Abstract
How organisms sense and respond to noxious temperatures is still poorly understood. Further, the mechanisms underlying sensitization of the sensory machinery, such as in patients experiencing peripheral neuropathy or injury-induced sensitization, are not well characterized. The genetically tractable Drosophila model has been used to study the cells and genes required for noxious heat detection,which has yielded multiple conserved genes of interest. Little is known however about the cells and receptors important for noxious cold sensing. Although, Drosophila does not survive prolonged exposure to cold temperatures (≤10 ºC), and will avoid cool, preferring warmer temperatures in behavioral preference assays, how they sense and possibly avoid noxious cold stimuli has only recently been investigated.
Here we describe and characterize the first noxious cold (≤10 ºC) behavioral assay in Drosophila. Using this tool and assay, we show an investigator how to qualitatively and quantitatively assess cold nociceptive behaviors. This can be done under normal/healthy culture conditions, or presumably in the context of disease, injury or sensitization. Further, this assay can be applied to larvae selected for desired genotypes, which might impact thermosensation, pain, or nociceptive sensitization. Given that pain is a highly conserved process, using this assay to further study thermal nociception will likely glean important understanding of pain processes in other species, including vertebrates.
Keywords: Neuroscience, Issue 122, sensory neuroscience, thermosensation, Drosophila neurons, thermal nociception, pain, cold nociception, behavioral assay, dendritic arborization neurons, transient potential receptor (TRP) channels.
Introduction
Drosophila has proven to be highly useful for the identification of novel conserved genes and neuronal circuits that underlie complex behaviors. Flies provide a sophisticated genetic toolkit and a simplified nervous system that allow for precise genetic and neuronal manipulation1,2,3,4 to dissect the cellular and molecular bases of nociception5,6,7. Larvae are particularly useful for these analyses, given that behavioral assays for gentle touch8,9,10, noxious heat11,12,13 and mechanical sensation of noxious stimuli4,11 have already been established, and the transparent larval cuticle allows for live or fixed imaging of the epidermis and underlying sensory neurons. Recently, an assay for noxious cold has also been developed7, which we describe in more detail here.
Using a fine, conical-tipped cold probe, we show that Drosophila larvae exhibit a set of cold-specific reactive behaviors, distinct from behaviors observed during normal locomotion, following gentle touch, or after harsh mechanical or high temperature stimuli7,8,11. The cold-specific behaviors include a robust full-body contraction (CT), a 45-90º raise of the posterior segments (PR) and a simultaneous raise of the anterior and posterior segments into a U-Shape (US). The prevalence of these behaviors increases with decreasing temperatures but each peaks at slightly different cold temperatures. Recent work suggests that CT responses are mediated by different peripheral sensory neurons than those that respond to noxious heat or harsh mechanical stimuli7.
Much like vertebrate nociceptors, Drosophila multiple dendritic (md) peripheral sensory neurons have complex dendritic structures that arborize over the epidermis1. md neurons are present in every larval body segment, projecting their axons to the ventral nerve cord14. md sensory neurons are separated into four different classes (I-IV) based on dendritic morphology and have varying sensory functions4,9,10,15,16,17. While class IV neurons are required for larval lateral body roll responses to high temperatures or harsh mechanical stimuli4, class III neurons are required for gentle touch responses9,10 and are not only activated by cold, but also are required for the cold-evoked behavioral responses7. Both class III and class IV neurons utilize discrete transient receptor potential (TRP) channels to facilitate behavioral responses to noxious7,11,18 and non-noxious stimuli9,10,17,19. Further, larval nociception is sensitized following injury, at the cellular20 and behavioral levels12,21.
The assay described here allows for the quantification of either normal, or potentially altered behavioral responses to cold temperatures ranging from noxious cold (≤ 10 ºC), innocuous cool (11-17 ºC), to ambient temperatures (18-22 ºC). The cold temperatures used in this assay are capable of directly activating class III sensory neurons, eliciting robust, reproducible calcium increases and cold-evoked behavioral responses, which can be qualitatively and quantitatively analyzed7. This assay can be applied to larvae of virtually any genotype as well as to larvae exposed to diverse environmental conditions (altered nutrition, injury, pharmacological agents) to determine both genetic and environmental factors that impact cold nociception, nociceptive sensitization or nociceptive plasticity. Given that thermosensation is ubiquitous across many species, this assay provides a valuable tool for the study of nociception and may uncover novel gene targets or neuronal interactions that will improve our understanding of vertebrate nociception.
The custom-built cold probe (see cold probe, Table of Materials) utilizes a closed loop temperature controlled Peltier device, which cools the aluminum shaft and conical tip through thermal conduction. A thermistor is embedded inside the aluminum conical tip reports the real-time temperature on the control unit. A heat sink and fan are attached to the thermoelectric module to regulate the Peltier effect's heat load (Qc) so the desired temperature range of (22-0 °C) can be achieved (see Thermal Control Unit, Table of Materials). The noxious cold stimulus of the cold probe tip is applied by hand to the dorsal midline, to segment(s) equidistant from anterior and posterior ends (roughly segment A4, see Figure 1A) of the larva. In response to cold stimuli, larvae generally produce one of three cold-evoked behaviors within a 10 s cutoff: a full body contraction (CT), a 45-90º raise of anterior and posterior segments into a U-Shape (US), or a raise of the posterior segments (PR) (described in Results). None of these behaviors are performed during normal peristaltic locomotion or foraging behavior. These behaviors are also distinct from gentle touch responses and the aversive rolling response to high temperature or noxious mechanical stimuli.
Protocol
1. Preparation of Larvae
- Raise stocks or genetic crosses in a 25 ºC incubator.
- If culturing a cross, use 20-25 virgin females and 15-20 males per vial containing regular cornmeal fly media. Allow females to lay eggs for approximately 48 h before transferring them to a new vial of food.
4-5 days after egg lay, collect 3rd instar larvae of the desired genotype by gently squirting a stream of water into the mushy food and larvae, and pour out the contents into a medium-sized, clean petri dish (60 mm x 15 mm).
Next, gently sort larvae by size using forceps or a paintbrush to preclude larger "wandering 3rd instar" or small sickly larvae from being tested. Discard larvae containing any undesired genetic markers or balancers.
Have a fellow lab member label dishes or containers where sorted larvae will be placed so that the experimenter can be "blind" to the experimental condition or genotype of the larvae if applicable. The experimenter can apply decoded labels to collected data after the experiment using a key generated by a lab-member.
Using a scoopula, transfer a dime-sized amount of fresh food into a (35 mm x 10 mm or 60 mm x 15 mm) clean, labeled Petri dish filled half-way with room temperature water.
Gently move the sorted larvae onto the food (to prevent starvation or desiccation if testing is anticipated to take more than 20 min) using forceps or paintbrush.
2. Cold Probe Assay
Turn on the cold probe unit allowing a few min for it to cool to the desired temperature. If setting to lower temperatures, condensation will likely form along the probe.
Wipe away any excess moisture with a laboratory wipe before application to the larva. When not in immediate use, place insulating cap on probe to both insulate and to keep the tip clean and prevent damage.
Place a mid 3rd instar larva onto a thin piece of dark moveable vinyl (typically, use a small piece cut from a notebook binder) under a bright field microscope. The black vinyl aids in contrast visualization and in moving the larva without touching it to align it properly with the probe.
Adjust the microscope (see Table of Materials) and light unit (see Table of Materials) to medium brightness (50-75% max brightness) to provide contrast and prevent the larva from drying out too quickly.
Discard any larvae that do not first exhibit normal peristaltic locomotion as they could confound the results. The larva should be moist from the Petri dish of water otherwise the larva will stick to the vinyl inhibiting normal locomotion. Water should not puddle around the larva.
Advance the probe by hand towards the larva at a 90º angle to the anteroposterior body axis, using the vinyl to move the larva into correct position (see Figure 1A).
Orient the tip of the probe to gently lay across the mid dorsal surface of the larva with the probe at approximately a 45º angle to the microscope stage.
Upon probe contact, start a laboratory timer. Apply enough downward pressure to slightly indent the surface of the larval cuticle while still allowing forward or backward movement.
Hold the probe in place for up to 10 s or until a cold-evoked behavioral response is observed - whichever occurs first. Then remove the probe.
Record the observed behavioral response and the response latency. Larvae that do not respond within 10 s are considered "non-responders". Latency can also be recorded for responders and used to measure changes in response robustness/amplitude.
Discard larva and prepare the next.
Repeat steps 2.3-2.11 until the desired number of test larvae is reached (three sets of n = 20-40 larvae were used here).
Representative Results
Drosophila larvae move with a peristaltic motion that includes occasional pauses, head turns, and changes in direction22. In response to focal application of a noxious cold stimulus however, larvae exhibit a set of unique behaviors, unlike the aversive lateral roll to noxious heat and mechanical stimuli. These behaviors are also different from responses to gentle touch8,9,10 or to the touch of a room temperature probe. The cold-evoked behaviors are as follows: scrunching their anterior and posterior segments towards the center of their body in a contraction (CT), raising of their anterior and posterior segments (approximately 45-90°) into the air, making a U-shape (US), or raising only their posterior segments (approximately 45-90°) into the air (PR) (Figure 1). The experimenter should be advised that these behavioral descriptions are qualitative guidelines rather than quantitative cut-offs. These responses are observed over a range of cold temperatures from 3-18 ºC (Figure 2A). However, different behaviors peak over different cold ranges (Figures 2B and 2C): 3-8 ºC for PR and US and 9-14 ºC for CT. CT is the only cold-evoked response occasionally observed in small percentage of larvae in response to gentle touch, using a room temperature (RT) probe (Figure 2). Latency information can also be collected and statistically compared using this assay (see below for statistical tests used), which may yield interesting information following injury or other environmental conditions.
To compare latencies of the different cold behaviors, cold-evoked responses were measured up to a 20 s cut-off at three cold-to-cool temperatures (3, 10 and 20 ºC) as well as to a room temperature (RT) probe and plotted as cumulative responses observed over time (Figure 3). First, we observed that most responses to cold temperatures (3 ºC and 10 ºC) are observed between 1 and 10 s after probe application (Figure 3A-3B), while CT responses appear to have longer frequencies at innocuous temperatures (20 ºC and room temperature (RT), Figure 3C-3D). Second, although US, PR and CT response versus latency curves were not significantly different from each other at 3 ºC (Figure 3A) at 10 ºC, 20 ºC and RT, the CT response versus latency curves were significantly different from US and/or PR, while US and PR curves were not significantly different from each other at any temperature tested (by Long-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon test, Figure 3B-3D). Interestingly, CT responses have significantly more "slow responders" (latency 3-10 s) than PR or US at 3 ºC when comparing latency data categorically (Figure 3E). For this type of categorical comparison, cold-evoked responses were grouped into three latency categories: less than 4 s, between 4-10 s, and between 11-20 s (Figure 3E-3H). The percentage of responders for each behavior is represented as a proportion of responders falling within each speed category (results were statistically compared between behaviors by a r x c contingency table). Again, this analysis revealed no significant differences between PR and US latency responses (Figure 3E-3H), but CT responses were significantly different from PR at 3 ºC and 10 ºC, and from US at all temperatures tested including the RT probe (Figure 3E-3F). Together, these data show that a 10 s assay cut-off is more than sufficient to collect meaningful data sets, and that the cold-evoked CT response occurs at relatively longer latencies than US or PR responses. Since there is some variability in responses from larva to larva, we tested three sets of 20 larvae at each temperature and reported the standard error of the mean for each response (Figure 3E-3H).
Since larval size (and therefore the relative size of the cold stimulus) can vary greatly between early and late 3rd instar larvae, early, middle and late 3rd larvae were tested at 3 ºC and 10 ºC and their responses were compared (Figure 4). Four to five day old larval cultures were used to collect larvae that were then grouped into early, mid or late 3rd instar stages roughly by their length/size (Figure 4). Interestingly, some response variability was seen between developmental stages depending on the temperature being tested. At 3 ºC, early 3rd instar larvae showed fewer PR and US responders and more CT responders (Figures 4A-4C). At 10 ºC however, no response varied substantially across developmental stages (Figures 4D-4F). Comparing the total percent responders at the 10 s cut-off for each behavior at 3 ºC or 10 ºC reveals that only early 3rd instar larvae have significantly different PR and CT responses at 3 ºC (Figures 4G and 4H).
Class III multiple dendritic sensory neurons are directly activated by cold temperatures and are required for the CT response to the cold probe7. Robust CT responses can also arise when a cold stimulus is applied to anterior regions of the larva (the head) (Figure 5B). To determine if cold-evoked responses resulting from direct stimulation of the head also require md neurons (including class III) a cold stimulus was gently applied to the head of larvae (Figure 5A) expressing a tetanus toxin transgene in all md sensory neurons effectively electrically silencing them3. When larvae are stimulated on the head with cold (6 ºC), a large percentage of larvae still produced a CT response, despite md neurons being silenced (Figure 5B). This data suggests md neurons are not required for a cold-evoked CT when probing the head, and this response must therefore require other types of sensory neurons, which could be identified using this tool and assay.
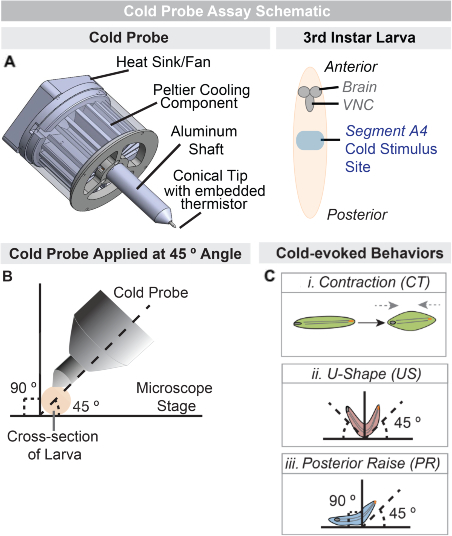 Figure 1: Cold probe assay. (A) Diagram of cold probe application to 3rd instar control (w1118) larva. The aluminum cold probe tip, set to the desired temperature, is lightly placed on the dorsal A4 segment of the larva for up to 10 s allowing free range of movement. (B) A cross-section of the larva indicating the angle of the probe as it is applied. The probe should be held at an approximate 45º angle to the microscope stage (dashed line), and perpendicularly to the anteroposterior body axis of the larva during the stimulation. (C) Diagram of the three cold-evoked behaviors observed: (i) Contraction (CT) of the anterior and posterior towards the center of the larval body (grey arrows), (ii) U-Shape (US): a 45-90 (dashed lines) raise of the anterior and posterior, and (iii) Posterior Raise (PR): a 45-90 (dashed lines) raise of the posterior of the head and tail towards the center of the larval body (grey arrows).
Figure 1: Cold probe assay. (A) Diagram of cold probe application to 3rd instar control (w1118) larva. The aluminum cold probe tip, set to the desired temperature, is lightly placed on the dorsal A4 segment of the larva for up to 10 s allowing free range of movement. (B) A cross-section of the larva indicating the angle of the probe as it is applied. The probe should be held at an approximate 45º angle to the microscope stage (dashed line), and perpendicularly to the anteroposterior body axis of the larva during the stimulation. (C) Diagram of the three cold-evoked behaviors observed: (i) Contraction (CT) of the anterior and posterior towards the center of the larval body (grey arrows), (ii) U-Shape (US): a 45-90 (dashed lines) raise of the anterior and posterior, and (iii) Posterior Raise (PR): a 45-90 (dashed lines) raise of the posterior of the head and tail towards the center of the larval body (grey arrows).
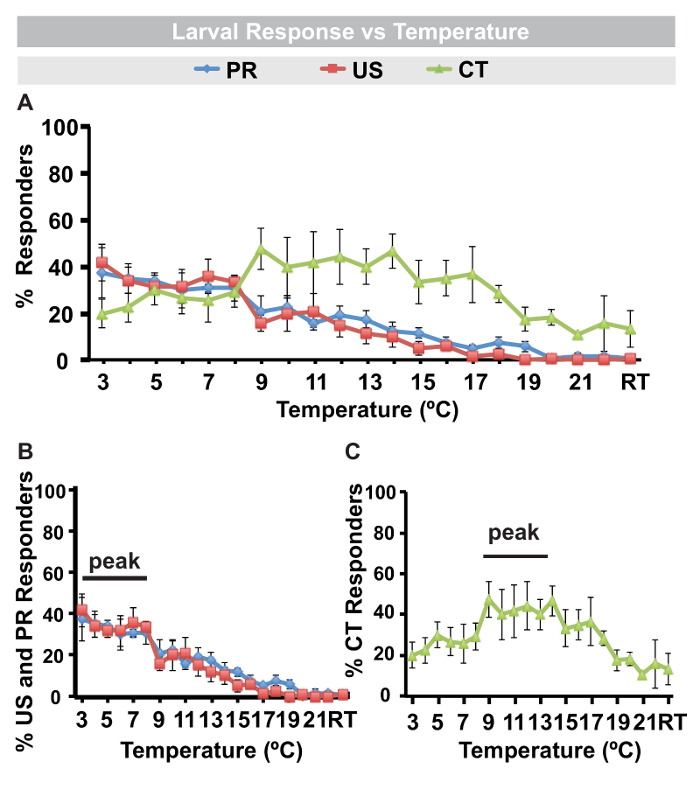 Figure 2: Cold-evoked response versus temperature. (A) Percent of responders in middle 3rd instar control (w1118) larvae over a range of cold temperatures (3-22 ºC) or room temperature (RT) probe. (B) Percent of PR, US and (C) CT responders at different cold temperatures with peak responses indicated. (A-C) PR: posterior raise, US: U-Shape, CT: Contract. Error bars are standard error of the mean for 3 sets of n = 40 larvae, responses averaged. Please click here to view a larger version of this figure.
Figure 2: Cold-evoked response versus temperature. (A) Percent of responders in middle 3rd instar control (w1118) larvae over a range of cold temperatures (3-22 ºC) or room temperature (RT) probe. (B) Percent of PR, US and (C) CT responders at different cold temperatures with peak responses indicated. (A-C) PR: posterior raise, US: U-Shape, CT: Contract. Error bars are standard error of the mean for 3 sets of n = 40 larvae, responses averaged. Please click here to view a larger version of this figure.
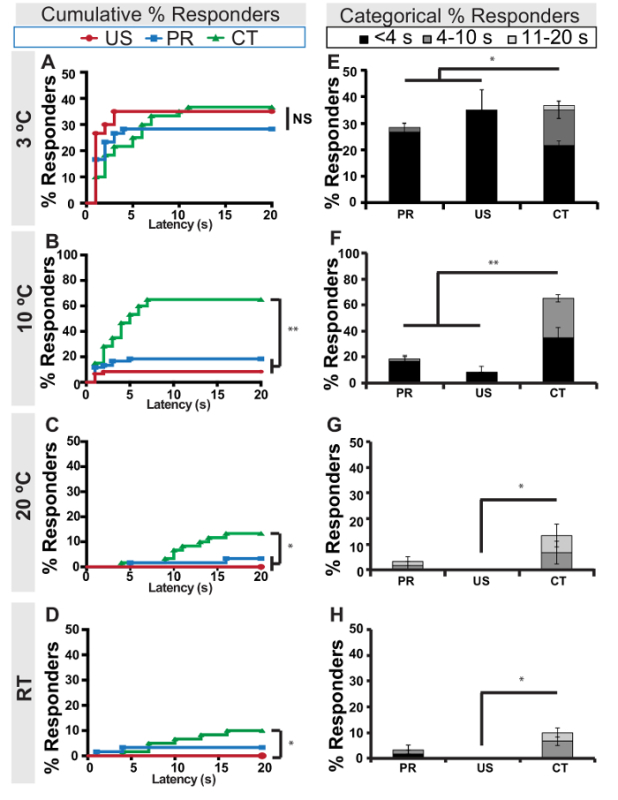 Figure 3: Cold-evoked response latency versus temperature. (A-D) Percent of cumulative US (red), PR (blue), or CT (green) responders to cold probe at various temperatures (3º C, 10 ºC, 20 ºC and RT) over time (1-20 s) in control (w1118) larvae. (E-H) Average proportions of fast (<4 s), slow (4-10 s), and late (11-12 s) responding larvae to cold probe at various temperatures (3º C, 10 ºC, 20 ºC and RT). (A-H) For each temperature, n = 3 sets of 40 larvae were tested. PR: posterior raise, US: U-Shape, CT: Contract, RT: Room temperature. (A-D) NS: no significant differences between data sets, * = p-value < 0.05, ** = p-value < 0.0001 by Long-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. (E-H) Error bars indicate s.e.m. * = p-value < 0.05, ** = p-value < 0.0001 by a r x c contingency table. Please click here to view a larger version of this figure.
Figure 3: Cold-evoked response latency versus temperature. (A-D) Percent of cumulative US (red), PR (blue), or CT (green) responders to cold probe at various temperatures (3º C, 10 ºC, 20 ºC and RT) over time (1-20 s) in control (w1118) larvae. (E-H) Average proportions of fast (<4 s), slow (4-10 s), and late (11-12 s) responding larvae to cold probe at various temperatures (3º C, 10 ºC, 20 ºC and RT). (A-H) For each temperature, n = 3 sets of 40 larvae were tested. PR: posterior raise, US: U-Shape, CT: Contract, RT: Room temperature. (A-D) NS: no significant differences between data sets, * = p-value < 0.05, ** = p-value < 0.0001 by Long-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. (E-H) Error bars indicate s.e.m. * = p-value < 0.05, ** = p-value < 0.0001 by a r x c contingency table. Please click here to view a larger version of this figure.
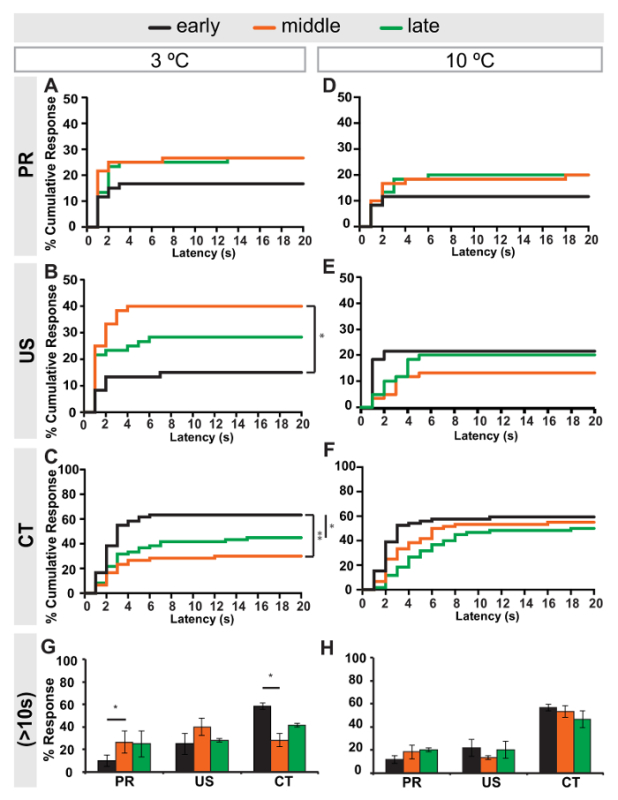 Figure 4: Cold-evoked response latency in early, middle and late 3rd instar larvae. Percent cumulative responders to a (A-C) 3 ºC or (D-F) 10 ºC cold probe over time (1-20 s) in early (black), middle (orange) or late (green) 3rd instar control (w1118) larvae. Responses versus latency are separated by behavior: PR (A, D), US (B, E), CT (A-F). * = p-value <0.05, ** = p-value < 0.001 by Long-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. (G-H) Percent of cumulative responders to a (G) 3 ºC or (H) 10 ºC cold probe within 10 s in early, middle or late 3rd instar larvae. Error bars indicate s.e.m. * = p-value < 0.05 by two-tailed Fisher's Exact test. n = 3 sets of 20 larvae. Please click here to view a larger version of this figure.
Figure 4: Cold-evoked response latency in early, middle and late 3rd instar larvae. Percent cumulative responders to a (A-C) 3 ºC or (D-F) 10 ºC cold probe over time (1-20 s) in early (black), middle (orange) or late (green) 3rd instar control (w1118) larvae. Responses versus latency are separated by behavior: PR (A, D), US (B, E), CT (A-F). * = p-value <0.05, ** = p-value < 0.001 by Long-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. (G-H) Percent of cumulative responders to a (G) 3 ºC or (H) 10 ºC cold probe within 10 s in early, middle or late 3rd instar larvae. Error bars indicate s.e.m. * = p-value < 0.05 by two-tailed Fisher's Exact test. n = 3 sets of 20 larvae. Please click here to view a larger version of this figure.
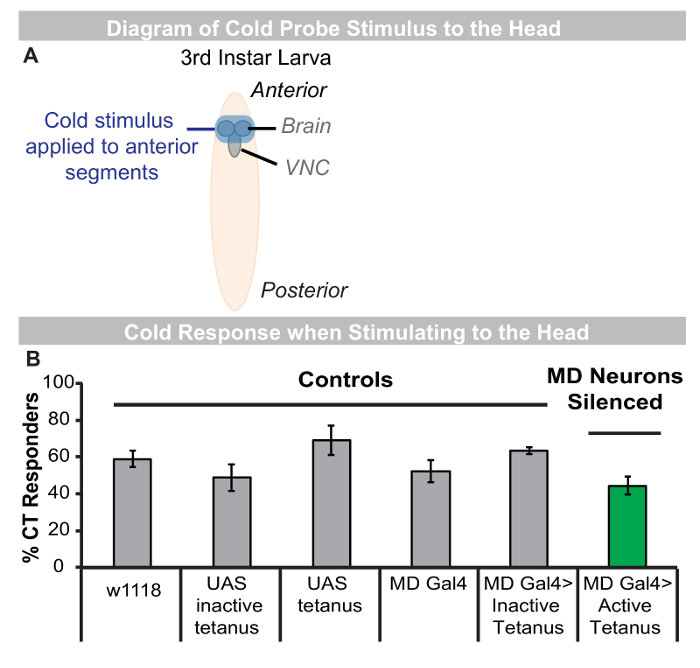 Figure 5: When stimulated in the head, cold-evoked CT responses do not require md sensory neurons. (A) Schematic of cold probe assay when anterior is stimulated in 3rd instar larva. (B) Average percent CT responders to cold probe (6 ºC) when larvae are stimulated in the most anterior segments. Grey bars: Genetic controls, Green bar: all md neurons silenced through expression of the tetanus toxin transgene. An inactive form of the tetanus toxin transgene was used as an additional control ('Inactive Tetanus'), n = 3 sets of 30 larvae per genotype. Green bar was not significantly reduced from all relevant genetic controls by two-tailed Fisher's Exact, test *p-value < 0.05. (B) Genotypes used (left to right): w1118 (control), UAS-inactive tetanus/+ (UAS alone control), UAS-tetanus/+ (UAS alone control), MD-Gal4/+ (Gal4 alone control), MD-Gal4/UAS-inactive tetanus (inactive tetanus control expressed in all md neurons), and MD-Gal4/UAS-active tetanus (experimental condition-active tetanus expressed in all md neurons). Please click here to view a larger version of this figure.
Figure 5: When stimulated in the head, cold-evoked CT responses do not require md sensory neurons. (A) Schematic of cold probe assay when anterior is stimulated in 3rd instar larva. (B) Average percent CT responders to cold probe (6 ºC) when larvae are stimulated in the most anterior segments. Grey bars: Genetic controls, Green bar: all md neurons silenced through expression of the tetanus toxin transgene. An inactive form of the tetanus toxin transgene was used as an additional control ('Inactive Tetanus'), n = 3 sets of 30 larvae per genotype. Green bar was not significantly reduced from all relevant genetic controls by two-tailed Fisher's Exact, test *p-value < 0.05. (B) Genotypes used (left to right): w1118 (control), UAS-inactive tetanus/+ (UAS alone control), UAS-tetanus/+ (UAS alone control), MD-Gal4/+ (Gal4 alone control), MD-Gal4/UAS-inactive tetanus (inactive tetanus control expressed in all md neurons), and MD-Gal4/UAS-active tetanus (experimental condition-active tetanus expressed in all md neurons). Please click here to view a larger version of this figure.
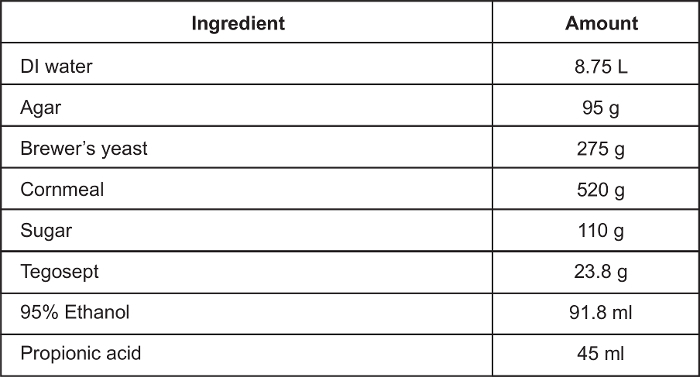 Table 1: Fly food recipe for cold probe assay.
Table 1: Fly food recipe for cold probe assay.
Discussion
The assay described here can be used to qualitatively and quantitatively assess nociception or nociceptive sensitization in larvae of various genetic backgrounds, environmental influences, and/or damage-induced conditions. Since this assay allows for focal application of a cold stimulus, with this tool one can assess the function of a subset of peripheral sensory neurons specifically in responding to cold temperatures. Interestingly, these cold-evoked behaviors seem to utilize different classes of sensory neurons than those activated by heat7,11, but the full thermal nociceptive circuit is not yet fully understood. Like many other local nociceptive assays this assay is subject to some variability between users depending on the application of the cold probe. However, the specific placement of the cold probe in the mid-body (avoiding the head and tail) does not appear to have a significant impact on cold responses7. Practice and repetition can minimize this inter-user variability.
We previously characterized the cold probe assay and reported the specific cold-responsive neurons and sensory channels required to produce the CT response7. Here we further characterize how latency data can be quantified and analyzed as another nociceptive measure, analyzing data from longer cut-off points to determine the most optimal time window for the assay. We found that the majority of cold-evoked responses occur within 10 second of stimulus application, making a 10 second cut off both economical and effective. Also, given that different cold-evoked behaviors peak at different temperatures, the latencies of these responses are important to analyze separately, as done here, to fully appreciate the robustness of the different responses at different temperatures. We also show, depending on the behavior being analyzed, how larval size may impact cold-evoked responses. Therefore, keeping larval size consistent in all behavioral experiments will be necessary. Lastly, we show how this assay can be used to identify, or rule out, sets of sensory neurons involved in cold nociception, which appears to vary depending on the location of cold stimulation to the larva.
Now that a baseline cold nociception assay exists, we can incorporate methods of damage and/or sensitization to ask more complicated questions about pain biology. For example, it is known that lateral body roll responses to innocuous or noxious heat are sensitized after UV damage to the epidermis, requiring specific genetic mediators and TRP channels12,20,21. Whether similar UV damage to the epidermis results in sensitized cold responses and whether similar genetic pathways are involved is unknown. Likewise, Drosophila are easily manipulated to induce wounds23,24 or expose to pharmaceutical drugs25,26, which can be used to interrogate morphological and genetic changes to nociceptive neurons and circuits.
There are a number of statistical tests that should be considered using this assay. The statistical test we used most commonly is the Fischer's exact test with a Bonferroni correction for multiple comparisons, or the chi-square test. These tests are used when comparing average responders in bar graph form (i.e. in Figure 3E-3H, Figure 4G-4H and Figure 5B). For comparing cumulative latencies in line graph form, we used Long-rank (Mantel-Cox) test27,28 and the Gehan-Breslow-Wilcoxon test29,30 which are both typically used to compare survival distributions. We suggest three sets of 20-40 larvae for each genotype/experiment to allow for calculation of standard error of the mean between repeated sets.
To conclude, this assay allows for precise application of a noxious cold stimulus and the resulting behavioral responses in Drosophila larvae have been characterized. There is still much we do not know about the cells and genes required for nociception in flies or vertebrates. With this assay, one can utilize the highly tractable Drosophila system to quickly screen for conserved genes crucial for baseline nociception, identify nociceptive circuits, and assess cellular and genetic changes that occur after tissue damage to cause nociceptive sensitization. Ultimately, this assay will help the pain field gain valuable information about pain biology in a system that can be readily applied to more complex animal models.
Disclosures
A United States patent application (CL) is pending on the design of the cold probe. Additional information on details of cold probe part numbers and construction, as well as additional consulting on tool design will be provided upon request.
Acknowledgments
We thank Sarah Wu and Camille Graham for developing early phases of the cold probe assay, the Bloomington Drosophila Stock Center for fly stocks, and Galko lab members for critically reading the manuscript. This work was supported by NIH NRSA (NIH F31NS083306) to HNT, and by NIH R01NS069828, R21NS087360 and a University of Texas MD Anderson Clark Fellowship in Basic Research to MJG.
References
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129(12):2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13(19):2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14(2):341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Hwang RY, et al. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17(24):2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Galko MJ. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev Dyn. 2012;241(1):16–26. doi: 10.1002/dvdy.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinkeviciute G, Gentile C, Neely GG. Drosophila as a tool for studying the conserved genetics of pain. Clin Genet. 2012;82(4):359–366. doi: 10.1111/j.1399-0004.2012.01941.x. [DOI] [PubMed] [Google Scholar]
- Turner HN, et al. The TRP Channels Pkd2, NompC, and Trpm Act in Cold-Sensing Neurons to Mediate Unique Aversive Behaviors to Noxious Cold in Drosophila. Curr Biol. 2016. [DOI] [PMC free article] [PubMed]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12(6):1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Caldwell JC, Tracey WD. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr Biol. 2012;22(22):2124–2134. doi: 10.1016/j.cub.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493(7431):221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113(2):261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19(10):799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A, Gilstrap AV, Galko MJ. Local and global methods of assessing thermal nociception in Drosophila larvae. J Vis Exp. 2012. p. e3837. [DOI] [PMC free article] [PubMed]
- Grueber WB, et al. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134(1):55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35(2):383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20(5):429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468(7326):921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely GG, et al. TrpA1 regulates thermal nociception in Drosophila. PLoS One. 2011;6(8):e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cameron S, Chang WT, Rao Y. Control of directional change after mechanical stimulation in Drosophila. Mol Brain. 2012;5:39. doi: 10.1186/1756-6606-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, et al. Tachykinin acts upstream of autocrine Hedgehog signaling during nociceptive sensitization in Drosophila. Elife. 2015;4:e10735. doi: 10.7554/eLife.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DT, et al. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 2011;21(18):1525–1533. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrigan D, Pepin DJ. How Maggots Move - Allometry and Kinematics of Crawling in Larval Diptera. J. Insect Physiol. 1995;41(4):329–337. [Google Scholar]
- Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2(8):E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra S, Wang Y, Brock AR, Galko MJ. Using Drosophila larvae to study epidermal wound closure and inflammation. Methods Mol Biol. 2013;1037:449–461. doi: 10.1007/978-1-62703-505-7_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar AC, Das TK, Shokat KM, Cagan RL. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature. 2012;486(7401):80–84. doi: 10.1038/nature11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63(2):411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RD. Multistate life-tables and regression models. Math Popul Stud. 1992;3(4):259–276. doi: 10.1080/08898489209525345. [DOI] [PubMed] [Google Scholar]
- Mantel N. Ranking procedures for arbitrarily restricted observation. Biometrics. 1967;23(1):65–78. [PubMed] [Google Scholar]
- Breslow N. A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika. 1970;57(3):579–594. [Google Scholar]
- Gehan EA. A generalized wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52:203–223. [PubMed] [Google Scholar]


