Abstract
Mild traumatic brain injury (mTBI) is a significant health concern. The majority who sustain mTBI recover, although ~20 % continue to experience symptoms that can interfere with quality of life. Accordingly, there is a critical need to improve diagnosis, prognostic accuracy, and monitoring (recovery trajectory over time) of mTBI. Volumetric magnetic resonance imaging (MRI) has been successfully utilized to examine TBI. One promising improvement over standard volumetric approaches is to analyze high-dimensional shape characteristics of brain structures. In this study, subcortical shape and volume in 76 Service Members with mTBI was compared to 59 Service Members with orthopedic injury (OI) and 17 with post-traumatic stress disorder (PTSD) only. FreeSurfer was used to quantify structures from T1-weighted 3 T MRI data. Radial distance (RD) and Jacobian determinant (JD) were defined vertex-wise on parametric mesh-representations of subcortical structures. Linear regression was used to model associations between morphometry (volume and shape), TBI status, and time since injury (TSI) correcting for age, sex, intracranial volume, and level of education. Volumetric data was not significantly different between the groups. JD was significantly increased in the accumbens and caudate and significantly reduced in the thalamus of mTBI participants. Additional significant associations were noted between RD of the amygdala and TSI. Positive trendlevel associations between TSI and the amygdala and accumbens were observed, while a negative association was observed for third ventricle. Our findings may aid in the initial diagnosis of mTBI, provide biological targets for functional examination, and elucidate regions that may continue remodeling after injury.
Keywords: Mild traumatic brain injury, Military, mTBI, Subcortical structures, Volumetric measures, Shape analyses, Service members
Introduction
Traumatic brain injury (TBI) affects many individuals and is a major health concern among U.S. Service Members and Veterans. Utilizing clinical and medical information from the Defense Medical Surveillance System (DMSS) and Theater Medical Data Store (TMDS), estimates indicate that more than 330,000 service members have sustained a TBI since 2000 (https://dvbic.dcoe.mil/sites/default/files/DoD-TBI-Worldwide-Totals%202000-2015Q1-May15-2015.pdf). TBI occurs both during deployment related operations and in garrison, with the majority of TBI diagnoses (~80 %) occurring in the mild range of severity according to the VA/DoD criteria (i.e., loss of consciousness < 30 min, post-traumatic amnesia <24 h, or alteration of consciousness <24 h, no abnormal day-of-injury imaging findings [22]). A diagnosis of a mild TBI (mTBI) suggests that most Service Members and Veterans will experience full cognitive and functional recovery [29, 69]. However, there are a number of individuals (~20 %) who continue to experience residual symptoms that could impact unit readiness, return to duty, safety of troop operations, and troop retention [13, 27, 67]. Thus, there is significant interest in improving diagnosis, prognostic accuracy, monitoring recovery or decline, and assessing treatment effects, especially for those experiencing persistent symptoms.
One of the primary clinical and research tools employed in examining the effects of TBI on brain structure has been medical imaging, especially quantitative or volumetric magnetic resonance imaging (MRI; [5, 6, 17, 61, 72]). Across the range of TBI severity, these studies have demonstrated a number of consistent findings including global atrophy (i.e., ventricle-to-brain ratio; [5, 65]), white and gray matter atrophy [6, 47, 64], regional vulnerability (temporal and frontal poles [4]) specific subcortical nuclei atrophy (i.e., thalamus, [43]), and cortical thinning [38, 66, 73]). Recent advances in automated volumetric analysis (i.e., FreeSurfer) not only dramatically hasten the work, but typically improve reliability and consistency in measurements, making it possible to examine multiple regions of interest (ROI) concurrently. For example, a FreeSurfer analysis of a mixed sample of TBI severity pediatric patients demonstrated volumetric changes across a range of structures, highlighting the diffuse nature of TBI [7]. Volumetric and cortical thickness measures have been especially useful when employed in research paradigms that assess the influence of various clinical factors and/or treatments on the evolution of TBI pathology [42, 66, 73]. In this manner, studies have implicated a number of clinical and functional measures that appear to be related to volumetric changes including neuropsychological measures of executive function, attention, memory, and processing speed [15, 40] and clinical features of injury including Glasgow Coma Scale (GCS) scores [19], loss of consciousness (LOC), and post-traumatic amnesia (PTA) [8, 60].
One method for detecting subtle volumetric changes is shape analysis. This method uses geometric modeling of the three-dimensional surfaces from volumetric data to examine the expansions and contractions along the entire surface of an ROI. Utilizing these methods, a number of studies have demonstrated different and specific spatial patterns that suggest localized changes in the complex cytoarchitecture of subcortical nuclei. For example, shape analyses of subcortical nuclei in moderate and severe TBI show reductions in localized areas of the hippocampus (head and tail), thalamus, and basal ganglia [40, 51], demonstrating vulnerability of specific regions within these complex subcortical nuclei. Furthermore, the significant shape findings were found to be functionally relevant in these samples and were associated with white matter changes as measured by other diffusion MRI sequences, including diffusion weighted imaging (DWI). However, these studies were in moderate and/or severe TBI participants, and to the authors’ knowledge there have been no published studies of shape analyses exclusively in mTBI.
Importantly, mTBI or concussion, poses several challenges to clinicians and researchers alike when looking for objective evidence of injury. Volumetric/morphometric changes in certain ROIs have been demonstrated in small cohorts of mTBI patients [34, 41, 44], but these findings are often equivocal or inconsistent across studies. There are a number of possible methodological (i.e., group analyses, sensitivity of gross volumetric measurements, different analysis tools) and even clinical (i.e., subtleness of the injury, unique spatial distributions of injury) reasons for the lack of reproducible findings. However, the increased sensitivity of shape analyses for detecting subtle differences in surfaces of subcortical structures as applied to other neurological disorders [35, 46, 70] may extend or improve the detection of differences between groups in volumetric studies of mTBI. Ultimately, these analyses may potentially prove more useful in detecting injury, predicting outcomes, evaluating long term structural change and plasticity, and/or evaluating therapeutic effects of interventions.
In this study, the volumetric measures from FreeSurfer output and shape information of subcortical ROIs in a cohort of U.S. Military Service Members with symptomatic mTBI were examined. The volumetric and shape measurements from the mTBI cohort were compared to the measurements from a cohort of orthopedically injured (OI) and PTSD only (no TBI history) U.S. Military Service Member controls. We hypothesized that the shape analyses would be more sensitive to injury compared to gross volumetric analyses and that shape would be associated with both demographic and/or clinical features in the mTBI participants.
Methods
Participants
Participants included 153 (134 male, 18 female) U.S. Service Members recruited at a large military treatment facility (MTF). The study consisted of three demographically comparable groups including symptomatic mTBI, orthopedic injury (OI) only control, and PTSD only control participants. Basic demographic data for each of the groups is found in Table 1.
Table 1.
Demographic variables for each group
| mTBI group | PTSD only group | OI only group | Total group | Significance | |
|---|---|---|---|---|---|
| N | 76 | 17 | 59 | 152 | N/A |
| Age | 32.85 years (9.25) | 37.47 years (6.55) | 36.95 years (6.75) | 34.92 years (8.33) | F = 11.96 |
| p < 0.001 | |||||
| (mTBI group different) | |||||
| Sex (% female) | 6.4 % | 5.9 % | 18.9 % | 11.2 % | χ2 = 6.33 |
| p < 0.04 | |||||
| (OI group different) | |||||
| Race | 73.7 % W | 58.8 % W | 65.5 % W | 66.9 % W | χ2 = 35.54 |
| 10.5 % AA | 17.6 % AA | 31.0 % AA | 19.4 % AA | p < 0.001 | |
| 15.8 % other | 23.6 % other | 3.5 % other | 13.7 % other | OI has more AA and less other | |
| Education | 60.5 % | HS 41.2 % HS | 22.4 % HS | 43.8 %HS | χ2 = 39.09 |
| 32.6 % C | 47.1 % C | 53.4 % C | 40.9 % C | p < 0.01 | |
| 6.9 % PG | 11.7 % PG | 24.2 % PG | 15.3 % PG | (OI group different) | |
| Branch of Service | 93.4 % USA | 94.1 % USA | 72.4 % USA | 84.4 % USA | χ2 = 19.66 |
| 2.6 % USAF | 5.9 % USAF | 20.7 % USAF | 10.7 % USAF | p = 0.003 | |
| 4 % other | 0 % other | 6.9 % other | 4.9 % other | (OI group has more USAF) | |
| Years in Service | 10.81 years (7.97) | 14.29 years (6.38) | 15.64 years (6.51) | 13.03 years (7.59) | F = 14.27 |
| p < 0.001 | |||||
| (mTBI group different) | |||||
| Number of deployment | 36.8 % 1X | 47.4 % 1X | 31.6 % 1X | 36.9 % 1X | χ2 = 14.69 |
| 11.8 % | 2X 26.3% 2X | 36.8% 2X | 34.4 % 2X | p < 0.14 | |
| 9.2 % 3X | 15.8 % 3X | 26.3 % 3X | 20.6 % 3X | ||
| 21.1 % 4X | 5.3 % 4X | 5.3 % 4X | 6.3 % 4X | ||
| 2.6 % 5X | 5.3 % 6X | 1.3 % 5X | |||
| 0.6 % 6X | |||||
| Prior psychiatric diagnosis | 21.8 % | 5.9 % | 22.9 % | 19.4 % | χ2 = 3.08 |
| p = 0.54 | |||||
| Prior learning disability | 5.3 % | 0 % | 1.7 % | 3.1 % | χ2 = 2.99 |
| p = 0.24 | |||||
| Time since injury | 308.6 days (176.6) | N/A | N/A | N/A | N/A |
| (Median 270 days) | |||||
| LOC | 42.1 % | N/A | N/A | N/A | N/A |
| AOC | 69.7% | N/A | N/A | N/A | N/A |
| PTA | 7.9 % | N/A | N/A | N/A | N/A |
| Prior head injury (subjective reporting) | 31.6 % no prior | None reported/denied | None reported/denied | N/A | N/A |
| 19.7 % 1 prior | |||||
| 9.2 % 2 prior | |||||
| 13.2 % 3 prior | |||||
| 3.9 % 4 prior | |||||
| 19.8 % > 5 prior | |||||
| 2.6 % no data | |||||
| Method of injury | 57.9 % blast | N/A | N/A | N/A | N/A |
| 42.1 % other |
The p value for the group comparison are also shown
W white, AA African American, O other race, HS high school, C College (Associates and/or Bachelor’s Degree), PG post graduate degree, USA United States Army, USAF United States Air Force
TBI participants
MTBI participants were 76 (71 male, 5 female) Service Members recruited consecutively from referrals to a large MTF TBI clinic. Each was diagnosed as having sustained mTBI using the Veteran Affairs (VA)/Department of Defense (DoD) Clinical Practice Guidelines (i.e., loss of consciousness (LOC) <30 min, alteration of consciousness (AOC) <24 h, post-traumatic amnesia (PTA) <24 h, and no day of injury imaging abnormalities [22]). Furthermore, participants with mTBI were required to have persistent cognitive symptoms as measured by the Neurobehavioral Symptom Inventory (NBSI or NSI; positive responses to the four cognitive questions at the moderate level). Additional inclusion criteria included an age range between 18 and 55 years, having sustained head injury during deployment (OEF/OIF/OND) activities, having sustained head injury three to 24 months prior to recruitment, and be able to speak, read, and write in English.
Orthopedic injured only participants
Orthopedic injured (OI) only groups have been a standard control group in many TBI studies as they are considered to share many of the same injury-related experiences including hospitalizations, pain and/or other medication use, and injury-related stress [46, 59]. For these reasons, we recruited an orthopedic injured (OI) only control that consisted of 59 (47 male, 12 female) Service Members identified through the Orthopedic Clinic at the same MTF. Potential participants were excluded if they had any self-reported or objective evidence of a previous closed head injury (regardless of severity), and/or a current PTSD diagnosis (DSM-IV criteria) as identified by the standardized Clinician-Administered PTSD Scale (CAPS) structured interview. In addition, OI only control participants were required to be within a similar age range to mTBI participants, deployed within the past 3–24 months, and able to speak, read, and write in English.
PTSD only participants
Given that post-traumatic stress disorder (PTSD) has a significant brain imaging signal [53, 68] and is a significant comorbid condition observed in Service Members with mTBI [29–32], a small cohort of Service Members with PTSD without TBI (N = 17; 16 male, 1 female) were recruited from the Behavioral Health Clinic, Warrior in Transition Battalion, and/or Orthopedic Clinic at the same large regional MTF. In addition, each participant in this group was required to meet the categorical criterion for PTSD diagnosis using the CAPS (DSM-IV criteria). PTSD only participants were selected using similar inclusion/exclusion criteria including age range, deployment history (within the past 3–36 months), and English proficiency. Furthermore, PTSD symptoms were required to be combat related, and participants were excluded if symptoms were primarily related to any other stressors or events. It should be noted that the deployment history inclusion criterion had to be extended by 12 months in this particular medical setting as PTSD-associated symptoms requiring intensive treatment were often being identified and treated after longer post-deployment intervals. For this reason, fewer potential Service Members with a diagnosis of PTSD were seen in this active duty MTF medical setting.
In addition, participants (regardless of experimental group) were excluded if they had any MRI contraindications (i.e., claustrophobia, shrapnel, and pregnancy), neurologic comorbidities (i.e., seizures, psychosis, and bipolar disorder), history of moderate/severe TBI, spinal cord injuries, were on scheduled narcotic pain medications, or were unable to use their dominant hand.
Recruitment procedures
Potential participants were identified by clinic personnel and were then interviewed by study staff who after explanation of the research (including aims, risks, and benefits) obtained written informed consent. Each participant then underwent medical record review to further determine eligibility. Those participants meeting basic inclusion/exclusion criteria underwent an additional screening interview by a trained TBI medical professional to further rule in/out closed head injury and/or concussion during military service using a semi-structured interview. Participants meeting basic inclusion/exclusion criteria were then scheduled for additional study procedures. This research was approved and monitored by the local hospital IRB (protocol #3743378) and Human Research Protection Office (HRPO) at the US Army Medical Department Medical Research and Materiel Command (USAMRMC) (protocol #A-17660).
Demographic and clinical variables
Basic demographic information was collected from each participant. Variables included age, sex, education, rank, branch of military service, number of deployments, and years in service. Service Members with prior psychiatric (i.e., major depressive disorder, anxiety disorders, posttraumatic stress disorder (PTSD)) or learning disability [i.e., attention deficit hyperactivity disorder (ADHD)] diagnoses were not excluded, but these diagnoses were documented for each participant. Each participant’s subjective reporting was then verified independently via a medical chart review and a binary (0 = no diagnosis, 1 = at least one diagnosis) variable was created for analysis purposes. For the mTBI group, clinical variables including time since injury (TSI, day of assessment—day of injury), duration of LOC, duration of AOC, PTA duration, mechanism of injury (blast vs. non-blast), and number of self-reported prior head injuries and blast exposures were captured. Descriptive statistics for these variables are seen in Table 1.
Imaging acquisition
Each participant underwent multimodal MRI utilizing the same 3 Tesla Siemens Verio Syngo scanner running version MR B17. Sequences administered included a T1- weighted MPRAGE, T2-weighted, fluid attenuated inversion recovery (FLAIR), susceptibility weighted imaging (SWI), diffusion tensor imaging (DTI), blood oxygenation level dependent (BOLD) resting state and task-related functional imaging, and chemical shift imaging (CSI) spectroscopy. For this report, only the volumetric T1- weighted sequence (1 mm3 isotropic voxels) acquired utilizing a 32-channel head coil was examined (sequence parameters: field of view (FOV) = 256 mm, repetition time (TR) = 2300 ms, echo time (TE) = 2.98 ms, flip angle = 9°, and a slice thickness = 1 mm). Scan time was approximately 9:50.
Imaging post-processing volumetric procedures
After each T1-weighted sequence data were visually inspected for artifacts that might affect processing (i.e., motion, complete anatomy coverage, inhomogeneity) and examined for consistent scan acquisition (same scan parameters), the raw DICOM data were submitted to the FreeSurfer 5.3 (MGH Martinos Center, Boston, MA) processing pipeline. All the data were batch-processed using one of two identical MacPro dual processor (2 × 3.06 GHz 6-core Intel Xeon) machines running the same Mac OS X software (Version 10.8). Though these procedures are described in detail elsewhere [18] the following processes were employed for each imaging data set.
The data were first processed using the recon-all command to produce fully segmented and labeled images (aseg.mgz). Upon completion, the aseg.mgz files were visually inspected for any obvious abnormal results (i.e., skull stripping errors). For the purposes of this study, we examined a number of volumes statistically by plotting the distribution of the measures to reveal possible volumetric outliers. Statistical outliers were then re-inspected visually, manual edits were conducted if needed, and the volumes were recalculated for each of the ROIs. Finally, the volumetric data for each structure of interest were extracted from the aseg.stats file. For this study, the volumes of the following ROIs were examined: lateral ventricles, third ventricle, fourth ventricle, brain stem, thalamus, caudate, pallidum, nucleus accumbens, hippocampus, amygdala, cerebellum white/gray matter, and anterior and posterior corpus callosum volumes.
Imaging post-processing shape procedures
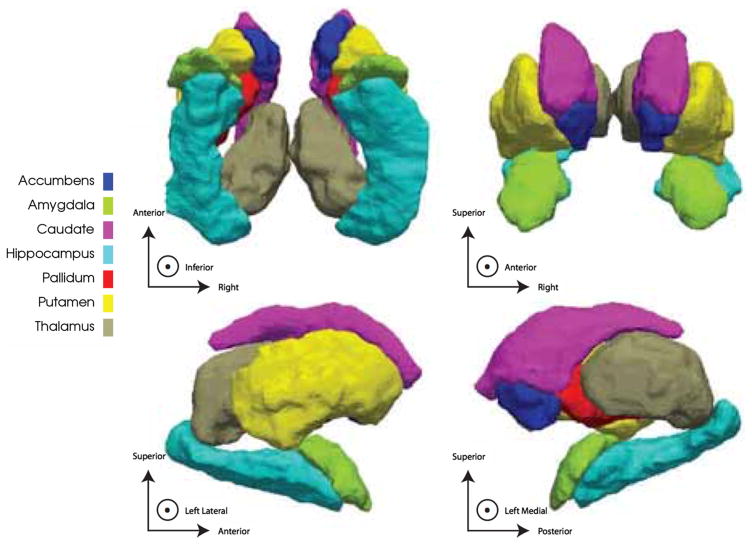
Following segmentation and manual inspection of the ROIs with FreeSurfer, two shape descriptors were defined on the surfaces of seven bilateral (14 total) subcortical structures: thalamus, putamen, pallidum, amygdala, accumbens, caudate and hippocampus. A parametric representation of each surface was obtained using the Medial Demons method detailed in [23, 25]. In brief, each surface was conformally mapped to a spherical template. These spherical maps were rigidly rotated to a probabilistic atlas. Next, the spherical demons [24] algorithm was used to nonlinearly warp the spherical maps on the basis of curvature. Two surface-based functions were defined at this stage; first, the global orientation function which defines the direction of the surface; and second, the local thickness of the surface with respect to a skeletonized medial core. Finally, the spherical demons algorithm was implemented a second time using both the newly defined medial core in conjunction with surface-based curvature to match each surface to the atlas. Each surface was composed of a number of vertices that scaled roughly with the structure’s average volume and also offered a sufficient resolution of the surface to describe variation in its local topology. Two shape features were defined at each surface vertex: (1) the radial distance (RD), a proxy for thickness (the distance from each surface point to the core 2D line skeleton), and (2) the log of the Jacobian determinant (JD) which indicates local surface area dilation or contraction. Because changes in surface area can be driven by underlying changes in either concavity or convexity of the surface we report the underlying RD along with regions of significant change in JD to discern the direction of this change. Across all 14 subcortical surfaces there were a total of 27,120 vertices. Supplemental Table 1 outlines the number of vertices constituting each surface. Figure 1 illustrates the subcortical surfaces to which shape analyses were applied.
Fig. 1.
Illustration of subcortical surfaces reported in this study. Note that all images are in radiological convention throughout this manuscript, that is, the right hemisphere appears on the left side of the image
The value of these shape descriptors lies in their ability to reveal local regions of variation within a structure’s surface. This is valuable complementary information in addition to the standard simple volumetric descriptions of a structure which are only able to report gross overall variation in a single direction (i.e., increased or decreased volume). The addition of localized descriptions of topology allows investigators to report subtler changes in the morphometry of a surface whose signal may have been lost when averaged across the whole ROI, thereby revealing patches of significant variation. This also allows investigators to report regions of equal but opposite variation within a single surface which would have otherwise been canceled out had they been reduced to a single, scalar value.
While numerous shape descriptors have been developed, we have chosen to use the RD and the JD due to their relatively intuitive interpretations. RD measures how thick the structure is at a given point defined on its surface while the JD is a measure of regional surface area deformation following registration. RD, JD and volume, therefore, all provide insight into different aspects of the structure’s topology. They are all, of course, moderately correlated with one another since they are descriptions of the same structure, but their combined use remains valuable.
Statistical procedures
All statistical procedures were coded and run using R-project version 3.2.0. The following basic statistical regression model was utilized for both the volumetric and shape analyses:
where Main Effect is one of group (TBI, ortho or PTSD status; modeled dichotomously), TSI (modeled continuously), MOI (modeled dichotomously), premorbid mental health diagnosis, and/or attention disorder/learning disability. Premorbid mental health diagnosis was modeled dichotomously with a positive status for any participant having prior history of any of major depressive disorder, anxiety disorders, and post-traumatic stress disorder. Similarly, participants having either a prior history of ADHD or a learning disability were collapsed into a single group. These groups were collapsed due to the small numbers of participants having individual cases of sub-diagnoses which would have been poorly estimated individually. Associations with TSI and method of injury (MOI) were assessed only within the TBI cohort as these variables were not tracked or of primary interest in the orthopedic or PTSD only cohorts. For shape analyses, the outcome Y is simply the vertex-specific RD or JD value, while for volumetric analyses, Y is the global volumetric measure. For each model we report the t value derived from the Student’s t test assessing the significance of the slope derived from the regression of the outcome, Y, on the main effect after correcting for the other covariates. Given the significant number of variables (see Supplementary Table 1), the analyses were corrected for multiple comparisons using a false discovery rate (FDR) correction. Main effect variables were selected based on interest in both exploring group differences and/or common variables identified as important in other research/clinical studies. The first main effect examined was group, which included mTBI, OI only controls, and/or PTSD only controls. For each model the following variables of interest were examined for main effects on the volume of interest or shape vertices: group (TBI, OI only, PTSD only); time since injury (TSI) for the mTBI patients only, mechanism of injury (MOI) for mTBI patients only (binary variable: blast vs other); and premorbid mental health diagnosis (binary variable: depression, anxiety, PTSD vs. no diagnosis), and premorbid attention disorder/learning disability (binary variable: attn/LD vs. no diagnosis). Several covariates were utilized regardless of model examined. These were selected based on the previous literature and are common covariates observed in many imaging studies. Covariates included age, sex, total intracranial volume (TICV), and education. All covariates were modeled as fixed effects due to their continuous nature, and in the case of education, due to the small number of categories which would limit the estimation variance across the categories.
Results
Demographic Variables
As noted in Table 1, there were a number of significant demographic differences between the groups. Namely, the OI only controls were older (5 years on average) and more educated (more post high school degrees) while the mTBI included more women. Other demographic variables are provided as a characterization of the sample in Table 1.
Mild traumatic brain injury versus orthopedic injury
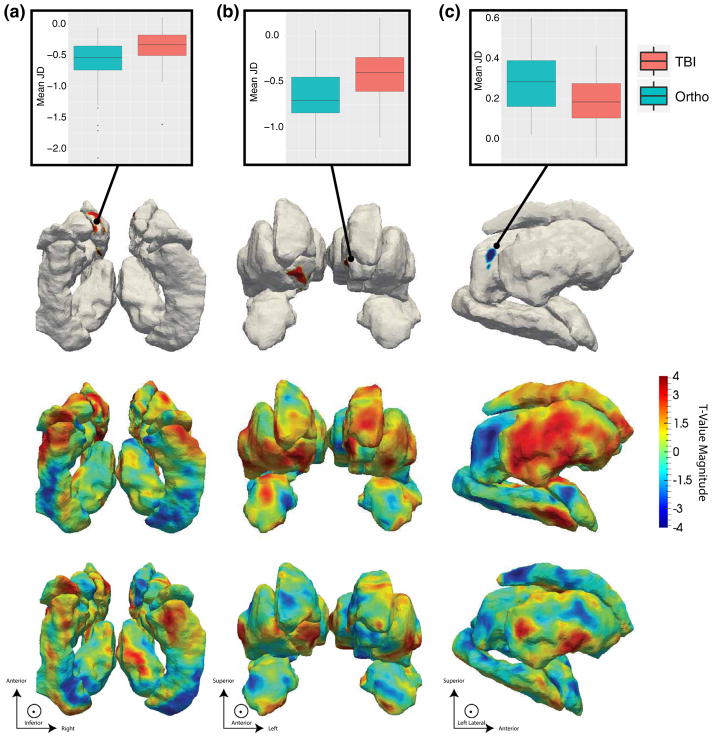
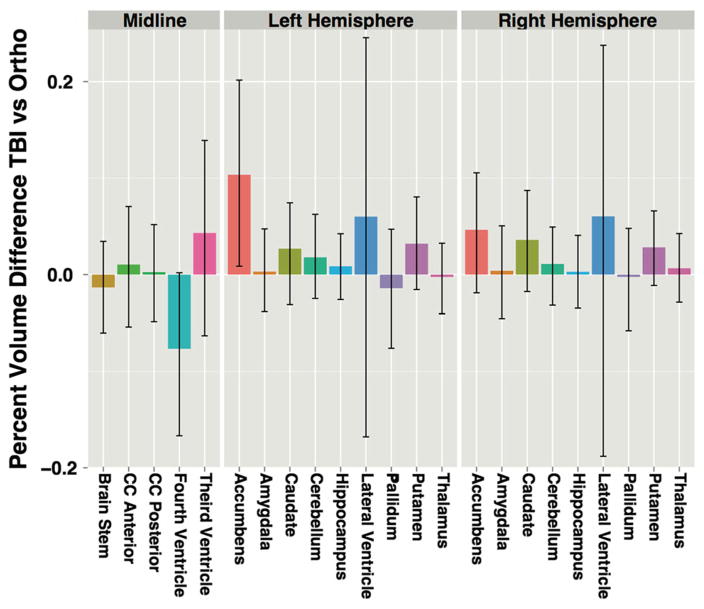
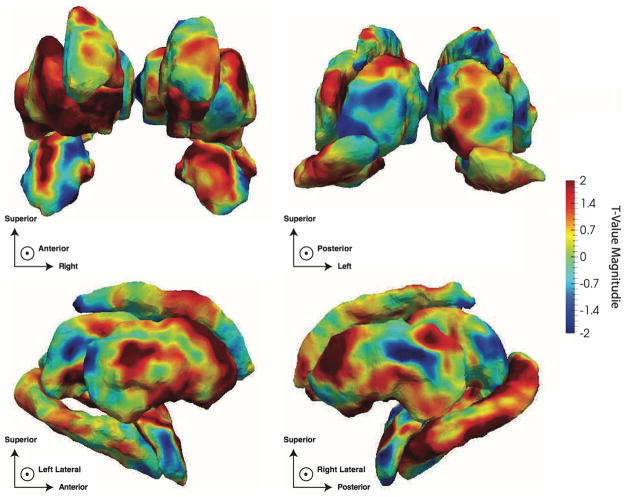
The surface areas (JD measure) of the bilateral anterior medial accumbens and left anterior medial caudate were significantly expanded in the mTBI group, relative to OI controls (Fig. 2a, b). Additionally, the surface area of the left posterolateral thalamus was significantly reduced in the mTBI group (Fig. 2c). No significant RD or volumetric measures were identified in association with the mTBI and OI contrast (see Supplemental Tables 2 and 3 for volumetric statistics). However, with the exceptions of bilateral pallidum, brainstem, and fourth ventricle, subcortical regions in mTBI participants tended to be larger, although not to a significant degree (Fig. 3).
Fig. 2.
t-Value maps of the regression of the Jacobian determinant (JD) on the ortho-TBI contrast from a inferior, b anterior and c left perspectives. Note that all images are in radiological orientation, i.e. left–right flipped. The top row plots the average JD within regions differing significantly between TBI and Orthopedic groups. The second row illustrates FDR-thresholded t value maps in which only highlighted regions are significant. The third row maps Jacobian t values across the entire set of surfaces. The bottom row compliments this by mapping the associated t values for the local thickness (RD) measure to determine whether changes in local surface area result from concavity or convexity. Here, the directions of the JD and RD associations agree within regions of significance indicating that regions in red result from dilation while regions in blue are suggestive of atrophy. All coefficient t values had 125 degrees of freedom
Fig. 3.
Percent volume change in TBI participants relative to orthopedic controls by region and hemisphere with bootstrapped 95 % confidence intervals
Time since injury
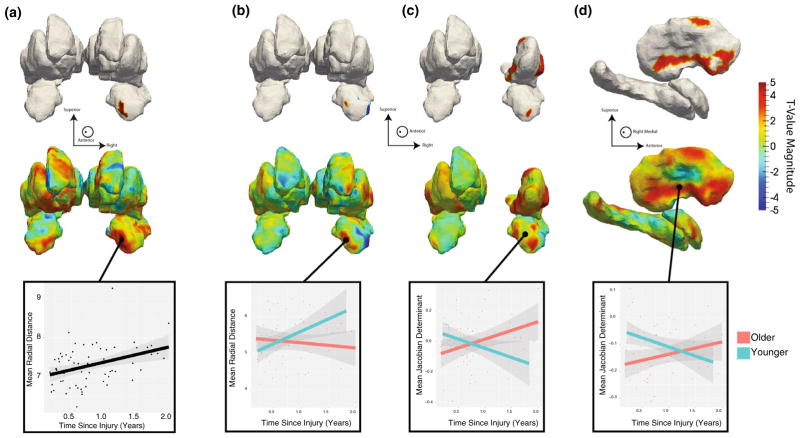
TSI was significantly positively associated with RD of the right anterior amygdala (Fig. 4) indicating increased thickness of this region in participants further removed from their injury. Among the vertices that were significant in this association, 75 % of the RD values were significantly associated with TICV (mean T = 3.08); none were significantly associated with age, sex or education. Volumetric associations with TSI failed to survive FDR correction. However, trend-level associations were observed with the right amygdala (p = 0.18, T = 3.06, df = 65; βTSI = 136.10; 8 % increase per year) and left accumbens area (p = 0.18; T = 3.14; df = 65; βTSI = 78.79; 18 % increase per year) with these measures being larger in subjects further removed from their injury. The third ventricle space (p = 0.14; T = −3.52; df = 65; βTSI = −179.51; 20 % decrease per year) tended to be reduced in size over time (Supplemental Table 3 and Fig. 1). No other structures’ shape or volume was significantly associated with TSI prior to or following FDR correction.
Fig. 4.
t Value maps of a the regression of thickness features (RD) on TSI shown from an anterior perspective showing a significant increase in the thickness of the right anterior amygdala with extended TSI. The top row of maps is FDR-thresholded with the remaining highlighted regions being those that are significantly associated. The second row of maps is unthresholded showing the global distribution of associations. Wherever significant associations are present we plot the least squares fit of the association for the vertices surviving FDR correction. All coefficient t values in a had 60 degrees of freedom. b–d Plot associations between the thickness (b) or surface area (JD measure) with the interaction of TSI and age. b Reveals a significant differential association between the thickness of the right amygdala between older and younger participants with the younger cohort having a more rapid expansion of this region than older participants. Identify regions of the c anterior right amygdala and d right pallidum with significantly differing trajectories of surface area changes. In both cases, the older participants exhibit gains in surface area at extended TSI while younger participants show significant reductions in the surface area of these regions. Associations in b–d had 59 degrees of freedom. Note that these figures are in radiological orientation, left–right flipped
Given the possibility of nonlinear effects of age and to further elucidate the relationship between age and TSI, post hoc analyses were conducted to examine the potential interaction of these variables. Age was dichotomized into younger (below the average age of mTBI participants; 33 years old) and older (>33 years old) and the results demonstrated an interaction effect (Fig. 4b–d) where younger mTBI participants had a robust increase in thickness measures (RD) in the right amygdala with increased TSI while the older participants had a flat or slightly negative slope in thickness with increasing TSI. In addition, there appeared to be an interaction between the age and TSI JD values in the right amygdala and right putamen, where older participants show additional dilation of the surfaces with increasing TSI and the younger participants have less dilation of the surfaces with increasing TSI.
Method of injury
No significant shape or volumetric differences were observed between participants with mTBI resulting from a blast versus those who did not experience blast injuries, after controlling for multiple comparisons. Associations of mechanism of injury with subcortical morphometry before multiple comparison corrections are shown in Fig. 5 and the effect sizes are calculated in Supplementary Table 4.
Fig. 5.
Association of surface area (JD) with method of injury (MOI). Positive associations of surface area and MOI are shown in warmer colors and are seen to be more spatially concentrated in anterior lateral regions of the subcortical structures. While these effects are slightly below statistical significance, the trend of spatial distribution is suggestive of the nature of a blast injury
Prior clinical history
No significant shape or volumetric differences were observed (1) between mTBI and PTSD participants; (2) between PTSD and OI participants; (3) between participants with and without a prior history of ADHD or learning disability; (4) between participants with and without a prior history of psychiatric disturbances. Null results not shown.
Discussion
Using state-of-the-art surface-based shape descriptors and volumetric analyses, we investigated whether a distinct profile of subcortical brain abnormalities could be identified in cognitively symptomatic US military Service Members 3–24 months post mTBI. We compared Service Members with mTBI to both those with strictly orthopedic injuries and those with PTSD only. We further investigated whether the morphometry of these structures expressed a relationship with TSI, MOI, and several other clinical variables of interest. The main findings of our study include increased surface area (i.e., dilation) of bilateral accumbens and left caudate and reduced surface area (i.e., atrophy) of left thalamus in Service Members with mTBI, relative to OI only controls. Additionally, we observed a time-dependent increase in the thickness (RD measure) of the right amygdala with participants further removed from their injuries having thicker amygdalae. Though the volumetric (i.e., FreeSurfer volumes) group comparisons did not reveal any significant differences between the groups, the TSI RD amygdala finding was partially corroborated by trend-level increases in the volume of the right amygdala with respect to TSI (see Figure Supplementary Fig. 1A and 1B). Additional post hoc analyses of this finding also revealed a more complex relationship between TSI and age of injury (Fig. 4) such that older participants may have an attenuated recovery trajectory in the amygdala and putamen. However, additional studies (prospective studies) will be needed to further corroborate and elucidate these findings.
Diffuse and regional cerebral atrophy has been noted in a number of TBI studies, especially when including participants with more severe injuries. Abnormal findings (i.e., primarily atrophy) have been reported in number of gray matter ROIs, including the basal ganglia, thalamus, cingulate gyrus, and mammillary bodies [1, 7, 45]. However, volumetric research focused on mTBI is limited, and results are often equivocal or with varied ROI differences. This occludes ROIs that are consistently affected in mTBI patient samples, if any. Nevertheless, studies of mTBI patients (especially those with persistent symptoms) remain important as elucidation of a consistent pattern of injury in this unique clinical population may lend important insights into why a number of mTBI patients develop chronic functional and clinical symptoms.
Similar to the findings in this study, thalamic lesions and volume abnormalities appear to be a consistent finding across TBI studies [20] regardless of the methods used to quantify the differences in thalamic size (i.e., manual, automated, and voxel based methods). In one of the only studies to use shape analyses, global atrophic surface changes were seen in bilateral thalami in a sample of 21 moderate to severe TBI patients [40]. This is in contrast with our findings in this mTBI sample, of more localized (i.e., pulvinar) left thalamus changes. Regardless, functional deficits are common in patients with thalamic lesions or abnormalities and likely implicate the sensory integrative roles and cortical interconnectedness that the thalamus plays in cognitive function. Given that this study used a sample of mTBI patients that presented with cognitive complaints, it is possible that the abnormal thalamic findings observed could represent a common anatomic finding in patients with persistent symptoms. The pulvinar has known projections to association cortex in the posterior parietal area of the brain and is implicated in a number of cognitive symptoms, including executive dysfunction, visual spatial attention or salience, and working memory [26, 36, 40, 62]. However, any association with cognitive performance remains speculative in this sample as additional research would be required to determine the specific relationship between observed thalamic abnormalities and cognitive function.
This study is one of the first to implicate abnormalities in the nucleus accumbens in mTBI patients. This may be related to the fact that historically the nucleus accumbens was a difficult ROI to measure, primarily because of its border continuity with the caudate. However, using more automated methods like FreeSurfer to segment and label the nucleus accumbens has led to improvements in accuracy and consistency of measurement. The anatomical organization of the nucleus accumbens is heterogenous and it receives projections from a number of different areas of the brain (including the thalamus and amygdala). Given these heterogeneous projections to and from the nucleus accumbens, its functional importance is complicated though it has been implicated in a number of TBI relevant outcomes, including depression, addiction, emotional regulation, inattention, and learning [3, 9, 10]. Thus, any abnormal findings in this ROI are intriguing. Beyond these shape changes, TBI studies have demonstrated abnormal white matter integrity of the fiber tracts that project to and/or are adjacent to the nucleus accumbens and may be related to abnormalities in shape or volume [48]. Shear/strain forces in TBI, even at the mild range, often result in axonal damage to deep white matter structures which in turn may produce a Wallerian type degeneration that could alter structure [37]. This hypothesis certainly could be the focus of future investigations (i.e., longitudinal studies) in this patient population by examining the adjacent white matter tracts in combination with volume and/or shape over time. This would not only improve our understanding of the relationship between these measures, but could lend additional insights into the exact pathology that underlie these findings.
Basal forebrain abnormalities might also have clinical implications for recovery and rehabilitation efforts in mTBI. In a recent animal model of TBI, injury to the basal forebrain (a major cholinergic center of the brain) moderated the effects of intensive rehabilitation efforts by limiting functional recovery and attenuating the histopathological improvements observed in intact cholinergic system animals [71]. This model of rehabilitation focused on motor lesions and recovery of motor functionality in these animals, so broader implications for functional recovery beyond motor function remain speculative. Thus, this finding will require further investigation in future studies to fully understand the extent of the nucleus accumbens’ vulnerability and any functional implications abnormalities might have in recovery or rehabilitation efforts in this patient population.
It is possible that the differences in shape observed in nucleus accumbens, amygdala and posterior thalamus in the mTBI group versus the OI group are interrelated. These three structures are known to be interconnected [33, 57, 63], and involved with processing visual information for emotional [50] and reward salience [11, 74]. Nucleus accumbens and amygdala are both involved with fear conditioning [52], and nucleus accumbens volume has been correlated with trait anxiety [39]. A sample of individuals without diagnosis of PTSD, but exposed to a prolonged traumatic event (coal mine flood disaster) had smaller gray matter volumes of the right pulvinar versus a control population [12]. Anxiety, lack of motivation and mood symptoms are common complaints in patients who have sustained mTBI, but until more prospective studies are conducted, it is unclear if persistent symptoms are related directly to injury or the exposure to the traumatic event [30]. Prospective studies with premorbid assessments in the military population are lacking and should be encouraged.
In cross-sectional imaging studies of mTBI, TSI is an important clinical factor to consider [16, 54]. A number of studies in both human and animal models demonstrate TSI as one of the more consistent correlates of cognitive and imaging findings [2, 28, 55, 56]. Namely, with increasing TSI, most TBI (especially mTBI) patients demonstrate improved function [49] and fewer imaging abnormalities. In contrast to functional/cognitive outcomes, imaging studies reveal more persistent differences over time, though the temporal evolution and progression of these imaging abnormalities is not well characterized currently and may be moderated by other factors such as age of injury. Importantly, however, a number of structural, functional, and metabolic differences can remain evident for 6 months to more than a year post-injury [14, 21] and these findings require additional investigation in a prospective longitudinal manner. In this study, the shape analysis demonstrated amygdala surface dilation with increasing TSI. There was also a notable, though statistically nonsignificant, trend in the volumetric data suggesting that the amygdala was larger in mTBI patients further from injury. However, as noted, age of injury appears to moderate this finding with the younger participants demonstrating increased thickness and less surface dilation with increasing TSI (Fig. 4). Again, as this study is cross-sectional and because of the known segmentation variability noted in the amygdala, it is difficult to say that these findings are related to any reparative and/or compensatory pathological changes post-injury. However, given the known functional association that the amygdala has with emotional regulation and tone, further investigation is warranted especially in US Service Members and Veterans with mTBI, where PTSD is a common comorbidity [29, 31].
Visual inspection of the shape analyses before adjustment for multiple comparisons also revealed interesting findings that may inform future hypothesis in larger datasets. For example, in the group comparisons illustration (Fig. 2), the contrast between anterior (i.e., dilation) and posterior (i.e., reduction) regions of the brain may indicate a gradient of injury and reparative pathological changes that require further examination in a prospective manner. These findings may also be useful and informative when examining other MRI sequences and post-processing methods in combination with shape analyses. Examination of the MOI illustration prior to FDR correction (Fig. 5) reveals a pattern of difference occurring on the medial surfaces of several subcortical nuclei bordering the lateral ventricles with the blast induced mTBI participants having reductions in thickness. Recent evidence in blast studies suggest that structures adjacent to the ventricles may be at particular risk due to the fluid dynamics associated with the primary blast wave [58]. Again, these hypotheses remain speculative, but shape analyses may prove to be a useful tool in examining ROIs in this unique population.
Traditionally, TBI studies regardless of severity have incorporated volumetric methods in attempts to improve diagnosis, determine the extent of injury severity, and to inform clinical and functional prognosis. The results of this study demonstrate the additional, more nuanced information that shape descriptors provided that were especially useful in discriminating the mTBI patients from the controls groups. However, there are a number of limitations that should be considered when interpreting the data. For example, despite attempts to equate the groups on major demographic variables, there are a number of variables including age, sex, and education that are different between the groups. To deal with these issues, several statistical procedures were undertaken (namely including these variables as covariates) to account for demographic differences, though we recognize that this remains an issue. In addition, mTBI patients in this study only included cognitively symptomatic participants and as such the results may not generalize to the other military or veteran samples that might include all mTBI participants. Inclusion of an asymptomatic mTBI sample might have also proved to be another important “control” group when looking for consistent patterns of shape or volume differences. The sample size of the PTSD only group is also underpowered for this type of analysis. Any signal associated with PTSD may have not been visible with such a small sample size. It should be noted that the PTSD only group was challenging to recruit for several reasons, including the fact that many individuals who were deployed to active combat zones and who were having clinically significant PTSD symptoms were often exposed to a blast and/or had sustained a mTBI. The difficulty in recruitment may also reflect the prioritization of medical disorders in this specific treatment setting which results in potential participants presenting for PTSD assessment and treatment further from the deployment setting. However, this group of patients should remain a focus of investigation as many of the mTBI patients included in this sample manifest PTSD symptom. Finally, it is impossible to say for certain what these changes mean clinically or pathologically. Use of additional MRI sequences and post-processing methods to confirm and to extend these findings might be a good first step in trying to understand what is happening at the histopathology level. Despite these limitations, the significant shape findings remain intriguing and warrant consideration.
Though this study is one of the larger mTBI studies (i.e., sample size) to date, it is clear that additional analyses and/or studies are needed to further elucidate important clinical and functional relationships associated with mTBI in US Combat Soldiers and Veterans. Future studies using shape analyses should include measures of neuropsychological function or conducted in longitudinal settings as these opportunities would expand our understanding of this potentially useful tool, and provide additional measures that could be used in developing and/or evaluating treatments.
Supplementary Material
Acknowledgments
The view(s) expressed herein are those of the author and do not reflect the official policy or position of the Defense and Veterans Brain Injury Center, Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense, or the U.S. Government. We gratefully acknowledge the generous time and effort that the Service Members made in supporting this study. We also gratefully acknowledge the clinical effort and expertise of the Brooke Army Medical Center Brain Injury and Rehabilitation Service staff in the identification, recruitment, consenting, and treatment of Service Members who are a part of this study. This work is supported in part by the Defense and Veterans Brain Injury Centers, the Telemedicine and Advanced Technology Research Center (TATRC) at the U.S. Army Medical Research and Materiel Command (USAMRMC; W81XWH-13-2-0025), the Chronic Effects of Neurotrauma Consortium (CENC; PT108802-SC104835), the National Institutes of Health (NIH U54 EB020403), and the Veterans Administration (VA Merit Grant).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-016-8236-7) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest No conflicts of interest are noted for authors.
Ethical standard This research was approved and monitored by the local hospital IRB (protocol #3743378) and Human Research Protection Office (HRPO) at the US Army Medical Department Medical Research and Material Command (USAMRMC) (protocol #A-17660). Study procedures were thus in compliance with ethical standards as laid down by the 1964 Declaration of Helsinki.
References
- 1.Anderson CV, Wood DM, Bigler ED, Blatter DD. Lesion volume, injury severity, and thalamic integrity following head injury. J Neurotrauma. 1996;13:59–65. doi: 10.1089/neu.1996.13.59. [DOI] [PubMed] [Google Scholar]
- 2.Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology. 2009;23:283–296. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger-Sweeney J. The cholinergic basal forebrain system during development and its influence on cognitive processes: important questions and potential answers. Neurosci Biobehav Rev. 2003;27:401–411. doi: 10.1016/s0149-7634(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 4.Bigler ED. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. 2007;21:515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- 5.Bigler ED. Structural image analysis of the brain in neuropsychology using magnetic resonance imaging (MRI) techniques. Neuropsychol Rev. 2015;25:224–249. doi: 10.1007/s11065-015-9290-0. [DOI] [PubMed] [Google Scholar]
- 6.Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci. 2013;7:395. doi: 10.3389/fnhum.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigler ED, Abildskov TJ, Wilde EA, McCauley SR, Li X, Merkley TL, Fearing MA, Newsome MR, Scheibel RS, Hunter JV, Chu Z, Levin HS. Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. Neuroimage. 2010;50:1017–1026. doi: 10.1016/j.neuroimage.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Brezova V, Moen KG, Skandsen T, Vik A, Brewer JB, Salvesen O, Haberg AK. Prospective longitudinal MRI study of brain volumes and diffusion changes during the first year after moderate to severe traumatic brain injury. Neuroimage Clin. 2014;5:128–140. doi: 10.1016/j.nicl.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan RC, Li Z, Li K, Zeng YW, Xie WZ, Yan C, Cheung EF, Jin Z. Distinct processing of social and monetary rewards in late adolescents with trait anhedonia. Neuropsychology. 2016;30:274–280. doi: 10.1037/neu0000233. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Fu K, Feng C, Tang L, Zhang J, Huan Y, Cui J, Mu Y, Qi S, Xiong L, Ma C, Wang H, Tan Q, Yin H. Different regional gray matter loss in recent onset PTSD and non PTSD after a single prolonged trauma exposure. PLoS One. 2012;7:e48298. doi: 10.1371/journal.pone.0048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Combs HL, Berry DT, Pape T, Babcock-Parziale J, Smith B, Schleenbaker R, Shandera-Ochsner A, Harp JP, High WM., Jr the effects of mild traumatic brain injury, post-traumatic stress disorder, and combined mild traumatic brain injury/posttraumatic stress disorder on returning veterans. J Neurotrauma. 2015;32:956–966. doi: 10.1089/neu.2014.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis EL, Hua X, Villalon-Reina J, Moran LM, Kernan C, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Thompson PM, Asarnow RF. Tensor-based morphometry reveals volumetric deficits in moderate/severe pediatric traumatic brain injury. J Neurotrauma. 2016;33:840–852. doi: 10.1089/neu.2015.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Depue BE, Olson-Madden JH, Smolker HR, Rajamani M, Brenner LA, Banich MT. Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. Biomed Res Int. 2014;2014:691505. doi: 10.1155/2014/691505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodd AB, Epstein K, Ling JM, Mayer AR. Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. J Neurotrauma. 2014;31:1235–1248. doi: 10.1089/neu.2014.3337. [DOI] [PubMed] [Google Scholar]
- 17.Esopenko C, Levine B. Aging, neurodegenerative disease, and traumatic brain injury: the role of neuroimaging. J Neurotrauma. 2015;32:209–220. doi: 10.1089/neu.2014.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale SD, Johnson SC, Bigler ED, Blatter DD. Nonspecific white matter degeneration following traumatic brain injury. J Int Neuropsychol Soc. 1995;1:17–28. doi: 10.1017/s1355617700000060. [DOI] [PubMed] [Google Scholar]
- 20.Gooijers J, Chalavi S, Beeckmans K, Michiels K, Lafosse C, Sunaert S, Swinnen SP. Subcortical volume loss in the thalamus, putamen, and pallidum, induced by traumatic brain injury, is associated with motor performance deficits. Neurorehabil Neural Repair. 2015 doi: 10.1177/1545968315613448. [DOI] [PubMed] [Google Scholar]
- 21.Green RE, Colella B, Maller JJ, Bayley M, Glazer J, Mikulis DJ. Scale and pattern of atrophy in the chronic stages of moderate-severe TBI. Front Hum Neurosci. 2014;8:67. doi: 10.3389/fnhum.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidelines VDCP. Clinical practice guidelines. U.S. Department of Veteran Affairs; 2016. [Accessed 30 Mar 2016]. Management of concussion-mild traumatic brain injury (mTBI) http://www.healthquality.va.gov/guidelines. [Google Scholar]
- 23.Gutman BA, Jahanshad N, Ching CR, Wang Y, Kochunov PV, Nichols TE, Thompson PM. Medial demons registration localizes the degree of genetic influence over subcortical shape variability: an N = 1480 meta-analysis. Proc IEEE Int Symp Biomed Imaging. 2015;2015:1402–1406. doi: 10.1109/ISBI.2015.7164138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutman BA, Madsen SK, Toga AW, Thompson PM. A family of fast spherical registration algorithms for cortical shapes. Third international workshop, MBIA; 2013; Switzerland: Springer International Publishing; 2013. pp. 246–257. [Google Scholar]
- 25.Gutman BA, Wang Y, Rajagopalan P, Toga AW. Shape matching with medial curves and 1-D group-wise registration. 2012 9th IEEE international symposium on biomedical imaging; Barcelona: IEEE; 2012. pp. 716–719. [Google Scholar]
- 26.Han K, Chapman SB, Krawczyk DC. Altered amygdala connectivity in individuals with chronic traumatic brain injury and comorbid depressive symptoms. Front Neurol. 2015;6:231. doi: 10.3389/fneur.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haran FJ, Alphonso AL, Creason A, Campbell JS, Johnson D, Young E, Tsao JW. Analysis of post-deployment cognitive performance and symptom recovery in US Marines. PLoS One. 2013;8:e79595. doi: 10.1371/journal.pone.0079595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris JL, Yeh HW, Choi IY, Lee P, Berman NE, Swerdlow RH, Craciunas SC, Brooks WM. Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J Cereb Blood Flow Metab. 2012;32:2122–2134. doi: 10.1038/jcbfm.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoge CW, Castro CA. Treatment of generalized war-related health concerns: placing TBI and PTSD in context. JAMA. 2014;312:1685–1686. doi: 10.1001/jama.2014.6670. [DOI] [PubMed] [Google Scholar]
- 30.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in US Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 31.Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1:269–277. doi: 10.1016/S2215-0366(14)70235-4. [DOI] [PubMed] [Google Scholar]
- 32.Jaramillo CA, Cooper DB, Wang CP, Tate DF, Eapen BC, York GE, Pugh MJ. Subgroups of US IRAQ and Afghanistan veterans: associations with traumatic brain injury and mental health conditions. Brain Imaging Behav. 2015;9:445–455. doi: 10.1007/s11682-015-9402-8. [DOI] [PubMed] [Google Scholar]
- 33.Jones EG, Burton H. A projection from the medial pulvinar to the amygdala in primates. Brain Res. 1976;104:142–147. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- 34.Jorge RE, Acion L, Starkstein SE, Magnotta V. Hippocampal volume and mood disorders after traumatic brain injury. Biol Psychiatry. 2007;62:332–338. doi: 10.1016/j.biopsych.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, Leaver A, Woods RP, Narr KL. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79:282–292. doi: 10.1016/j.biopsych.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karmonik C, Clark J, Fung SH, Grossman RG, High W, Jiang Y. Comparison of functional network integrity in TBI and orthopedic controlpatientsusing graph-theoretical analysis. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:1366–1369. doi: 10.1109/EMBC.2013.6609763. [DOI] [PubMed] [Google Scholar]
- 37.Kelley BJ, Farkas O, Lifshitz J, Povlishock JT. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp Neurol. 2006;198:350–360. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 38.King JB, Lopez-Larson MP, Yurgelun-Todd DA. Mean cortical curvature reflects cytoarchitecture restructuring in mild traumatic brain injury. Neuroimage Clin. 2016;11:81–89. doi: 10.1016/j.nicl.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn S, Schubert F, Gallinat J. Structural correlates of trait anxiety: reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J Affect Disord. 2011;134:315–319. doi: 10.1016/j.jad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Leunissen I, Coxon JP, Caeyenberghs K, Michiels K, Sunaert S, Swinnen SP. Subcortical volume analysis in traumatic brain injury: the importance of the fronto-striato-thalamic circuit in task switching. Cortex. 2014;51:67–81. doi: 10.1016/j.cortex.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Levine B, Kovacevic N, Nica EI, Cheung G, Gao F, Schwartz ML, Black SE. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70:771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- 42.Little DM, Kraus MF, Joseph J, Geary EK, Susmaras T, Zhou XJ, Pliskin N, Gorelick PB. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutkenhoff ES, McArthur DL, Hua X, Thompson PM, Vespa PM, Monti MM. Thalamic atrophy in antero-medial and dorsal nuclei correlates with six-month outcome after severe brain injury. Neuroimage Clin. 2013;3:396–404. doi: 10.1016/j.nicl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP, Grossman RI. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am J Neuroradiol. 2002;23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- 45.Maller JJ, Thomson RH, Pannek K, Bailey N, Lewis PM, Fitzgerald PB. Volumetrics relate to the development of depression after traumatic brain injury. Behav Brain Res. 2014;271:147–153. doi: 10.1016/j.bbr.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 46.Mathias JL, Dennington V, Bowden SC, Bigler ED. Community versus orthopaedic controls in traumatic brain injury research: how comparable are they? Brain Inj. 2013;27:887–895. doi: 10.3109/02699052.2013.793398. [DOI] [PubMed] [Google Scholar]
- 47.Mayer AR, Hanlon FM, Ling JM. Gray matter abnormalities in pediatric mild traumatic brain injury. J Neurotrauma. 2015;32:723–730. doi: 10.1089/neu.2014.3534. [DOI] [PubMed] [Google Scholar]
- 48.McCauley SR, Wilde EA, Bigler ED, Chu Z, Yallampalli R, Oni MB, Wu TC, Ramos MA, Pedroza C, Vasquez AC, Hunter JV, Levin HS. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. J Neurotrauma. 2011;28:503–516. doi: 10.1089/neu.2010.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 50.Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Palacios EM, Sala-Llonch R, Junque C, Fernandez-Espejo D, Roig T, Tormos JM, Bargallo N, Vendrell P. Long-term declarative memory deficits in diffuse TBI: correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex. 2013;49:646–657. doi: 10.1016/j.cortex.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Pietrzak RH, Averill LA, Abdallah CG, Neumeister A, Krystal JH, Levy I, Harpaz-Rotem I. Amygdala-hippocampal volume and the phenotypic heterogeneity of posttraumatic stress disorder: a cross-sectional study. JAMA Psychiatry. 2015;72:396–398. doi: 10.1001/jamapsychiatry.2014.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasad MR, Swank PR, Ewing-Cobbs L. Long-term school outcomes of children and adolescents with traumatic brain injury. J Head Trauma Rehabil. 2016 doi: 10.1097/HTR.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am. 2014;37:1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riedy G, Senseney JS, Liu W, Ollinger J, Sham E, Krapiva P, Patel JB, Smith A, Yeh PH, Graner J, Nathan D, Caban J, French LM, Harper J, Eskay V, Morissette J, Oakes TR. Findings from structural MR imaging in military traumatic brain injury. Radiology. 2015;279:207–215. doi: 10.1148/radiol.2015150438. [DOI] [PubMed] [Google Scholar]
- 57.Roozendaal B, de Quervain DJ, Ferry B, Setlow B, McGaugh JL. Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. J Neurosci. 2001;21:2518–2525. doi: 10.1523/JNEUROSCI.21-07-02518.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar A, Yashwanth B, Sarkar S. Analysis of blast induced intracranial pressure dynamics in cerebrospinal fluid leading to traumatic brain injury. Int J Emerg Multidiscip Fluid Sci. 2013;3:135–144. [Google Scholar]
- 59.Satz PS, Alfano MS, Light RF, Morgenstern HF, Zaucha KF, Asarnow RF, Newton S. Persistent post-concussive syndrome: a proposed methodology and literature review to determine the effects, if any, of mild head and other bodily injury. J Clin Exp Neuropsychol. 1999;21:620–628. doi: 10.1076/jcen.21.5.620.870. [DOI] [PubMed] [Google Scholar]
- 60.Schonberger M, Ponsford J, Reutens D, Beare R, O’Sullivan R. The Relationship between age, injury severity, and MRI findings after traumatic brain injury. J Neurotrauma. 2009;26:2157–2167. doi: 10.1089/neu.2009.0939. [DOI] [PubMed] [Google Scholar]
- 61.Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte I, Lin AP, Westin CF, Kikinis R, Kubicki M, Stern RA, Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strangman GE, O’Neil-Pirozzi TM, Supelana C, Goldstein R, Katz DI, Glenn MB. Regional brain morphometry predicts memory rehabilitation outcome after traumatic brain injury. Front Hum Neurosci. 2010;4:182. doi: 10.3389/fnhum.2010.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tasker RC. Changes in white matter late after severe traumatic brain injury in childhood. Dev Neurosci. 2006;28:302–308. doi: 10.1159/000094156. [DOI] [PubMed] [Google Scholar]
- 65.Tate DF, Khedraki R, Neeley ES, Ryser DK, Bigler ED. Cerebral volume loss, cognitive deficit, and neuropsychological performance: comparative measures of brain atrophy: II. Traumatic brain injury. J Int Neuropsychol Soc. 2011;17:308–316. doi: 10.1017/S1355617710001670. [DOI] [PubMed] [Google Scholar]
- 66.Tate DF, York GE, Reid MW, Cooper DB, Jones L, Robin DA, Kennedy JE, Lewis J. Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav. 2014;8:102–109. doi: 10.1007/s11682-013-9257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tator CH, Davis HS, Dufort PA, Tartaglia MC, Davis KD, Ebraheem A, Hiploylee C. Postconcussion syndrome: demographics and predictors in 221 patients. J Neurosurg. 2016:1–11. doi: 10.3171/2015.6.JNS15664. [DOI] [PubMed] [Google Scholar]
- 68.van Rooij SJ, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med. 2015;45:2737–2746. doi: 10.1017/S0033291715000707. [DOI] [PubMed] [Google Scholar]
- 69.Vanderploeg RD, Belanger HG, Horner RD, Spehar AM, Powell-Cope G, Luther SL, Scott SG. Health outcomes associated with military deployment: mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Arch Phys Med Rehabil. 2012;93:1887–1895. doi: 10.1016/j.apmr.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 70.Wade BS, Valcour VG, Wendelken-Riegelhaupt L, Esmaeili-Firidouni P, Joshi SH, Gutman BA, Thompson PM. Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. Neuroimage Clin. 2015;9:564–573. doi: 10.1016/j.nicl.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Connor JM, Nagahara AH, Tuszynski MH. Rehabilitation drives enhancement of neuronal structure in functionally relevant neuronal subsets. PNAS. 2016;113:2750–2755. doi: 10.1073/pnas.1514682113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilde EA, Bouix S, Tate DF, Lin AP, Newsome MR, Taylor BA, Stone JR, Montier J, Gandy SE, Biekman B, Shenton ME, York G. Advanced neuroimaging applied to veterans and service personnel with traumatic brain injury: state of the art and potential benefits. Brain Imaging Behav. 2015;9:367–402. doi: 10.1007/s11682-015-9444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilde EA, Merkley TL, Bigler ED, Max JE, Schmidt AT, Ayoub KW, McCauley SR, Hunter JV, Hanten G, Li X, Chu ZD, Levin HS. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int J Dev Neurosci. 2012;30:267–276. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilke M, Kagan I, Andersen RA. Effects of pulvinar inactivation on spatial decision-making between equal and asymmetric reward options. J Cognit Neurosci. 2013;25:1270–1283. doi: 10.1162/jocn_a_00399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.