Abstract
The IL-2/IL-2R pathway is implicated in type 1 diabetes (T1D). While its role in regulatory T cell (Treg) biology is well characterized, mechanisms that influence IL-2 responses in effector T cells (Teff) are less well understood. We compared IL-2 responses in 95 healthy control and 98 T1D subjects. In T1D, low IL-2 responsiveness was most pronounced in memory Teff. Unlike Treg, CD25 expression did not influence the Teff responses. Reduced IL-2 responses in memory Teff were not rescued by resting, remained lower after activation and proliferation, and were absent in type 2 diabetes. Comparing basal IL-2 responses in resting versus activated cells, memory Teff displayed lower, but more sustained, responses to IL-2 overtime. These results suggest that T1D–associated defects in the Teff compartment are due to intrinsic factors related to activation. Evaluation of both Teff and Treg IL-2R signaling defects in T1D subjects may inform selection of therapies.
Keywords: IL-2, type 1 diabetes, CD4 T cell, signaling, activation
1. Introduction
Type 1 diabetes (T1D) is a complex autoimmune disease caused by the selective destruction of insulin producing beta cells in the pancreatic islet. This immune-mediated destruction of beta cells ultimately leads to a life-long dependence on exogenous insulin treatment. While the etiology of T1D is unknown, it is clear that both genetic and environmental factors contribute to disease susceptibility and progression. Beta-cell specific T cells are known to play a key role in T1D, yet there is also strong evidence that systemic immunological defects impact T1D pathogenesis. Gaining a better understanding of the character of the global immune response may lead to discovery of new areas for disease prevention and intervention.
Low dose IL-2 therapy augments the frequency of regulatory CD4 T cells (Treg), an IL-2 dependent cell-type that constitutively expresses high levels of CD25. There is great interest in using low dose IL-2 therapy to boost Tregs in T1D given that genetic variants in the IL-2/IL-2R Pathway are associated with T1D, and defects in both IL-2R signaling and Treg stability have been observed in T1D in humans and mouse models [1–5]. Beneficial clinical responses have been observed in low dose IL-2 trials in graft versus host disease, vasculitis, lupus and alopecia patients [6]. In these clinical settings, IL-2 therapy clearly augments Treg, but may also impact other cell types [2]. In a recent dose-escalation Phase I adaptive trial in T1D, a range of IL-2 doses that increase Treg frequency in peripheral blood was identified [7]. However, in the same trial, other cell types also responded to IL-2 including Teff at all doses tested and there was significant heterogeneity across subjects. This finding confirms initial trials with IL-2 therapy in T1D that suggest there is a narrow dosing window that varies across subjects to achieve Treg- specifc tolerogenic effects [8, 9]. To this point, it is imperative to better understand patient heterogeneity and underlying mechanisms of disease by clearly defining the degree, cell source, and cause of variability in IL-2/IL-2R signaling.
It remains to be determined to what degree IL-2/IL-2R signaling variability is due to differences in the intrinsic or extrinsic regulation of this pathway. Intrinsically in humans, genome wide association studies have identified multiple T1D–associated single nucleotide polymorphisms (SNPs) that are located in genes (IL-2, IL2RA, and PTPN2) that encode proteins in the IL-2/IL-2R signaling pathway [3,4]. Yet, this does not explain low IL-2 response in all subjects. IL-2R signaling is tuned at multiple levels with expression level of CD25 being key. Other intrinsic factors including signaling molecules and miRNA have been shown to control signaling in model systems [10, 11]. Extrinsically, metabolic factors and secreted receptors [12–14] regulate IL-2R signaling in other disease settings. In this study, we examine IL-2 responsiveness in 98 T1D subjects and 95 healthy controls and explore underlying mechanisms in Teff Our results demonstrate that disease impacts IL-2/IL-2R signaling most prominently in the CD4 Teff compartment. Furthermore, these effects are most likely acquired and intrinsic given that they are present in memory (but not naive) cells, are not present in type 2 diabetic (T2D) subjects, persist following activation and proliferation, and are altered by activation state.
2. Materials and Methods
2.1 Human subjects and sample handling
Peripheral blood mononuclear cells (PBMC) were collected from subjects enrolled in the Benaroya Research Institute (BRI) Immune Mediated Disease Registry and Repository. Written informed consent was obtained from all subjects according to IRB approved protocols at BRI, Seattle Washington, USA. Age and gender matched healthy controls were selected for each experiment based on lack of personal or family history of autoimmunity or asthma. Control and disease subjects were included in daily experiments to avoid batch effects.
2.2 Flow cytometric analysis
Thawed PBMC were rested for 1 hour at 37°C in serum free X-vivo media prior to stimulation with 25 IU/mL IL-2 for 10 minutes, consistent with a dose previously used to show differences in both Treg and Teff between control and T1D subjects [15, 16]. Stains and analyses were performed as described previously [15, 16]. Phosphorylated Signal of transducer and activator transcription 5 (pSTAT5) protein was detected in cell types identified with monoclonal antibodies (Table S1). To accurately measure IL-2 responses in CD25hi Treg, cells, an independent stain of CD25, FOXP3 and CD127 was used to determine percentage of CD25hi cells composed of >95% FOXP3 and CD127lo. This percentage of CD25hi was then applied to Treg analysis of pSTAT5 on a per subject basis as shown in Fig S1. For selected experiments, cells were stained for CD71 and HLA-DR concurrent with the IL-2 stimulation. To permit direct comparisons between samples acquired across days, instrument standardization was performed using 8 peak rainbow calibration beads (Spherotech, Lake Forest, IL) adjusting PMT voltages so that 7th peak mean fluorescent intensities for each parameter were consistent. Data were acquired using an LSR-Fortessa (BD Biosciences) with FACS Diva software and analyzed with FlowJo software version 9.5 (Tree Star, Ashland, OR). Samples were eliminated from analysis if viability upon thaw was <70%. Populations with less than 500 events were eliminated from analysis.
2.3 Genotyping
IL2RArs12722495, IL2RArs2104286, IL2RArs11594656, PTPN2rs478582 and PTPN2rs1893217 were genotyped as described previously [16].
2.4 In vitro activation cultures
Memory CD4 T cells were selected using no-touch Miltenyi memory CD4 T cell selection kits (mean purity of 97%; range 92–100). Memory CD4 T cells were then depleted of CD25hi cells prior to activation using positive CD25 Miltenyi MACS selection as performed previously [17, 18]. Cells were activated half maximally with plate-bound anti-CD3 (1 µg/mL, clone UCHT1) and soluble anti-CD28 (0.5 µg/mL clone CD28.2). Subjects were excluded if <10% of cells were activated by day 3 (1 control, 2 T1D). For some experiments, cells were labelled with CFSE prior to culture as per manufacturer’s instructions (Invitrogen).
2.5 Statistics
A two-tailed t-test or Mann Whitney test was used to compare two groups depending on distribution of the data. For comparisons of multiple groups, Kruskal-Wallis test was performed. All statistical calculations were performed using GraphPad Prism v6.05. Comparisons with p <0.05 were considered significantly different.
3. Results and Discussion
3.1 Attenuated IL-2 responses in T1D are prominent in memory CD4 Teff
Heterogeneity of subjects and cell types can confound some immunological findings in smaller studies. To better understand this heterogeneity, we compared IL-2 responses in T cell subsets in a large number (98 T1D and 95 healthy control) of subjects matched for age, gender and ethnicity (Table 1). We chose a dose of IL-2 known to elicit differences in all T cell subsets, and that is not driven exclusively by IL-2 availability and CD25 expression level. Importantly, this enabled measurement of IL-2 responsiveness in CD25hi Treg and CD25lo Teff, both of which are activated even with low doses of IL-2 in vivo [7]. This IL-2 response assay was highly reproducible (Fig. 1A, Fig. S1). We were able to perform post-hoc disease and selected genetic analysis due to substantial variation in subject characteristics including age of diagnosis, disease duration, and distribution of selected SNPs in the IL-2/IL-2R pathway.
Table 1.
Subject characteristics
| Cohort | Controls | T1D | T-mid1 | T-lo1 |
|---|---|---|---|---|
| n | 95 | 98 | 6 | 6 |
|
|
||||
| Age (yrs.); mean ± SD (range) | 32.8 ± 7.5 (18–49) | 32.6 ± 7.6 (18–48) | 29.5 ± 8.0 (18–38) | 28.6 ± 3.5 (24–33) |
|
|
||||
| Gender (% male) | 48% | 46% | 66% | 66% |
|
|
||||
| Caucasian/white (%) | 100 | 100 | 100 | 100 |
|
|
||||
| Disease Duration (yrs.); mean ± SD (range) | NA | 13.6 ± 10.9 | 8.6 ± 5.3 | 7.0 ± 2.0 |
| (0.1–39) | (1.4–16.2) | (0.3–19.4) | ||
|
|
||||
| Age of diagnosis (yrs.); mean ± SD (range) | NA | 19.3 ± 10.1 | 19.6 ± 4.8 | 22.2 ± 6.3 |
| (0.9–46) | (11–27) | (13–31) | ||
|
|
||||
| IL-2 associated Genetics2 | ||||
|
|
||||
| IL2RArs2104286 (A, risk) | 0.764 | 0.799 | 1.00 | 1.00 |
|
|
||||
| IL2RArs11594656 (T, risk) | 0.723 | 0.758 | 0.833 | 0.666 |
|
|
||||
| PTPN2rs1893217 (C, risk) | 0.163 | 0.180 | 0.00 | 0.00 |
|
|
||||
| PTPN2rs478582 (T, risk) | 0.544 | 0.611 | 0.500 | 0.500 |
|
|
||||
T-mid and T-lo cohorts were pre-selected as T1D subjects with IL-2 responses comparable to controls (T-mid) and <2 SD of controls (T-lo). To eliminate the impact of alleles known to alter IL-2R signaling, IL2RArs12722495, IL2RArs2104286, and PTPN2rs1893217 were held constant.
Allele frequencies for each risk allele are shown. IL2RArs12722495 was also typed. However, the risk allele was only detected at an allele frequency of 0.082 in controls and 0.061 in T1D, limiting accurate analysis.
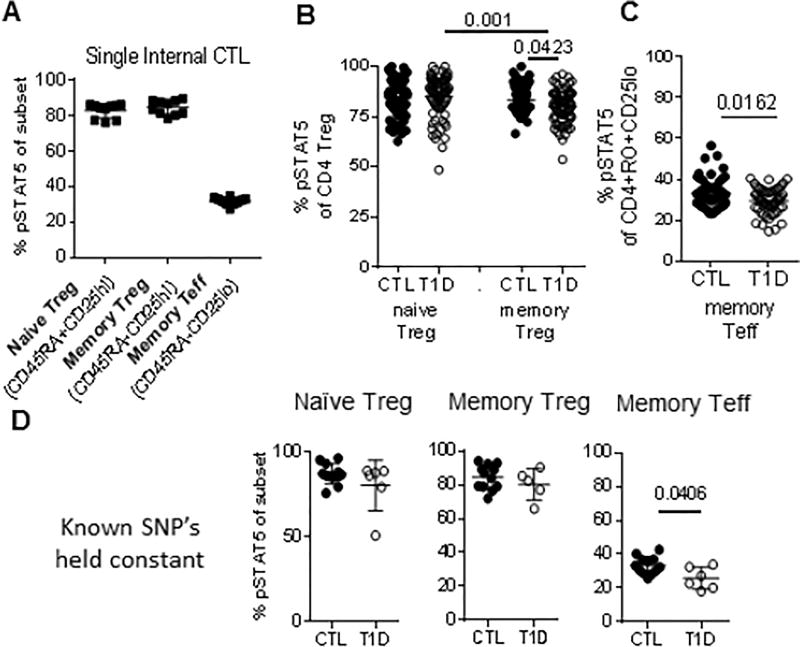
Figure 1. Prominent T1D-associated reduction in IL-2 responses in memory Teff.
PBMC from an internal control (n=10), experimental controls (n=83) and T1D (n=75) subjects were thawed, rested and stimulated with IL-2 as described in the methods. Gating was performed on live, singlet, lymphocytes using CD3, CD4, CD45RA and CD25 to identify T cell subsets as shown in Fig. S1 matching parallel stains of the same sample for CD25, FOXP3 and CD127 to define CD25hi FOXP3 Treg (A) Assay coefficient of variation for naive Treg (4.98%), memory Treg (4.79%) and memory Teff (6.61%) were determined using multiple vials from the same internal control, same draw thawed over the time-course of the experiment. Response to IL-2 in (B) Treg populations and (C) Teff was compared between experimental controls and T1D. Responses to IL-2 in naïve Teff were negligible (<10%) and did not differ between cohorts. Experimental cell types and cohorts in B differed by Kruskal-Wallis test (p<0.0001). All differences between groups in B and C were determined using a Mann-Whitney test. (D) The same subjects as in A were held constant for the common alleles PTPN2rs1893217 T/T, PTPN2rs478582 T/T, IL2RArs2104286 A/A and IL2RArs12722495 A/A. Cohorts were compared using a Mann Whitney test. CTL, closed circles; T1D, open circles.
In our large experimental cohorts, we found reduced IL-2R signaling in memory, but not naïve, Treg of T1D subjects as compared to controls (Fig. 1B). Decreased memory Treg response is consistent with our previous findings using smaller cohorts [15, 16], and in the study by Yang et al. using a lower in vitro IL-2 dose with extended kinetics [19]. Another study with a smaller cohort did not find such differences [20], and a study in autoantibody positive pre-T1D only found differences linked to CD25 genotype, but not disease [21]. While in previous work we functionally linked reduced IL-2 responses in T1D to reduced FOXP3 persistence and induction [15], Marwaha linked IL-17 production in memory FOXP3+ cells with a CD25 risk allele [21], and Yang et al. found differences in the proportion of Treg subsets when focusing on IL-2hi and lo outliers [19]. Collectively, studies to date demonstrate that subtle reductions in IL-2R signaling in naïve Treg of selected subjects and memory Treg of T1D subjects overall leadto reduced Treg stability and function. This is also consistent with subtle changes in steady-state signaling that result in functional consequences in potentially pathogenic T cells through altered balances in transcription factors and epigenetic status of STAT5-dependent genes [22–24].
Using the same subjects and assay, we next asked whether reduced IL-2 responses applied across cell types. Analyzing response to IL-2 in the memory CD4 Teff compartment, we conclusively found that in T1D IL-2 signaling was significantly reduced as compared to controls (Fig. 1C). Decreased memory Teff IL-2 responses are consistent with our previous findings using smaller cohorts [15, 16]. Other studies either did not compare Treg and Teff responses in the same subjects [21, 25], or used assays optimized for detecting IL-2 response in Treg driven by limiting amounts of IL-2 [19], or smaller cohorts size [20].
3.2 Low responses to IL-2 in memory Teff of T1D are independent of known T1D-associated genetic risk alleles
One cause of variability across cell types and subjects may be genetics. Thus, we analyzed the role of selected SNPs in the IL-2/IL-2R pathway previously associated with T1D and compared these to disease specific differences in IL-2 responsiveness. We detected association of PTPN2rs1893217 with reduced IL-2 response in memory Teff from control subjects, consistent with previous results [16, 19, 26] and association of PTPN2rs478582 with diminished IL-2 responsiveness only in naïve Treg from T1D subjects as observed by Yang et.al. [19] (data not shown). Neither of the PTPN2 SNPs completely explained the overall reduced IL-2R signaling observed in memory Treg and Teff of T1D. When restricting our analysis to individuals held constant for risk alleles at PTPN2 and IL2RA, we found reduced response to IL-2 in memory Teff of T1D compared with control subjects (Fig. 1D) suggesting that disease drives reduced IL- 2 responses in memory Teff of T1D through extrinsic factors, intrinsic factors and/or unknown genetics, or epigenetic changes.
3.3 CD25 expression level does not influence low response to IL-2 in memory CD4 Teff of T1D
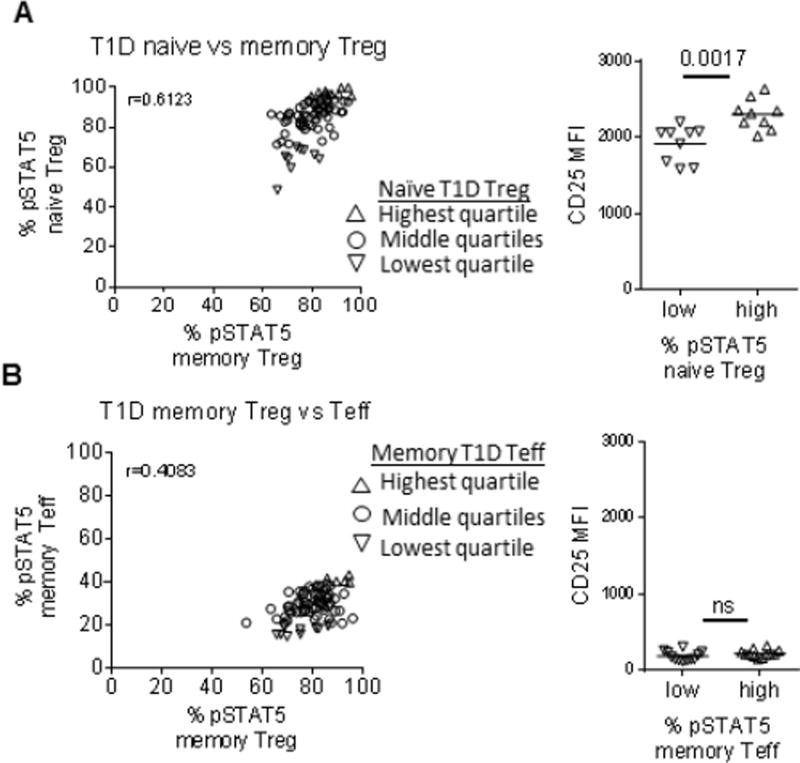
Expression level of CD25 is known to be one of the key determinants of IL-2 response. We compared responses to IL-2 in different CD4 T cell populations of T1D to determine whether CD25 expression correlated with IL-2 responsesin Teff cells. As expected, naïve and memory Treg responses in the same T1D subjects were significantly correlated (Fig. 2A), with lower IL-2 responders in the naïve Treg subset associating with lower CD25 expression levels. These data support a strong role for CD25 expression level in each individual in determining Treg IL-2 responses in T1D, as shown by others [19, 27, 28] and in children with T1D [25]. By comparison, IL-2 responsiveness in memory Treg was not as well correlated with memory Teff within the same subject (Fig. 2B). We found no significant difference in CD25 expression between high and low IL-2 responders in the memory Teff compartment, and other studies have not found differences in expression of the low affinity IL-2 receptor (CD122) between controls and T1D [10, 15]. This suggests that different factors may influence IL-2 responses in Treg versus Teff, resulting in a more pronounced effect in Teff observed here.. Since less is known about reduced IL-2 responses in Teff, , and low IL-2 responses in Teff are associated with differential effector responses [23, 29], we subsequently focused on this population to understand underlying mechanisms of reduced IL-2 signaling in T1D.
Figure 2. CD25 expression level does not define high and low IL-2 responders in resting memory Teff of T1D.
IL-2 responses from Fig. 1 in (A) naïve Treg versus memory Treg and (B) memory Treg versus memory Teff were compared using a Spearman correlation. CD25 mean fluorescence intensity (MFI) was compared between the highest (open up triangles) and lowest (open down triangles) quartiles for memory Teff and naïve Treg. Differences between groups were determined using a Mann-Whitney test.
3.4 Extrinsic factors in vivo do not attenuate IL-2 response in memory CD4 Teff of T1D
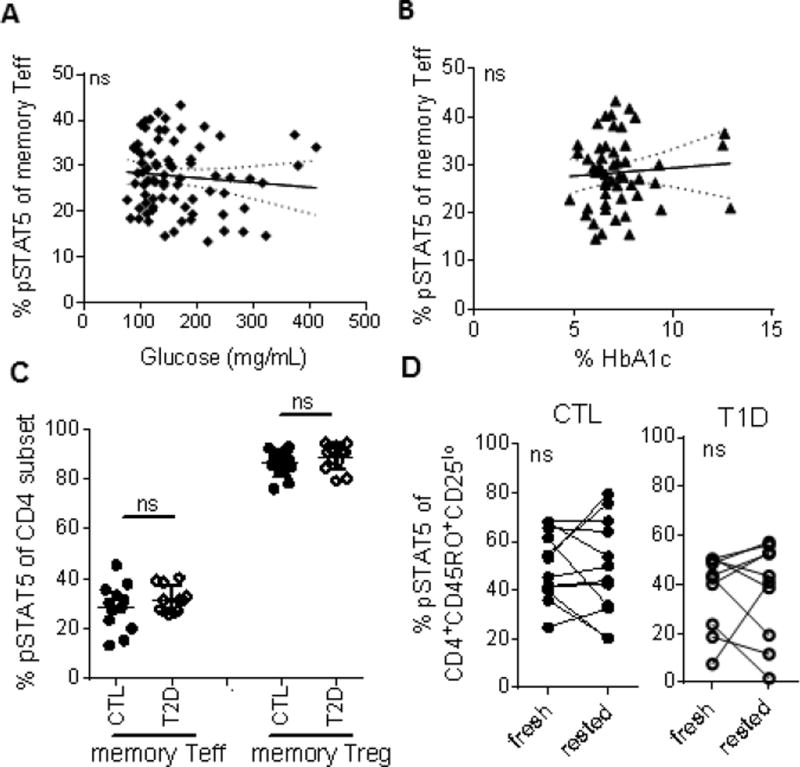
To determine whether extrinsic in vivo factors alter IL-2R signaling, we took three independent approaches. We first compared clinical metabolic measures of T1D subjects with IL-2 responses and found no correlations between IL-2 responses and glucose or hemoglobin A1c levels at time of draw (Fig. 3A, B). We also did not find a correlation with duration of disease or age at diagnosis (Fig. S2) in adult T1D subjects. Next, to test whether overt metabolic dysfunction alters IL-2 response, we assayed IL-2 responses in type 2 diabetic (T2D). Responses to IL-2 were not reduced in memory CD4 Teff or Treg of T2D subjects compared with control subjects (Fig. 3C). Lastly, we isolated fresh cells from PBMC and asked whether IL-2 signaling increased when Teff cells were removed from exposure to the in vivo microenvironment. Resting cells overnight in fresh media did not restore IL-2R signaling in T1D (Fig. 3D) even though extrinsically-driven lipid raft-associated defects in signaling of T cells from SLE subjects can be restored by incubation in fresh media [30],). Together, these data suggest that extrinsic factors in vivo do not play a dominant role in reducing IL-2R signaling in Teff from T1D. Instead, intrinsic factors are likely involved, consistent with our observation that IL-2 signaling is constant over-time in the same subject [15, 19]; this suggests a stable phenotype as opposed to transient alterations in signaling caused by in vivo oscillations in inflammatory or metabolic state.
Figure 3. In vivo factors of T1D do not overtly reduce IL-2 response in memory CD4 Teff of T1D subjects.
For a subset of subjects for whom baseline (A) glucose and (B) HbA1c values were available at time of draw, response to IL-2 as measured in Fig. 1 was compared to clinical measures. Solid lines represent linear regression and dashed lines represent 95% confidence intervals. No comparisons showed significant relationships. (C) Responses to IL-2 were assessed in thawed PBMC of age, gender, race, ethnicity and BMI (body mass index) matched control and type 2 diabetic (T2D) subjects as in Fig. 1. (D) Memory CD4 T cells were isolated from fresh blood of control and T1D subjects using no-touch Miltenyi MACS beads. Responses to IL-2 were assessed immediately after isolation (fresh) and after overnight rest (rested) in culture media. Time-points were compared using a paired t-test. CTL, closed circles; T1D, open circles; T2D, open diamonds.
3.5 In vitro activation through the T cell receptor enhances low IL-2 response
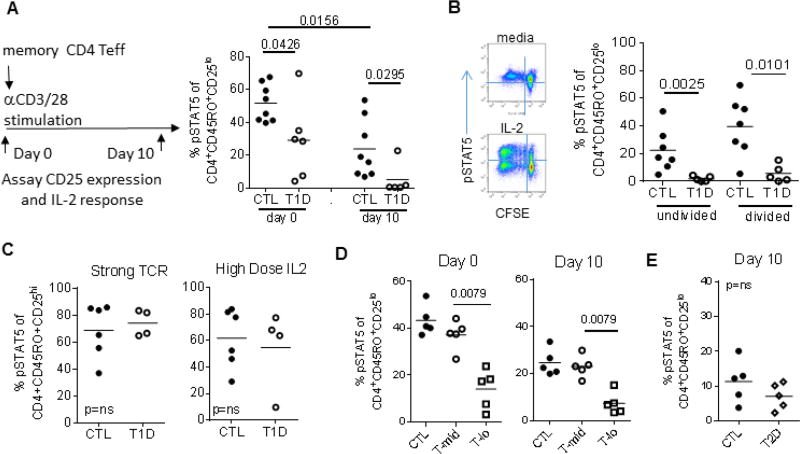
We next designed in vitro experiments to query possible intrinsic factors that may alter IL-2R signaling in T1D. We first compared IL-2 responses before and after in vitro activation comparing controls and T1D subjects held constant for variants in the IL-2R signaling pathway to eliminate known genetic influences. Memory CD4 Teff cells were activated with half- maximal TCR stimulation and response to IL-2 was measured when cells came back to rest at day 10 (Fig 4A). The frequency of activated memory CD4 Teff cells was similar between control and T1D at day 3 and day 10 as measured by CD25 expression (Fig. S3). Response to IL-2, as measured by pSTAT5, decreased with activation in both controls and T1D (Fig. 4A) suggesting that IL-2 responses are influenced by activation. Importantly, the difference between T1D and control T cells was evident both at the initiation of culture and after 10 days when cells returned to rest. Thus, half-maximal T cell receptor activation reduced IL-2 responses in all subjects, with a sub-group of T1D subjects having more profound loss of IL-2 responses both prior to and after activation.
Figure 4. Loss of IL-2 response is augmented with in vitro TCR activation.
(A) Isolated memory CD4+CD45RO+CD25− T cells from controls (n=8) and T1D (n=6) held constant for IL2RArs12722495, IL2RArs2104286, and PTPN2rs1893217 were activated half-maximally with plate-bound anti-CD3 (1 µg/mL) and soluble anti-CD28 (0.2 µg/mL) and assayed for IL-2 responses as diagramed on days 0 and 10. (B) Additional control (n=7) and T1D (n=5) subjects selected and stimulated as in A were labelled with CFSE prior to activation. Representative plots at day 10 with media (top) and IL-2 (bottom) are shown. Responses to IL-2 were measured in undivided (G0, right quadrants) and divided (all divisions, left quadrants) CD25lo cells. (C) Cells from control (n=6) and T1D (n=4) subjects were activated with 5 µg/mL plate-bound anti-CD3 and 1 µg/mL soluble anti-CD28 or multiple high doses (100 IU/mL) IL-2 on days 0, 3 and 7. Activation in high dose anti-CD3 cultures resulted in >70% of cells expressing high levels of CD25 through-out culture. (D) T1D with IL-2 responses comparable to controls (T-mid, open circles), and T1D with IL-2 responses <2 SD of controls (T-lo, open squares) and (E) age, gender and BMI matched controls and T2D were compared using the same cultures, assays and analyses. For all assays, responses to IL-2 were compared between groups using a Mann-Whitney test prior to culture and after 10 days when cells had come to rest. CTL, closed circles; T1D, open circles; T2D, open diamonds.
To determine whether low IL-2 signaling was due to differences in proliferation or outgrowth of non-responding cells, we CFSE labelled the memory CD4 Teff cells. The number of cell divisions by day 10 did not significantly differ between control and T1D subjects, nor did the frequency of undivided cells (data not shown). Following activation and proliferation, response to IL-2 was lower in Teff cells from T1D than controls in both the undivided and divided populations (Fig. 4B). Importantly, CD4 memory Teff did not lack the ability to respond to IL-2 since pSTAT5 of T1D subjects could be restored to comparable levels as controls when strong stimulation through the T-cell receptor or high doses of IL-2 were used (Fig. 4C). Rescue with high dose IL-2 is also consistent with the rescue of IL-2R signaling in vivo in recent onset T1D patients treated with rapamycin and IL-2 [24]. Thus, half-maximal activation through the TCR does not rescue IL-2 response in T1D, but instead, may augment it. This suggests that blunted IL-2 responsiveness may be an acquired, regulated phenotype consistent with diminished signaling in memory, but not naïve, T cells.
There exists, however, substantial heterogeneity in IL-2 responses across T1D subjects [19]. Thus, to more precisely test the hypothesis that reduced IL-2R signaling is intrinsic and acquired, we measured IL-2 response in memory CD4 T cells of subjects held constant for known risk alleles and defined by divergent IL-2 responses in prior assays: T-mid (T1D with pSTAT5 comparable to controls) and T-lo (T1D with pSTAT5 < 2 standard deviations below controls). Using this stratification, phenotypes were reproducible following isolation of memory Teff and measurement of IL-2 response on day 0 prior to culture (Fig. 4D). On day 10, the frequency and expression level of CD25 were similar (Fig S3), however responses to IL-2 remained markedly lower than controls only in T-lo subjects, but not T-mid subjects. Importantly, this was not due to overt metabolic differences since T cells from T2D subjects responded similarly to controls by day 10 of in vitro cultures (Fig. 4E). Together, these data suggest intrinsic factors related to activation likely influence basal IL-2 response, and the effect is more pronounced in T-lo subjects, but not T-mid consistent with heterogeneity of many T1D phenotypes [19, 31, 32].
3.6 Attenuation of IL-2 response is most profound in quiescent, not activated, memory CD4 Teff of T1D
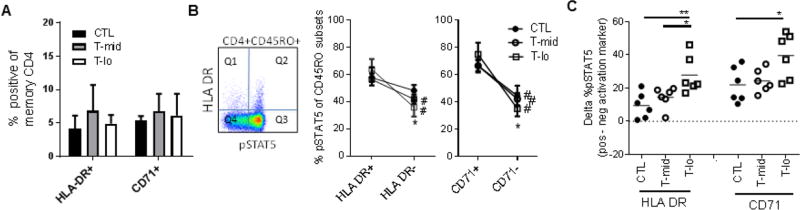
To further explore the role of activation on basal IL-2 responses in memory CD4 Teff cells, we compared basal IL-2 responses in cells defined by the presence or absence of known activation markers. Recently in vivo activated memory CD4 T cells expressing HLA-DR or CD71 composed less than a quarter of all memory CD4 T cells and we did not find a significant difference in the frequency of expression of activation markers between cohorts (Fig. 5A). Thus, the majority of memory CD4 Teff cells display a resting basal phenotype. Consistent with previous data, responses to IL-2 were lower in the T-lo cohort as compared to controls in the resting cells (Fig. 5B). This resulted in a greater difference between responses to IL-2 in activated versus resting T cells in the T-lo cohort (Fig. 5C). To rule out the possibility that this was due to variation in CD25 expression, we co-stained cells with CD25 and found that the level of CD25 expression on resting memory CD4 T cells did not differ (Fig. S4).
Figure 5. Dichotomy in IL-2 response in activated versus quiescent memory cells is greatest in T1D subjects with low basal response to IL-2.
Thawed PBMC were stained for the activation markers CD71 and HLA-DR in addition to CD4, CD45RO and pSTAT5. (A) Percent of memory Teff CD4+CD45RO+ cells expressing activation markers in CTL, T-mid and T-lo cohorts (n=6/group) as defined in Fig. 4 are shown. No significant differences by ANOVA were observed between groups for any marker. (B) The frequency of pSTAT5+ cells in CD4+CD45RO+ memory cells stratified by expression of HLA-DR and CD71 was determined as shown for a representative plot. Proportion pSTAT5+ cells in activated cells (Q2/(Q1+Q2)) and resting cells (Q3/(Q3+Q4)) was determined for all subjects (n=6/group). Gates are based on no IL-2 stimulation controls. Significance between activated (HLA-DR+, CD71+) and resting (HLA-DR−, CD71−) subsets for the same subject was determined using a paired t-test and is noted with a number symbol (#). Significance between cohorts for each CD4 memory T cell subset was determined using an unpaired t-test. Asterisk (*) denote a significant difference between T- lo and CTL. (C) Magnitude of the difference between the frequency of pSTAT5+ cells in activated and resting subsets was compared within activation markers using an ANOVA (CD71, p=0.0251; HLA DR, p=0.0066). Individual cohorts were then compared using Tukey’s multiple comparison test. * <0.05, ** < 0.05. CTL, closed circles; T-mid, open circles; T-lo, open squares.
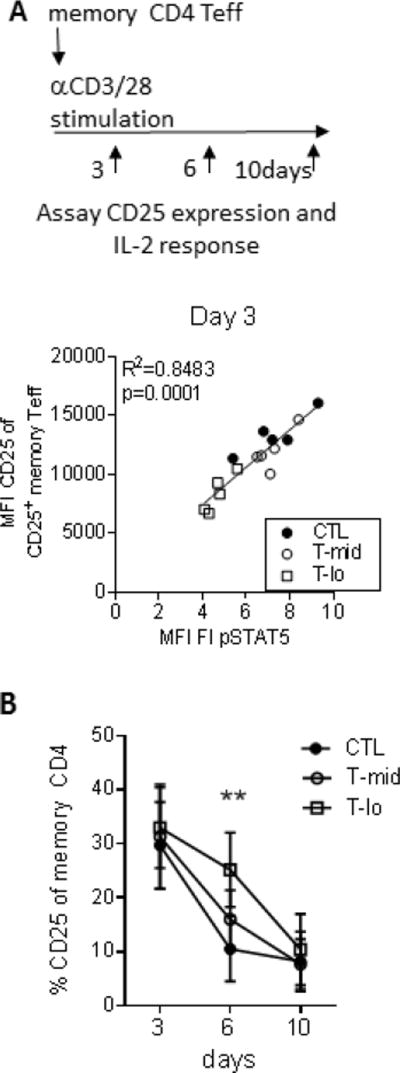
3.7 Weaker, but more sustained activation is evident in memory CD4 Teff of T1D
We measured activation and IL-2 response kinetics overtime to better understand how activation in T-lo subjects may influence IL-2 response. The level of CD25 expression upon activation directly correlated with the level of pSTAT5 at day 3, and T-lo displayed lower responses than T-mid and control subjects (Fig. 6A). Nevertheless, the frequency of CD25+ cells was similar across all groups at day 3 (Fig. 6B), as was also seen in Fig. 4. Interestingly, the frequency of CD25+ cells was maintained longer (through day 6) only in T-lo subjects. By day 10, the frequency and expression level of CD25 were similar across all groups, however responses to IL-2 remained markedly lower only in T-lo subjects (Fig. 6B). Taken together, activation in vitro reveals kinetic differences in responses to IL-2 in T-lo subjects that implicate a lower, but more sustained activation resulting in a lower response to IL-2 at rest (day 0 and 10).
Figure 6. Weaker, but more protracted activation is associated with low IL-2 response in resting memory Teff cells of T1D.
Isolated memory CD4+CD45RO+CD25− T cells from controls, T1D with IL-2 responses comparable to controls (T-mid) and T1D with IL-2 responses <2 SD of controls (T-lo) were activated half-maximally with plate-bound anti-CD3 and soluble anti-CD28 as in Fig. 4. Activation on day 3, 6 and 10 of culture was measured by flow cytometry. (A) On day 3, mean fluorescence intensity (MFI) of CD25 and fold increase (FI) of MFI pSTAT5 (IL-2 stimulated/unstimulated) were compared within the same subjects using linear regression. T-lo were significantly lower for pSTAT5 MFI (p=0.008) and CD25 MFI (p=0.016) than CTL and T-mid using a Mann Whitney test. (B) Frequency of CD25+ memory CD4 T cells (mean +/− SD) was compared between CTL, T-mid, and T-lo (n=5/group) at each time-point. Groups only differed at day 6 (Kruskal-Wallis, p=0.009). ** p<0.005, T-lo vs T-mid and CTL. CTL, closed circles; T-mid, open circles; T-lo, open squares.
These results suggest IL-2 responsiveness is modulated through a regulated process that involves intrinsic factors in memory CD4 Teff of some T1D subjects. The specificity of diminished response to IL-2 offers clues to the underlying mechanisms controlling this phenotype. Reduced IL-2 signaling is present in Teff of T1D, but not multiple sclerosis [16]. Deficiencies in IL-2 signaling have also been observed in naive T cells of systemic lupus erythematosus patients [33], but not memory cells [16],. Thus, reduced IL-2 signaling is observed in some, but not all, autoimmune subjects.
Reduced IL-2 responses are also observed in some advanced cancer settings and in subjects chronically infected with hepatitis C [34–36] suggesting that this phenotype in Teff may be linked to chronic activation or differentiation state. Molecularly, this may be caused by unknown genetics, epigenetics, differentiation, or divergence in signaling pathways as has been seen in other settings [34, 36–38]. T cell receptor activation is known to modulate cytokine signaling [39, 40]. Enhanced negative feedback has been observed in T cells of HIV subjects in the MAPK pathway [41, 42]. Phosphatases, SOCS proteins, PIM kinases and microRNAs are all examples of inhibitors that can negatively regulate cytokine responses and are activated upon T cell receptor engagement [1, 35, 43, 44]. In fact, in some T1D subjects, we found that high expression of the inhibitor PTPN2 reduces basal IL-2R signaling [15]. Further array-based and functional experiments are required to reveal inhibitory mechanisms that may vary by subject, but have a similar underlying cause and outcome.
4 Conclusion
This comprehensive study expands our understanding of underlying mechanisms of reduced IL-2R signaling in a cell-type specific manner, underscores the heterogeneity across subjects and cell types, and highlights the predominance of acquired, disease-associated IL-2R signaling defects in the Teff compartment in T1D that may be linked to chronic activation or differentiation state. Together, these Treg and Teff IL-2 signaling defects could inform selection of therapies for an individual. For example, the preponderance of reduced IL-2 signaling in Teff of T1D may favor Th17 and follicular helper cell differentiation [23, 29] or impact activation induced cell death, and therefore may be best therapeutically targeted through alternative pathways alone or in combination with IL-2 based therapies. By comparison, subjects with higher IL-2 response in Treg may respond to Treg-selective IL-2 therapy alone.
Supplementary Material
Highlights.
Low response to IL-2 is most prominent in memory CD4 Teff of T1D subjects
Multiple factors influence T1D–associated low IL-2 response in Teff
Low, but sustained TCR activation correlates with low basal IL-2R signaling
Activation enhances low IL-2 responsiveness suggesting a regulated response
Acknowledgments
We are very grateful to the investigators and staff of the BRI Diabetes Clinical Research Program for subject recruitment, as well as the BRI Translational Research Clinical Core for sample processing and handling. We thank all members of the Human Immunophenotyping Core who helped with standardization and acquisition of flow cytometry data in the large cohorts of controls and T1D subjects. We thank Anne Hocking for review of the article and assistance with submission.
Grant Support
This work was supported by a JDRF Career Development Award 3-2012-205 to SAL, and NIH grants AI101990, AI083455, and DK097672 to JHB.
Abbreviations
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- CTL
control
- Treg
regulatory T cell
- Tefff
effector T cell
- SNP
single nucleotide polymorphism
- pSTAT5
phosphorylated Signal Transducer and Activator of Transcription
- PBMC
peripheral blood mononuclear cells
- FDR
false discovery rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Long A, Buckner JH. Intersection between genetic polymorphisms and immune deviation in type 1 diabetes. Curr.Opin.Endocrinol.Diabetes Obes. 2013;20:285–291. doi: 10.1097/MED.0b013e32836285b6. [DOI] [PubMed] [Google Scholar]
- 2.Long SA, Buckner JH, Greenbaum CJ. IL-2 therapy in type 1 diabetes: "Trials" and tribulations. Clin.Immunol. 2013 doi: 10.1016/j.clim.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzwajg M, Churlaud G, Hartemann A, Klatzmann D. Interleukin 2 in the pathogenesis and therapy of type 1 diabetes. Current diabetes reports. 2014;14:553. doi: 10.1007/s11892-014-0553-6. [DOI] [PubMed] [Google Scholar]
- 4.Hulme MA, Wasserfall CH, Atkinson MA, Brusko TM. Central role for interleukin-2 in type 1 diabetes. Diabetes. 2012;61:14–22. doi: 10.2337/db11-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James CR, Buckle I, Muscate F, Otsuka M, Nakao M, Oon JS, Steptoe RJ, Thomas R, Hamilton-Williams EE. Reduced interleukin-2 responsiveness impairs the ability of T cells to compete for IL- 2 in nonobese diabetic mice. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.7. [DOI] [PubMed] [Google Scholar]
- 6.Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15:283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 7.Todd JA, Evangelou M, Cutler AJ, Pekalski ML, Walker NM, Stevens HE, Porter L, Smyth DJ, Rainbow DB, Ferreira RC, Esposito L, Hunter KM, Loudon K, Irons K, Yang JH, Bell CJ, Schuilenburg H, Heywood J, Challis B, Neupane S, Clarke P, Coleman G, Dawson S, Goymer D, Anselmiova K, Kennet J, Brown J, Caddy SL, Lu J, Greatorex J, Goodfellow I, Wallace C, Tree TI, Evans M, Mander AP, Bond S, Wicker LS, Waldron-Lynch F. Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial. PLoS Med. 2016;13:1002139. doi: 10.1371/journal.pmed.1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartemann A, Bensimon G, Payan C, Jacqueminet S, Bourran O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised double-blind placebo-controlled trial. The Lancet Diabetes and Endocrinology. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 9.Truman LA, Pekalski ML, Kareclas P, Evangelou M, Walker NM, Howlett J, Mander AP, Kennet J, Wicker LS, Bond S, Todd JA, Waldron-Lynch F. Protocol of the adaptive study of IL-2 dose frequency on regulatory T cells in type 1 diabetes (DILfrequency): a mechanistic non-randomised repeat dose open-label response-adaptive study. BMJ Open. 2015;5:009799. doi: 10.1136/bmjopen-2015-009799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, Malek TR. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms support the use of low-dose IL-2 therapy in Type-1 diabetes. Diabetes. 2015 doi: 10.2337/db14-1322. [DOI] [PubMed] [Google Scholar]
- 11.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat.Rev.Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien MW, Lin MH, Huang SH, Fu SH, Hsu CY, Yen BL, Chen JT, Chang DM, Sytwu HK. Glucosamine Modulates T Cell Differentiation through Down-regulating N-Linked Glycosylation of CD25. The Journal of biological chemistry. 2015;290:29329–29344. doi: 10.1074/jbc.M115.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, Zuchegna C, Romano A, De Simone S, Procaccini C, La Rocca C, Carrieri PB, Maniscalco GT, Salvetti M, Buscarinu MC, Franzese A, Mozzillo E, La Cava A, Matarese G. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol. 2015;16:1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong C, Luckey MA, Ligons DL, Waickman AT, Park JY, Kim GY, Keller HR, Etzensperger R, Tai X, Lazarevic V, Feigenbaum L, Catalfamo M, Walsh ST, Park JH. Activated T cells secrete an alternatively spliced form of common gamma-chain that inhibits cytokine signaling and exacerbates inflammation. Immunity. 2014;40:910–923. doi: 10.1016/j.immuni.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang ZY, Pihoker C, Sanda S, Greenbaum C, Buckner JH. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T cells of type 1 diabetic subjects. Diabetes. 2010;59:407–415. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerosaletti K, Schneider A, Schwedhelm K, Frank I, Tatum M, Wei S, Whalen E, Greenbaum C, Kita M, Buckner J, Long SA. Multiple Autoimmune-Associated Variants Confer Decreased IL-2R Signaling in CD4(+)CD25(hi) T Cells of Type 1 Diabetic and Multiple Sclerosis Patients. PLoS.ONE. 2013;8:83811. doi: 10.1371/journal.pone.0083811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long SA, Walker MR, Rieck M, James E, Kwok WW, Sanda S, Pihoker C, Greenbaum C, Nepom GT, Buckner JH. Functional islet-specific Treg can be generated from CD4(+)CD25(−) T cells of healthy and type 1 diabetic subjects. Eur.J.Immunol. 2009;39:612–620. doi: 10.1002/eji.200838819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JH, Cutler AJ, Ferreira RC, Reading JL, Cooper NJ, Wallace C, Clarke P, Smyth DJ, Boyce CS, Gao GJ, Todd JA, Wicker LS, Tree TI. Natural Variation in Interleukin-2 Sensitivity Influences Regulatory T-Cell Frequency and Function in Individuals With Long-standing Type 1 Diabetes. Diabetes. 2015;64:3891–3902. doi: 10.2337/db15-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marwaha AK, Panagiotopoulos C, Biggs CM, Staiger S, Del Bel KL, Hirschfeld AF, Priatel JJ, Turvey SE, Tan R. Pre-diagnostic genotyping identifies T1D subjects with impaired Treg IL-2 signaling and an elevated proportion of FOXP3+IL-17+ cells. Genes and immunity. 2017;18:15–21. doi: 10.1038/gene.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, Heuts F, Kogimtzis A, Patel S, Rosenthal M, Ono M, Sansom DM, Narendran P, Walker LS. Follicular helper T cell signature in type 1 diabetes. J Clin Invest. 2015;125:292–303. doi: 10.1172/JCI76238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dovat S, Payne KJ. STAT5 alters the state of transcriptional networks driving aggressive leukemia. Nat Immunol. 2017;18:597–598. doi: 10.1038/ni.3747. [DOI] [PubMed] [Google Scholar]
- 25.Parackova Z, Kayserova J, Danova K, Sismova K, Dudkova E, Sumnik Z, Kolouskova S, Lebl J, Stechova K, Sediva A. T regulatory lymphocytes in type 1 diabetes: Impaired CD25 expression and IL-2 induced STAT5 phosphorylation in pediatric patients. Autoimmunity. 2016:1–9. doi: 10.1080/08916934.2016.1217998. [DOI] [PubMed] [Google Scholar]
- 26.Long SA, Cerosaletti K, Wan JY, Ho JC, Tatum M, Wei S, Shilling HG, Buckner JH. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes Immun. 2011;12:116–125. doi: 10.1038/gene.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, Gasteiger G, Feng Y, Fontenot JD, Rudensky AY. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. 2016;17:1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dendrou CA, Plagnol V, Fung E, Yang JH, Downes K, Cooper JD, Nutland S, Coleman G, Himsworth M, Hardy M, Burren O, Healy B, Walker NM, Koch K, Ouwehand WH, Bradley JR, Wareham NJ, Todd JA, Wicker LS. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat.Genet. 2009;41:1011–1015. doi: 10.1038/ng.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker LS, von Herrath M. CD4 T cell differentiation in type 1 diabetes. Clinical and experimental immunology. 2016;183:16–29. doi: 10.1111/cei.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J.Clin.Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia. 2016;59:13–20. doi: 10.1007/s00125-015-3789-z. [DOI] [PubMed] [Google Scholar]
- 32.Arif S, Leete P, Nguyen V, Marks K, Nor NM, Estorninho M, Kronenberg-Versteeg D, Bingley PJ, Todd JA, Guy C, Dunger DB, Powrie J, Willcox A, Foulis AK, Richardson SJ, de Rinaldis E, Morgan NG, Lorenc A, Peakman M. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63:3835–3845. doi: 10.2337/db14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comte D, Karampetsou MP, Kis-Toth K, Yoshida N, Bradley SJ, Kyttaris VC, Tsokos GC. Brief Report: CD4+ T Cells From Patients With Systemic Lupus Erythematosus Respond Poorly to Exogenous Interleukin-2. Arthritis Rheumatol. 2017;69:808–813. doi: 10.1002/art.40014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J.Clin.Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Natarajan K, Xie Y, Burcu M, Linn DE, Qiu Y, Baer MR. Pim-1 kinase phosphorylates and stabilizes 130 kDa FLT3 and promotes aberrant STAT5 signaling in acute myeloid leukemia with FLT3 internal tandem duplication. PLoS.ONE. 2013;8:74653. doi: 10.1371/journal.pone.0074653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortarini R, Vegetti C, Molla A, Arienti F, Ravagnani F, Maurichi A, Patuzzo R, Santinami M, Anichini A. Impaired STAT phosphorylation in T cells from melanoma patients in response to IL-2: association with clinical stage. Clin.Cancer Res. 2009;15:4085–4094. doi: 10.1158/1078-0432.CCR-08-3323. [DOI] [PubMed] [Google Scholar]
- 37.Belot MP, Fradin D, Mai N, Le Fur S, Zelenika D, Kerr-Conte J, Pattou F, Lucas B, Bougneres P. CpG methylation changes within the IL2RA promoter in type 1 diabetes of childhood onset. PLoS One. 2013;8:68093. doi: 10.1371/journal.pone.0068093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc.Natl.Acad.Sci.U.S.A. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herndler-Brandstetter D, Brunner S, Weiskopf D, van RR, Landgraf K, Dejaco C, Duftner C, Schirmer M, Kloss F, Gassner R, Lepperdinger G, Grubeck-Loebenstein B. Post-thymic regulation of CD5 levels in human memory T cells is inversely associated with the strength of responsiveness to interleukin-15. Hum.Immunol. 2011;72:627–631. doi: 10.1016/j.humimm.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, August A. The signaling symphony: T cell receptor tunes cytokine-mediated T cell differentiation. J.Leukoc.Biol. 2014 doi: 10.1189/jlb.1RI0614-293R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu YL, Shan L, Huang H, Haupt C, Bessell C, Canaday DH, Zhang H, Ho YC, Powell JD, Oelke M, Margolick JB, Blankson JN, Griffin DE, Schneck JP. Sprouty-2 regulates HIV-specific T cell polyfunctionality. J.Clin.Invest. 2014;124:198–208. doi: 10.1172/JCI70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi H, Cho SY, Schwartz RH, Choi K. Dual effects of Sprouty1 on TCR signaling depending on the differentiation state of the T cell. J.Immunol. 2006;176:6034–6045. doi: 10.4049/jimmunol.176.10.6034. [DOI] [PubMed] [Google Scholar]
- 43.Malek TR. The biology of interleukin-2. Annu.Rev.Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 44.Katz G, Pobezinsky LA, Jeurling S, Shinzawa M, Van LF, Singer A. T cell receptor stimulation impairs IL-7 receptor signaling by inducing expression of the microRNA miR-17 to target Janus kinase 1. Sci.Signal. 2014;7:83. doi: 10.1126/scisignal.2005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.