.6 |
| IL-6 (Ln), per unit higher | 1.5 | 1.2–2.0 | IL-6 (Ln), per unit higher | 1.6 | 1.2–2.0 |
Age centered at age 76 years,
Conduction velocity, m/sec = 43.6 ± 5.4
3.2 Resting Heart Rate
Poor motor amplitude (<1 mV) was associated with 2.3 beats/min (p=0.003) higher resting HR in fully adjusted models adjusting for age, sex, race, study site, diabetes, impaired fasting glucose, beta blocker use, tricyclic antidepressant use, CRP, IL-6 and total cholesterol (n=1,729 in this final model). Although statistically significant, each standard deviation of worse motor nerve amplitude was associated with resting HR of less than one BPM higher (β=0.9, p<0.001) in fully adjusted models. (Model details included in Table 5.) No evidence existed for an interaction between diabetes and motor amplitude. In final models, though several factors were significantly related to resting heart rate, overall R2 values were low (R2=0.10) in models for continuous and poor amplitude. In sensitivity analyses which removed participants taking medications influencing heart rate (n=880), results remained similar in magnitude and direction. Results were also similar when removing participants with diabetes (n=521) for the continuous amplitude models, although the relationship between poor amplitude and higher resting HR was attenuated to non-significance (β =1.4, p=0.10).
Table 5.
Fully Adjusted Linear Regression Models Estimating the Effects of Nerve Amplitude on Resting Heart Rate (n=1,729)
| Poor Motor Nerve Amplitude, <1 mV Model R2=0.10 |
Continuous Motor Nerve Amplitude, Effect per Standard Deviation Slower Model R2=0.10 |
||||||
|---|---|---|---|---|---|---|---|
| Independent Variable | Beta Estimate | Standard Error | P-Value | Independent Variable | Beta Estimate | Standard Error | P-Value |
| Poor Motor Nerve Amplitude <1 mV | 2.3 | 0.8 | 0.003 | Motor Amplitude, per SD Lower2 | 0.9 | 0.1 | <0.001 |
| Age1, Per Year Older | 0.1 | 0.1 | 0.4 | Age1, Per Year Older | 0.1 | 0.1 | 0.5 |
| Women | 1.4 | 0.5 | 0.005 | Women | 1.5 | 0.5 | 0.002 |
| Black Race | 0.4 | 0.5 | 0.5 | Black Race | 0.5 | 0.5 | 0.3 |
| Pittsburgh Site | −1.7 | 0.5 | <0.001 | Pittsburgh Site | −1.7 | 0.5 | <0.001 |
| Diabetes Status | Diabetes Status | ||||||
| Diabetes | 2.6 | 0.6 | <0.001 | Diabetes | 2.6 | 0.6 | <0.001 |
| Impaired Fasting Glucose | 1.8 | 0.7 | 0.006 | Impaired Fasting Glucose | 2.0 | 0.7 | 0.003 |
| Beta Blocker Use | −4.9 | 0.6 | <0.001 | Beta Blocker Use | −5.0 | 0.6 | <0.001 |
| Tricyclic Antidepressant Use | 4.1 | 1.6 | 0.01 | Tricyclic Antidepressant Use | 4.3 | 1.6 | 0.008 |
| IL-6 (Ln) | 1.5 | 0.4 | <0.001 | IL-6 (Ln) | 1.5 | 0.4 | <0.001 |
| CRP (Ln) | 0.7 | 0.2 | 0.001 | CRP (Ln) | 0.7 | 0.2 | 0.001 |
| Fasting Cholesterol (per SD higher) | 0.8 | 0.3 | 0.002 | Fasting Cholesterol (per SD higher) | 0.8 | 0.3 | <0.001 |
Age centered at age 76 years
Amplitude, mV, mean ± SD = 3.3 ± 2.0
3.3 Heart Rate Response and Heart Rate Recovery
No sensorimotor PN measures were associated with HR recovery in adjusted models. (Model details included in Appendix Table A1.)
4.0 Discussion
In community-dwelling older adults, we found that certain key measures of motor function were independently associated with worse cardiovascular autonomic function. Although both comprise the PNS, the sensorimotor and autonomic divisions are not generally examined simultaneously in older adults. Motor nerve function, specifically motor amplitude and conduction velocity, were associated with postural hypotension and modestly with resting HR, respectively. Participants with diabetes had worse sensorimotor and cardiovascular autonomic function; but consistent relationships were observed for participants with and without diabetes.
The PNS contains large and small nerve fibers, each responsible for distinct aspects of neurotransmission. Within the sensorimotor division, large myelinated fibers mediate motor control as well as touch, vibration, and position perception. Small, thinly myelinated or unmyelinated fibers provide information about pain in addition to cold and warm perception, respectively. The autonomic division is made up of thinly myelinated and unmyelinated fibers, and mediates HR, blood pressure, sweating, gastrointestinal and genitourinary function. Even though the observed relationships were between different PNS nerve fiber types, our associations are consistent with the notion of selective vulnerability where long nerve fibers (like the vagus nerve and peroneal motor nerve) are particularly prone to damage.
Age typically explains a portion of the declines within and across systems. However, age was only weakly associated with postural hypotension and HR (results not shown). Furthermore, age did not significantly attenuate any of the associations between sensorimotor and autonomic function. This supports an independent relationship between sensorimotor and cardiovascular autonomic function, and the relationships we observed were not simply due to age related declines in both. Nevertheless, the age range in this cohort was only 10 years, and an age effect may be detectable in a population with a wider age range.
Additionally, our associations were not explained by factors traditionally influencing PNS function. These associations were independent of sex, race, diabetes, and prevalent diseases. In particular, obesity and metabolic and cardiovascular factors have been shown to influence sensorimotor function in older adults48 and midlife women49, as well as vascular factors like peripheral arterial disease50, however, these factors generally did not reach statistical significance in our analyses. Multivariable models included few parameters beyond age, sex, race, study site and diabetes (which we included a priori regardless of significance)—suggesting a primary association between the PNS components. Total cholesterol and certain medications known to influence heart rate reached significance in resting heart rate models, while inflammatory markers reached statistical significance in both resting heart rate and postural hypotension models. The addition of IL-6 and CRP (resting heart rate only) did not attenuate the relationship between the measures of motor nerve function and cardiovascular autonomic function. The relationship of inflammation and cardiac autonomic function is consistent with prior literature indicating that higher levels of inflammation are associated with worse heart rate variability51. However, the effects of the observed relationships were small. For example, poor motor amplitude was associated with a resting heart rate of 2.3 bpm higher, which may have limited clinical relevance. In a previous study conducted in middle-aged men, mortality risk increased by about 16% per every 10 bpm higher resting HR.15 Overall R2 values in our multivariable models were low. This may indicate potential underlying mechanisms common to the pathogenesis of sensorimotor and autonomic dysfunction that were not captured by the included covariates. Alternatively, this may simply indicate weak associations, which could be due the complex nature of both the sensorimotor and cardiovascular autonomic nervous systems.
Future work should include comprehensive examinations of each aspect of sensorimotor and autonomic function to better understand the biologic mechanisms underlying these interrelationships. Recent work in a patient population with type 2 diabetes demonstrated that those with cardiac autonomic neuropathy have worse motor and sensory nerve function.31 Our observed relationships were for motor nerve function measures only, and our study did not include sensory nerve conduction testing. Some associations may have reached statistical significance through chance alone given the multiple comparisons. However, associations that remained after multivariable adjustment were generally highly significant (particularly the relationship between continuous motor amplitude and resting heart rate), reaching alpha levels of p≤0.01.
A major strength of our study was assessing sensorimotor PN function through a variety of motor and sensory nerve function measures in addition to self-reported symptoms—an important consideration since impairments in older adults are commonly asymptomatic.52 We included many diverse factors known to influence sensorimotor or autonomic function. The measures of cardiovascular autonomic function are clinically relevant and may feasibly be administered in a variety of settings. However other measures often utilized in clinical settings—for example, expiratory-inspiratory ratio, HR variability, and tilt-table testing—were not assessed. These measures are rarely included in epidemiologic studies, and HR variability in particular can be computationally challenging to analyze and difficult to interpret. Though it has limitations, HR variability analyzed using frequency domain methods may be useful for separating the effects of sympathetic and parasympathetic modulation. Including HR variability and other sensitive measures of autonomic function may aid in clarifying associations. The measures we used therefore are surrogates of autonomic function, and may be influenced by current acute physiologic and health status, potentially resulting in residual confounding. Precise measures of postural hypotension using beat-to-beat blood pressure monitoring techniques have recently provided unique insight into the relationship between postural hypotension and falls.16,17 Future work examining nerve conduction velocity and continuous beat-to-beat monitoring assessments of postural hypotension may be an important extension of this work, particularly as both have been related to falls in older adults.9,12,16–18 Two of our measures of cardiovascular autonomic function were dependent on completing a 400m endurance walking test—the Long Distance Corridor Walk. Our prior work in this cohort has indicated that participants who were able to complete the full 400m tend to be younger and were generally healthier and had fewer chronic conditions compared to participants who were ineligible or unable to complete the full test.5 Having these performance based only on a healthier subset may have limited our ability to detect any relationship between heart rate response/recovery with the measures of sensorimotor function. Similarly, a smaller portion of participants had data available for motor nerve conduction testing compared to the other sensorimotor measures, potentially also limiting the ability to assess the relationship between nerve conduction velocity and cardiovascular autonomic function.
5.0 Conclusions
We demonstrated that certain key aspects of worse sensorimotor PN function are independently associated with poor cardiovascular autonomic function indicators (resting HR and postural hypotension) in community-dwelling older adults. The clinical relevance of these hypotension and HR findings are unknown though they may be important because of the known association between both sensorimotor and cardiac autonomic function with poor outcomes in older adults. Future studies should assess these combined, but often underappreciated, PNS impairments in older adults to better understand the shared modifiable risk factors involved.
Acknowledgments
This work was supported by the National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050 (to E.S.S.), and National Institute of Nursing Research grant R01-NR12459. This research was supported in part by the Intramural Research Program of the National Institute of Health, NIA, and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30- AG024827) Pilot Grant (to E.S.S.). Brittney S. Lange-Maia was funded by a National Institute on Aging Training Grant T32-AG000181 (to A.B.N.) during this work. Funding sources were not involved in the study design, data collection/analysis/interpretation, or in writing this manuscript. A preliminary version of this work was presented at an oral presentation at the 2015 Annual Meeting of the Gerontological Society of America in Orlando, FL.
John M. Jakicic reports the following: Principal and Co-Investigator on research grants awarded to the University of Pittsburgh by the National Institutes of Health, Jawbone, Inc. and BodyMedia, Inc.; Co-Investigator on research grants awarded to the University of Pittsburgh by the American Heart Association, Ethicon/Covidien, Weight Watchers International, and HumanScale; Weight Watchers International Scientific Advisory Board for 2015 and 2016 ILSI North American Energy Balance and Active Lifestyle Committee through August 10, 2015; Honorarium as a consultant to NovoNordisk.
Abbreviations
- PN
Peripheral nerve
- Health ABC
Health, Aging and Body Composition Study
- HR
Heart rate
Appendix 1
Table A1.
Unadjusted and Minimally Adjusted (Model 1)1 Linear and Logistic Regression Models Estimating Effects of Motor and Sensory Nerve Function on Cardiovascular Autonomic Function
| Odds of Postural Hypotension (95% CI) |
Resting Heart Rate Beta Values |
Heart Rate Response2 Beta Values |
Heart Rate Recovery2 Beta Values |
|||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 11 | Unadjusted | Model 1 | Unadjusted | Model 1 | Unadjusted | Model 1 | |
| Motor Nerve Function | ||||||||
| Motor Nerve Amplitude, per SD worse3 | N=1863 | N=1863 | N=1324 | N=1324 | ||||
| 1.0 (0.8–1.1) |
0.9 (0.8–1.1) |
0.6 P<0.01 |
0.7 P<0.01 |
−0.5 0.19 |
<0.0 P>0.99 |
−0.2 P=0.27 |
0.1 P=0.66 |
|
| Poor Motor Nerve Amplitude <1 mV | N=1863 | N=1863 | N=1324 | N=1324 | ||||
| 1.0 (0.6–1.8) |
1.0 (0.5–1.8) |
1.7 P=0.03 |
1.8 P=0.02 |
−1.1 P=0.38 |
0.3 P=0.82 |
−0.7 P=0.42 |
0.2 P=0.83 |
|
| Motor Nerve Conduction Velocity, per SD slower4 | N=1756 | N=1755 | N=1263 | N=1263 | ||||
| 1.4 (1.2–1.7) |
1.3 (1.1–1.6) |
−0.2 P=0.33 |
−0.3 P=0.25 |
−1.2 P<0.01 |
−0.2 P=0.56 |
−0.4 P=0.04 |
0.1 P=0.67 |
|
| Poor Motor Nerve Conduction Velocity <40 m/s | N=1756 | N=1755 | N=1263 | N=1263 | ||||
| 1.8 (1.2–2.7) |
1.5 (1.0–2.3) |
0.05 P=0.93 |
0.03 P=0.97 |
−1.4 P=0.11 |
0.3 P=0.73 |
−0.8 P=0.25 |
0.4 P=0.56 |
|
| Sensory Nerve Function | ||||||||
| Vibration Perception Threshold, per unit higher | N=2312 | N=2311 | N=1585 | N=1585 | ||||
| 1.0 (1.0–1.0) |
1.0 (1.0–1.0) |
0.01 P=0.29 |
0.002 N=0.73 |
−0.02 P=0.04 |
0.005 P=0.66 |
−0.01 P=0.31 |
0.01 P=0.32 |
|
| Inability to detect vibration >131 μ | N=2312 | N=2311 | N=1585 | N=1585 | ||||
| 1.5 (0.8–2.7) |
1.3 (0.7–2.4) |
1.2 P=0.20 |
0.8 P=0.37 |
−1.4 P=0.35 |
0.4 P=0.79 |
0.7 P=0.50 |
1.8 P=0.10 |
|
| 1.4 or 10-g monofilament insensitivity | N=2355 | N=2354 | N=1617 | N=1617 | ||||
| 1.0 (0.7–1.4) |
1.0 (0.7–1.4) |
−0.3 P=0.42 |
−0.4 P=0.36 |
−0.9 P=0.19 |
−0.6 P=0.4 |
0.1 P=0.81 |
0.2 P=0.63 |
|
| Peripheral Neuropathy Symptoms | ||||||||
| Any Symptoms | N=2381 | N=2381 | N=1630 | N=1630 | ||||
| 1.0 (0.8–1.5) |
1.0 (0.7–1.5) |
0.9 P=0.06 |
0.4 P=0.35 |
0.4 P=0.54 |
0.4 P=0.61 |
0.4 P=0.38 |
0.3 P=0.59 |
|
Minimally adjusted models included age, sex, race, site, and diabetes
All heart rate response and heart rate recovery models were adjusted for 400m walking test completion time.
Amplitude, mV, mean ± SD = 3.3 ± 2.0
Conduction velocity, m/sec = 43.6 ± 5.4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Coauthor Disclosures:
No other coauthors report any disclosures or conflicts of interest.
References
- 1.Cowen T, Ulfhake B, King R. Aging in the peripheral nervous system. In: Dyck P, Thomas P, editors. Peripheral neuropathy. 4th. Vol. 1. Philadelphia, PA: Elsevier Saunders; 2005. p. 22. [Google Scholar]
- 2.Verdu E, Ceballos D, Vilches J, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 3.Ward RE, Boudreau RM, Caserotti P, et al. Sensory and motor peripheral nerve function and incident mobility disability. J Am Geriatr Soc. 2014;62(12):2273–9. doi: 10.1111/jgs.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strotmeyer E, De Rekeneire N, Schwartz A, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults. Diabetes Care. 2008;31(9):17671–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange-Maia BS, Newman AB, Cauley JA, et al. Sensorimotor peripheral nerve function and the longitudinal relationship with endurance walking in the health, aging, and body composition study. Arch Phys Med Rehabil. 2016;97(1):45–52. doi: 10.1016/j.apmr.2015.08.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward RE, Caserotti P, Faulkner K, et al. Peripheral nerve function and lower extremity muscle power in older men. Arch Phys Med Rehabil. 2014;95(4):726–33. doi: 10.1016/j.apmr.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward RE, Boudreau RM, Caserotti P, et al. Sensory and motor peripheral nerve function and longitudinal changes in quadriceps strength. J Gerontol A Biol Sci Med Sci. 2015;70(4):464–70. doi: 10.1093/gerona/glu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strotmeyer ES, de Rekeneire N, Schwartz A, et al. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: The health, aging and body composition study. J Am Geriatr Soc. 2009;57(11):2004–2010. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrucci L, Bandinelli S, Cavazzini C, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116(12):807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Finucane C, O’Connell MD, Fan CW, et al. Age related normative changes in phasic orthostatic blood pressure in a large population study: Findings from the irish longitudinal study on ageing (TILDA) Circulation. 2014;130(20):1780–9. doi: 10.1161/CIRCULATIONAHA.114.009831. [DOI] [PubMed] [Google Scholar]
- 11.Chang M, Havlik RJ, Corti MC, Chaves PH, Fried LP, Guralnik JM. Relation of heart rate at rest and mortality in the women’s health and aging study. Am J Cardiol. 2003;92(11):1294–9. doi: 10.1016/j.amjcard.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein LZ, Robbins AS, Josephson KR, Schulman BL, Osterweil D. The value of assessing falls in an elderly population: A randomized clinical trial. Ann Intern Med. 1990;113(4):308–316. doi: 10.7326/0003-4819-113-4-308. [DOI] [PubMed] [Google Scholar]
- 13.Rose KM, Tyroler HA, Nardo CJ, et al. Orthostatic hypotension and the incidence of coronary heart disease: The atherosclerosis risk in communities study. Am J Hypertens. 2000;13(6):571–578. doi: 10.1016/s0895-7061(99)00257-5. [DOI] [PubMed] [Google Scholar]
- 14.Jouven X, Empana J, Schwartz P, Desnos M, Courbon D, Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 15.Jouven X, Empana JP, Escolano S, et al. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. Am J Cardiol. 2009;103(2):279–83. doi: 10.1016/j.amjcard.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 16.McDonald C, Pearce M, Kerr SR, Newton J. A prospective study of the association between orthostatic hypotension and falls: definition matters. Age Ageing. 2016:1–7. doi: 10.1093/ageing/afw227. [DOI] [PubMed] [Google Scholar]
- 17.Juraschek SP, Daya N, Appel LJ, et al. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens. 2017;30(2):188–195. doi: 10.1093/ajh/hpw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finucane C, O’Connell MDL, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc. 2017;65(3):474–482. doi: 10.1111/jgs.14563. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitz LA. Orthostatic hypotension and falls. J Am Geriatr Soc. 2017;65(3):470–471. doi: 10.1111/jgs.14745. [DOI] [PubMed] [Google Scholar]
- 20.Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 21.Motataianu A, Balasa R, Voidazan S, Baijko Z. Cardiovascular autonomic neuropathy in context of other complications of type 2 diabetes mellitus. Biomed Res Int. 2013;2013:8. doi: 10.1155/2013/507216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinik AI, Strotmeyer ES, Nakave AA, Patel CV. Diabetic neuropathy in older adults. Clin Geriatr Med. 2008;24(3):407–435. doi: 10.1016/j.cger.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler D, Bucholtz S, Sohr C, Nourooz-Zadeh J, Roden M. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol. 2015;52:65–72. doi: 10.1007/s00592-014-0601-3. [DOI] [PubMed] [Google Scholar]
- 24.Huijberts MS, Schaper NC, Schalkwijk CG. Advanced glycation end products and diabetic foot disease. Diabetes Metab Res Rev. 2008;24(Suppl 1):S19–24. doi: 10.1002/dmrr.861. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes care. 1992;15(12):1835–43. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- 26.Tesfaye S, Chaturvedi N, Eaton S, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 27.Valensi P, Paries J, Attali JR. Cardiac autonomic neuropathy in diabetic patients: Influence of diabetes duration, obesity, and microangiopathic complications–the french multicenter study. Metabolism. 2003;52(7):815–20. doi: 10.1016/s0026-0495(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 28.Elliott J, Tesfaye S, Chaturvedi N, et al. Large-fiber dysfunction in diabetic peripheral neuropathy is predicted by cardiovascular risk factors. Diabetes care. 2009;32(10):1896–900. doi: 10.2337/dc09-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brownrigg JR, de Lusignan S, McGovern A, et al. Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart. 2014;100(23):1837–43. doi: 10.1136/heartjnl-2014-305657. [DOI] [PubMed] [Google Scholar]
- 30.Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: The DIAD study: A randomized controlled trial. JAMA. 2009;301(15):1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodica B, Bajko Z, Smaranda M, Voidazan S, Anca M. Influence of risk factors and diabetic complications on peripheral nerve function in type 2 diabetes mellitus. Acta Medica Marisiensis. 2015;61(1):40–46. https://doi.org/10.1515/amma-2015-0015. [Google Scholar]
- 32.Yun JA, Cha SA, Lim TS, et al. Cardiovascular autonomic dysfunction predicts diabetic foot ulcers in patients with type 2 diabetes without diabetic polyneuropathy. Medicine. 2016;95(12) doi: 10.1097/MD.0000000000003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilal N, Erdogan M, Ozbek M, et al. Increasing severity of cardiac autonomic neuropathy is associated with increasing prevalence of nephropathy, retinopathy, and peripheral neuropathy in turkish type 2 diabetics. J Diabetes Complications. 2008;22(3):181–5. doi: 10.1016/j.jdiacomp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Traon AP, Fontaine S, Tap G, Guidolin B, Senard J, Hanaire H. Cardiovascular autonomic neuropathy and other complications in type 1 diabetes. Clin Auton Res. 2010;20:153–160. doi: 10.1007/s10286-010-0062-x. [DOI] [PubMed] [Google Scholar]
- 35.Simonsick E, Newman A, Nevitt M, et al. Measuring higher level physical function in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2001:M644–649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 36.Ward RE, Boudreau RM, Vinik AI, et al. Reproducibility of peroneal motor nerve conduction measurement in older adults. Clin Neurophysiol. 2013;124(3):603–9. doi: 10.1016/j.clinph.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maser RE, Viggo K, Dorman JS, Drash AL, Becker DJ, Orchard TJ. Measuring subclinical neuropathy: Does it relate to clinical neuropathy? Pittsburgh epidemiology of diabetes study-V. J Diabet Complications. 1991;5(1):6–12. doi: 10.1016/0891-6632(91)90003-8. [DOI] [PubMed] [Google Scholar]
- 38.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz A, Vittinghoff E, Sellmeyer D, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31(3):391–396. doi: 10.1007/s10286-011-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: The health ABC long distance corridor walk. J Am Geriatr Soc. 2001;49(11):1544–8. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 41.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 42.Aboyans V, Criqui M, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 44.Cesari M, Penninx BWJH, Newman AB, et al. Inflammatory markers and cardiovascular disease (the Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92(5):522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 45.Leishear K, Boudreau RM, Studenski SA, et al. Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. J Am Geriatr Soc. 2012;60(6):1057–63. doi: 10.1111/j.1532-5415.2012.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 47.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 48.Callaghan BC, Xia R, Banerjee M, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care. 2016;39(5):801–807. doi: 10.2337/dc16-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ylitalo KR, Herman WH, Harlow SD. Performance-based physical functioning and peripheral neruopathy in a population-based cohort of women at midlife. Am J Epidemiol. 2013;177(8):810–817. doi: 10.1093/aje/kws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDermott M, Guralnik J, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: The InCHIANTI study. J Am Geriatr Soc. 2004;52(3):405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 51.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4(1):4–18. doi: 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregg E, Sorlie P, Pulose-Ram R, et al. Prevalence of lower-extremity disease in the U.S. adult population 40+ years of age with and without diabetes. Diabetes Care. 2004;27(27):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]


