Abstract
Lymphocyte extravasation into the central nervous system (CNS) is critical for immune surveillance. Disease-related alterations of lymphocyte extravasation might result in pathophysiological changes in the CNS. Thus, investigation of lymphocyte migration into the CNS is important to understand inflammatory CNS diseases and to develop new therapy approaches. Here we present an in vitro model of the human blood-brain barrier to study lymphocyte extravasation. Human brain microvascular endothelial cells (HBMEC) are confluently grown on a porous polyethylene terephthalate transwell insert to mimic the endothelium of the blood-brain barrier. Barrier function is validated by zonula occludens immunohistochemistry, transendothelial electrical resistance (TEER) measurements as well as analysis of evans blue permeation. This model allows investigation of the diapedesis of rare lymphocyte subsets such as CD56brightCD16dim/- NK cells. Furthermore, the effects of other cells, cytokines and chemokines, disease-related alterations, and distinct treatment regimens on the migratory capacity of lymphocytes can be studied. Finally, the impact of inflammatory stimuli as well as different treatment regimens on the endothelial barrier can be analyzed.
Keywords: Immunology, Issue 122, lymphocyte extravasation, lymphocyte migration, CNS homing, blood-brain barrier, HBMEC, endothelium
Introduction
Lymphocyte migration from the blood into tissues is crucial for immune surveillance. A sequence of specific molecular interactions ensures site specific extravasation into small intestine, skin, lymph nodes, the central nervous system (CNS), and other tissues1. Alterations in lymphocyte migration are involved in the pathophysiology of a number of wide spread diseases2. Migration into the immune-privileged CNS is tightly regulated and accordingly alterations of this process are involved in CNS-related diseases like encephalomyelitis3, neuromyelitis optica, stroke, and multiple sclerosis (MS)2,4,5,6,7. Therefore, it is important to study lymphocyte extravasation to better understand disease pathophysiology and to develop tools for amelioration of disease burden8,9,10,11,12.
Lymphocytes migrate into the CNS via distinct routes. Extravasation through postcapillary venules into the subarachnoid space via the blood-cerebrospinal fluid barrier within the choroid plexus and across the blood-brain barrier have been described1,13,14,15. Migration across the blood-brain barrier is conducted by the interaction of lymphocytes with endothelial cells14. In contrast to endothelial cells in the periphery, endothelial cells of the CNS express high amounts of tight junction molecules, thereby strictly limiting the amount of cells and proteins capable of crossing the blood-brain barrier16. Inflammation results in loosening of tight junctions and induces the expression of adhesion molecules; thus, enhancing lymphocyte migration into the CNS1,17,18.
Extravasation via the blood-brain barrier is a multistep process. Lymphocytes tether to the endothelial cells and then roll along the endothelium in a process mainly mediated by selectins1,15. Subsequently, interactions between chemokines secreted by the endothelium and the respective chemokine receptors expressed on lymphocytes induce conformational changes of integrins, thereby promoting firm adhesion to the endothelial cells1. Finally, lymphocytes either crawl along the endothelial barrier against the blood flow before transmigrating into the perivascular space, or stall immediately and directly transmigrate at the site of firm adhesion1,19,20. All these steps of lymphocyte extravasation can be analyzed in vitro using distinct techniques21. Time-lapse video microscopy is used to study the initial tethering and rolling15. Adhesion assays provide detailed information about firm arrest to endothelial barriers22. Transmigration assays as demonstrated here allow analysis of immune-cell transmigration21,23,24,25,26,27,28,29.
Using the human in vitro blood brain barrier model, we could recently show that a higher migratory capacity of CD56brightCD16dim/- NK cells compared to their CD56dimCD16+ counterparts was reflected by a predominance of this NK cell subset in the intrathecal compartment21. Thus, our experimental setup seems to be suitable to mimic the in vivo situation.
Protocol
1. Cell Culture of Human Brain Microvascular Endothelial Cells (HBMEC)
- Coating of cell culture flasks
- To prepare the fibronectin solution, add 10 mL PBS to a 15 mL centrifuge tube. Add 150 µL fibronectin and mix well.
- To cover the bottom a T-25 cell culture flask add 2 mL of the fibronectin solution. Incubate the cell culture flask for at least 3 h at 37 °C in the incubator. Fibronectin coated flasks can be stored for 2 weeks at 37 °C / 5% CO2.
- Seeding and cell culture of HBMEC
- Aspirate fibronectin solution from the bottom of the cell culture flask. Add 7.2 x 104 HBMEC/cm² suspended in 6 mL ECM-b medium (= ECM-b supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin, and 1% endothelial cell growth supplement). Incubate at 37 °C / 5% CO2. Check cell growth daily using a microscope.
- Change the medium every 3 days. Harvest or split cells, when HBMEC reach approximately 80% confluence. HBMEC should be used between passage 1 and 15 to avoid loss of physiological properties.
- Harvest HBMEC.
- Prepare accutase solution by mixing accutase (1x) with PBS at a ratio of 1:1. Keep accutase solution at 37 °C in a water bath until further use.
- Transfer ECM-b medium from the cell culture flask to a 15 mL centrifuge tube. Wash HBMEC by adding 5 mL PBS to the bottom of the cell culture flask. Aspirate PBS and repeat the washing step two more times.
- Add 2 mL pre-warmed accutase solution. Incubate at 37 °C for 2 min. Afterwards, HBMEC are re-suspended by firmly tapping the cell culture flask several times. Cell detachment is controlled using a microscope
- The ECM-b-medium previously stored in a 15 mL tube is added back to the cell culture flask as soon as HBMEC start to detach. Rinse the bottom of the flask repeatedly until most HBMEC are re-suspended.
- Transfer the cell suspension to a 15 mL centrifuge tube. Centrifuge at 300 x g for 10 min at room temperature. Discard supernatant and re-suspend cells in 1 mL ECM-b medium. Count cells and dilute the cell suspension to achieve a final concentration of 3 x 105 HBMEC per mL ECM-b medium.
2. Preparation of the Cell Culture Inserts
- Coating of cell culture inserts Important note: Avoid touching the membrane of the cell culture inserts.
- Add 100 µL fibronectin solution (see 1.1.1) to each cell culture insert (Figure 1A) and one well of a 96-well flat bottom plate (optical control well). Incubate for at least 3 h at 37 °C. After incubation aspirate fibronectin solution.
- Add 100 µL HBMEC suspension to the cell culture inserts and the optical control well. Add 600 µl ECM-b medium to the lower compartment of the cell culture inserts. Incubate for 3 - 4 days at 37 °C / 5% CO2 until barrier integrity (Figure 1B) is reached, check cell growth by microscopic evaluation of the HBMEC in the optical control well. Note: Cell growth beyond four days is not recommended.
- Optional: To mimic inflammatory conditions aspirate the medium from the lower compartment and replace it with ECM-b medium supplemented with 500 U/mL IFN-γ/TNF-α 24 h prior the migration assay.
3. Quality Control with Evans Blue on the Day of the Transmigration Assay
- Preparation of evans blue solution
- To prepare PBS/B27 solution mix 10 mL PBS with 200 µl B27 supplement using a 15 mL centrifuge tube. Dilute evans blue stock solution (20 mg/mL PBS) 1:1,000 with PBS/B27.
- Evans blue permeability assay
- Aspirate the medium from the lower compartment followed by the upper compartment of one cell culture insert containing a confluent HBMEC monolayer. Add 100 µL evans blue solution to the cell culture insert.
- Add 600 µL PBS/B27 to the lower compartment and incubate for 60 min at 37 °C / 5% CO2. Carefully remove the cell culture insert using forceps.
- Evans blue measurement
- Remove PBS/B27 from the lower compartment and transfer 100 µL each to two wells of a black polystyrol 96-well flat bottom plate. Insert plate in a Tecan Infinite M200 Pro plate reader and determine optimal z-position.
- Measure excitation of evans blue using respective settings (for example: excitation: 620 nm, emission: 680 nm, excitation bandwidth: 9 nm, emission bandwidth: 20 nm, 175x enhancement, 25 flashes, time of integration: 20 µs).
- To determine HBMEC barrier functions compare acquired data to a standard curve depicting evans blue permeation across HBMEC at different time points after seeding cells (Figure 1B, right).
4. Migration Assay
- Preparation of peripheral blood mononuclear cells (PBMC).
- Add 10 mL RPMI into a 15 mL centrifuge tube and add 200 µL B27 supplement. Count PBMC and centrifuge cells at 300 x g for 5 min. Re-suspend PBMC to a final concentration of 5 x 106 cells/mL RPMI/B27.
- Set-up of the migration assay
- Aspirate medium from the lower compartment followed by the upper compartment of cell culture inserts containing confluent HBMEC monolayers (Figure 1A). Per donor add 100 µL PBMC suspension each to the cell culture inserts and also to one well of a 24-well plate per (in vitro control).
- Add 600 µL RPMI/B27 to the lower compartment of the cell culture inserts and 500 µL to the PBMC of the in vitro control and incubate 6 h at 37 °C / 5% CO2.
- Harvesting of migrated PBMC
- Take out the cell culture insert using forceps and carefully rinse the bottom with 400 µL PBS without touching the membrane. Discard the cell culture insert.
- Add 20 µL flow count fluorospheres (approximately 1,000 beads/µL) to the lower compartment of the cell culture insert as well as to the in vitro control and mix well. Transfer 1 mL of resulting PBMC suspension to flow cytometry tubes.
5. Flow Cytometry
- Sample preparation
- Centrifuge PBMC at 300 x g for 5 min at room temperature.
- Prepare antibody solution by adding fluorochrome-conjugated antibodies to 100 µL flow cytometry buffer (PBS/1% BSA/2 mM EDTA) per sample. For the results presented below 1 µL CD4-FITC, 1 µLCD3-PerCP/Cy5.5, 1 µL CD56-PC7, 1 µL CD8-A700, and 1 µL CD16-A750 were used per sample.
- Re-suspend PBMC in 100 µL of the antibody solution and incubate for 30 min at 4 °C.
- Add 250 µL flow cytometry buffer and centrifuge at 300 x g for 5 min.
- Sample acquisition
- Re-suspend PBMC in the required amount (varies depending on the flow cytometer used) of flow cytometry buffer.
- Acquire stained PBMC using a flow cytometer with an active detector between 525 and 700 nm wavelength to detect flow count fluorospheres (excitation 488 nm, emission 525 - 700 nm). (The following steps are an example if a Gallios flow cytometer operated with Kaluza G software is used: (1) Start the computer. (2) When the operating system is fully loaded, start the flow cytometer by pressing the "cytometer on" button. (3) Load the respective acquisition protocol by pressing "open protocol" button. (4) Choose the required protocol and select "open". (5) Duplicate the protocol for every sample by clicking with the right mouse button on the protocol visible in the virtual carousel and a left click on the field "duplicate". (6) Label each sample in the sample list. (7) Transfer the samples to the indicated positions of the carousel and start the acquisition.)
- Sample analysis
- Open resulting flow cytometry data using the respective software. Determine number of subpopulations of interest for transmigrated PBMC as well as cells from in vitro control wells and flow count fluorospheres using the respective analysis software. (An example of the gating strategy is given in the results part (Figure 1 C: To analyze the transmigration of NK-cell subsets, first select lymphocytes in a sideward scatter channel (SSC) versus forward scatter channel (FSC) plot. Lymphocytes are then displayed in a CD3 versus CD56 plot and CD56+CD3- NK cells are selected. To distinguish between NK-cell subsets, NK cells are displayed in a CD56 versus CD16 plot and CD56brightCD16dim/- as well as CD56dimCD16+ NK cells are selected. In addition, flow count fluorospheres are selected from a FSC versus SSC plot and subsequently displayed in a plot of a channel with an emission between 525 and 700 nm versus time to determine their number.)
- To calculate the total cell number of each sample, normalize the detected number of cells using flow count fluorospheres:

- Determine percentage of migrated cells as ratio between total migrated cells and total cells in the in vitro control.
Representative Results
Representative results showing transmigration of NK-cell and T-cell subsets using the human blood-brain barrier model (Figure 1A) are shown. The integrity of the HBMEC monolayer was validated by staining of the tight junction molecule ZO-1, transendothelial electrical resistance (TEER) measurements, and evans blue permeation (Figure 1B). Following 3 - 4 days culture HBMEC expressed the tight junction molecule ZO-1 (Figure 1B, left). Furthermore, HBMEC grew in monolayers exhibiting transendothelial electrical resistance (Figure 1B middle) as well as reduced permeation for evans blue (Figure 1B, right). HBMEC monolayers were used to study the transmigration of NK cells including CD56brightCD16dim/- and CD56dimCD16+ NK-cell subsets and T cells including CD4+ and CD8+ T cells as two examples (Figure 1D+E, respectively). The percentage of migrated cells was calculated based on cell counts obtained by flow cytometry and normalized using flow count fluorospheres as an internal control (Figure 1C). The HBMEC monolayer was stimulated with IFN-γ and TNF-α 24h prior the assay to mimic inflammatory conditions. Cytokine stimulation resulted in increased migration of all analyzed lymphocyte populations. This might be due to an increased expression of adhesion molecules including ICAM-1 (data not shown). CD56brightCD16dim/- NK cells exhibited a higher migratory capacity when compared to their CD56dimCD16+ NK across both unstimulated (10.88% vs. 0.86%) and IFN-γγ/TNF-α stimulated (18.22% vs. 2.94%) HBMEC (Figure 1D). However, the relative increase of transmigration as a result of inflammation was higher for the CD56dimCD16+ NK-cell subset (+342% vs. +167% for CD56brightCD16dim/-). These results mimic the in vivo observations that CD56brightCD16dim/- NK cells are enriched in the intrathecal compartment. Thus, the blood-brain barrier model seems to be suitable to analyze basic principles of immune-cell diapedesis of rare lymphocyte populations into the CNS21. Finally, the transmigratory capacity of CD4 and CD8 T-cell subsets is shown (Figure 1E).
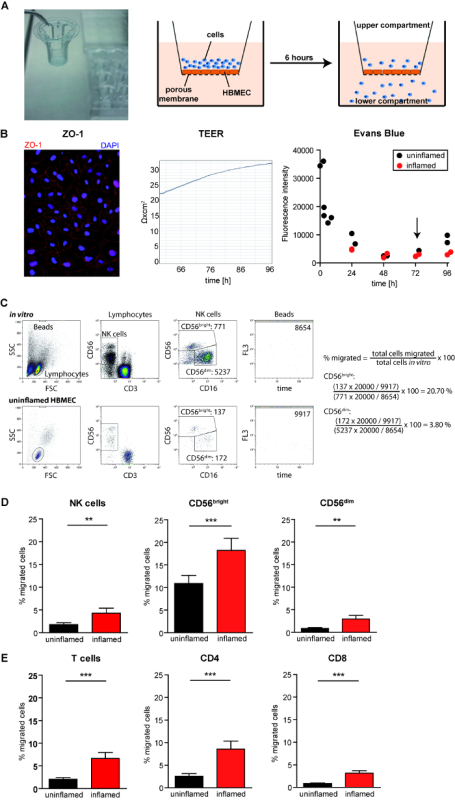 Figure 1: Differential Migration of NK-cell Subsets across Uninflamed and Inflamed HBMEC Monolayers. A. A picture of transwell inserts (left) and illustration of the experimental setup (right). B. Validation of HBMEC barrier functions. Left: immunohistochemical staining of the tight junction molecule ZO-1 using rabbit anti-human ZO-1 (abcam, 1:200) and goat anti-rabbit IgG-Cy3 (1:300) on HBMEC cultivated for 3 days. Center: transendothelial electrical resistance (TEER) of HBMEC between day 2 and day 4 of cultivation. Right: standard curve for evans blue permeation for HBMEC with ("inflamed", red) or without ("uninflamed", black) stimulation with 500 U/mL IFN-γ and TNF-α for 24 hours. Transmigration assays are performed 72 - 96 hours after seeding of the HBMEC (black arrow). C-E. PBMC derived from 16 healthy individuals were subjected to migration assays as described in the protocol section. 24 h prior to the assay, half of the cell culture inserts were stimulated with 500 IU/mL IFN-γ and TNF-α. Transmigrated cells were harvested and analyzed by flow cytometry. C. Representative results for PBMC derived from the in vitro control well (top) and after migration across uninflamed HBMEC (bottom). NK cells were gated from total lymphocytes as CD3-CD56+ cells and further distinguished into CD56brightCD16dim/- ("CD56bright") and CD56dimCD16+ ("CD56dim") NK-cell subsets. Flow count fluorospheres ("beads") were gated based on FSC/SSC characteristics and their number was determined in a FL3 versus time plot. Exemplary calculations to determine the percentage of migrated CD56bright and CD56dim NK cells are shown on the right. D. Percentage of migrated NK cells as well as CD56bright and CD56dim NK-cell subsets, and E. percentage of migrated T cells including CD4+ and CD8+ T-cell subsets following transmigration across uninflamed (black) or inflamed (red) HBMEC depicted as mean ± SEM. P-values were calculated by paired student t-test; **p<0.01, ***p<0.001. Please click here to view a larger version of this figure.
Figure 1: Differential Migration of NK-cell Subsets across Uninflamed and Inflamed HBMEC Monolayers. A. A picture of transwell inserts (left) and illustration of the experimental setup (right). B. Validation of HBMEC barrier functions. Left: immunohistochemical staining of the tight junction molecule ZO-1 using rabbit anti-human ZO-1 (abcam, 1:200) and goat anti-rabbit IgG-Cy3 (1:300) on HBMEC cultivated for 3 days. Center: transendothelial electrical resistance (TEER) of HBMEC between day 2 and day 4 of cultivation. Right: standard curve for evans blue permeation for HBMEC with ("inflamed", red) or without ("uninflamed", black) stimulation with 500 U/mL IFN-γ and TNF-α for 24 hours. Transmigration assays are performed 72 - 96 hours after seeding of the HBMEC (black arrow). C-E. PBMC derived from 16 healthy individuals were subjected to migration assays as described in the protocol section. 24 h prior to the assay, half of the cell culture inserts were stimulated with 500 IU/mL IFN-γ and TNF-α. Transmigrated cells were harvested and analyzed by flow cytometry. C. Representative results for PBMC derived from the in vitro control well (top) and after migration across uninflamed HBMEC (bottom). NK cells were gated from total lymphocytes as CD3-CD56+ cells and further distinguished into CD56brightCD16dim/- ("CD56bright") and CD56dimCD16+ ("CD56dim") NK-cell subsets. Flow count fluorospheres ("beads") were gated based on FSC/SSC characteristics and their number was determined in a FL3 versus time plot. Exemplary calculations to determine the percentage of migrated CD56bright and CD56dim NK cells are shown on the right. D. Percentage of migrated NK cells as well as CD56bright and CD56dim NK-cell subsets, and E. percentage of migrated T cells including CD4+ and CD8+ T-cell subsets following transmigration across uninflamed (black) or inflamed (red) HBMEC depicted as mean ± SEM. P-values were calculated by paired student t-test; **p<0.01, ***p<0.001. Please click here to view a larger version of this figure.
Discussion
Here we present a technique to investigate the transmigration of lymphocytes across the human blood-brain barrier. In vitro analysis of lymphocyte migration to the CNS is important to study basic processes of lymphocyte extravasation, potential disease-related alterations, and new therapeutic approaches.
Several modifications of the blood-brain barrier model are possible. For example, cells from the upper compartment could be analyzed to investigate the composition of the non-migrated cell population. Furthermore, treatment of the HBMEC monolayer with IFN-γ and TNF-α 24 hours prior to the assay can be used to mimic an inflamed blood-brain barrier in order to study the effect of inflammatory CNS disorders21,25. Similarly, treatment of HBMEC or lymphocytes with other substances allows investigating their effects on lymphocyte extravasation (e.g. their effect on adhesion molecules)30,31. The involvement of certain adhesion molecules can be studied using blocking antibodies for integrins or their ligands32. Furthermore, the experimental setup presented here allows analysis of chemotactic effects from chemokines or supernatants derived from astrocytes or other cells33,34. Replacement of HBMEC with primary human brain derived epithelial cells widens the spectrum of this experimental setup for investigation of the blood-cerebrospinal fluid barrier15. HBMEC should not be replaced with immortalized endothelial cells or cells derived from other organs to maintain CNS-specificity of the model. However, brain-derived endothelial cells from other species might be used to analyze transmigration in the respective animals35. In addition, retinoic acid or hydrocortisone have been described to increase barrier functions and might thus be employed36,37. In case of limited cell numbers the amount of cells subjected to the assay might be reduced, because our model provides linear recovery rates between 2 x 105 and 1 x 106 PBMC (data not shown). To analyze rare cell populations following transmigration it might be necessary to pool cells from several wells in order to obtain sufficient cells required for flow cytometry. Finally, our experimental setup could also be used to study the drug delivery into the CNS38. A pore size of 3 µm is typically used to allow lymphocyte transmigration, whereas a pore size of 0.4 µm prevents lymphocyte transmigration, but allows to study drug delivery39,40,41,42.
Despite the various applications of the experimental setup described here, several points have to be kept in mind to ensure meaningful results. The integrity of the porous membrane as well as the HBMEC monolayer is critical. Thus, it is mandatory to avoid physical damage of the membrane, for example with pipet tips. Addition of fluids or cell suspension to the upper part of the cell culture insert should be performed without direct contact. Droplets might be wiped off at the border of the cell culture insert. Horizontal placement of the pipet tip ensures protection of the membrane, when the abluminal membrane is rinsed at the end of the migration assay. The quality of the HBMEC monolayer can be analyzed by microscopic evaluation of confluence as well as evans blue permeation as demonstrated for the assessment of blood-brain barrier integrity in rodent experiments43. Centrifugation of HBMEC at low g forces and usage of early passages (i.e. 1 - 15) ensures quality and uniformity of the monolayer. To prevent dissolution from the porous membrane, it is important to adhere to the described order of aspiration and addition of medium, when working with the HBMEC monolayer.
Although adherence to the protocol described here ensures meaningful and reproducible results, this technique has some limitations. First of all, in vivo the blood-brain barrier is formed by a number of cells, which interact in various ways and strengthen the barrier function by affecting the formation of tight junctions1. Therefore, although this blood-brain barrier model is a good approximation of the in vivo situation, important aspects are missing. In addition, lymphocyte extravasation into the CNS across the blood-brain barrier is a multistep process. While each single step can be investigated separately, the technique presented here does not provide information on the whole process of extravasation under the influence of shear forces2,44,45,46. Finally, analysis of migrated cells from PBMC might be challenging depending on the frequency of the cells of interest, because usually only single-digit frequencies of cells transmigrate. Therefore, separation of the populations of interest prior to the assay or pooling of cells migrated across multiple cell culture inserts might be necessary. Other models for the analysis of leukocyte migration into the CNS cover some of the aspects missing in our model. Co-culture with astrocytes, pericytes, and/or neurons are used to mimic the complexity of the blood-brain barrier better47. Models including shear forces such as DIV-BBB reflect more the physiological conditions, thus, enabling a more sophisticated analysis of lymphocyte diapedesis48.
In summary, we present an easily accessible technique suitable for the investigation of qualitative and quantitative lymphocyte diapedesis across the human blood-brain barrier.
Disclosures
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.S.-M. and U.B. have no financial disclosures. T. S.-H. received travel and conference expenses from Biogen. N.S. received speaker and advisory board honoraria from Biogen and Novartis Pharma, as well as travel expenses from Biogen. H.W. received compensation for serving on Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen, Merck Serono, Novartis, and Sanofi-Genzyme. He also received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen, CSL Behring, Fresenius Medical Care, Glaxo Smith Kline, GW Pharmaceuticals, Lundbeck, Merck Serono, Omniamed, Novartis, and Sanofi-Genzyme. He received compensation as a consultant from Biogen, Merck Serono, Novartis, and Sanofi-Genzyme. H.W. received research support from Bayer Vital, Biogen, Genzyme, Merck Serono, Novartis, Sanofi-Aventis Germany, and Sanofi US. C.C.G. received speaker honoraria and travel expenses for attending meetings from Genzyme, Novartis Pharma GmbH, and Bayer Health Care.
Acknowledgments
This study has been supported by the Collaborative Research Centre CRC TR128 "Initiating/Effector versus Regulatory Mechanisms in Multiple Sclerosis-Progress towards Tackling the Disease" (Project A9 to H.W. and C.C.G., project B1 to N.S.).
References
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Takeshita Y, et al. An in vitro blood-brain barrier model combining shear stress and endothelial cell/astrocyte co-culture. J Neurosci Methods. 2014;232:165–172. doi: 10.1016/j.jneumeth.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado GC, et al. A novel model of demyelinating encephalomyelitis induced by monocytes and dendritic cells. J Immunol. 2006;177(10):6871–6879. doi: 10.4049/jimmunol.177.10.6871. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Illuminating neuromyelitis optica pathogenesis. Proc Natl Acad Sci U S A. 2012;109(4):1001–1002. doi: 10.1073/pnas.1119288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68(5):311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Lopes Pinheiro MA, et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 2016;1862(3):461–471. doi: 10.1016/j.bbadis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Meuth SG, Kieseier BC, Wiendl H. Immunotherapeutic approaches in MS: update on pathophysiology and emerging agents or strategies 2006. Endocr Metab Immune Disord Drug Targets. 2007;7(1):35–63. doi: 10.2174/187153007780059414. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Meuth SG, Stuve O, Kieseier B, Wiendl H. Multiple sclerosis therapy: an update on recently finished trials. J Neurol. 2007;254(11):1473–1490. doi: 10.1007/s00415-007-0684-7. [DOI] [PubMed] [Google Scholar]
- Wiendl H, Hohlfeld R. Multiple sclerosis therapeutics: unexpected outcomes clouding undisputed successes. Neurology. 2009;72(11):1008–1015. doi: 10.1212/01.wnl.0000344417.42972.54. [DOI] [PubMed] [Google Scholar]
- Schwab N, Schneider-Hohendorf T, Breuer J, Posevitz-Fejfar A, Wiendl H. JCV index and L-selectin for natalizumab-associated PML risk stratification. Journal of Neuroimmunology. 2014;275(1-2):24. [Google Scholar]
- Schwab N, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81(10):865–871. doi: 10.1212/WNL.0b013e3182a351fb. [DOI] [PubMed] [Google Scholar]
- Takeshita Y, Ransohoff RM. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev. 2012;248(1):228–239. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab N, Schneider-Hohendorf T, Wiendl H. Trafficking of lymphocytes into the CNS. Oncotarget. 2015;6(20):17863–17864. doi: 10.18632/oncotarget.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Hohendorf T, et al. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med. 2014;211(9):1833–1846. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16(9):449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502(2):236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Rudolph H, et al. Postarrest stalling rather than crawling favors CD8+ over CD4+ T-cell migration across the blood-brain barrier under flow in vitro. Eur J Immunol. 2016. [DOI] [PMC free article] [PubMed]
- Bartholomaus I, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462(7269):94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Gross CC, et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci U S A. 2016;113(21):E2973–E2982. doi: 10.1073/pnas.1524924113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CC, Brzostowski JA, Liu DF, Long EO. Tethering of Intercellular Adhesion Molecule on Target Cells Is Required for LFA-1-Dependent NK Cell Adhesion and Granule Polarization. Journal of Immunology. 2010;185(5):2918–2926. doi: 10.4049/jimmunol.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzke B, et al. Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann Clin Transl Neurol. 2015;2(2):119–130. doi: 10.1002/acn3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel K, et al. Blockade of the kinin receptor B1 protects from autoimmune CNS disease by reducing leukocyte trafficking. J Autoimmun. 2011;36(2):106–114. doi: 10.1016/j.jaut.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Schneider-Hohendorf T, et al. Regulatory T cells exhibit enhanced migratory characteristics, a feature impaired in patients with multiple sclerosis. Eur J Immunol. 2010;40(12):3581–3590. doi: 10.1002/eji.201040558. [DOI] [PubMed] [Google Scholar]
- Huang YH, et al. Specific central nervous system recruitment of HLA-G(+) regulatory T cells in multiple sclerosis. Ann Neurol. 2009;66(2):171–183. doi: 10.1002/ana.21705. [DOI] [PubMed] [Google Scholar]
- Dehmel T, et al. Monomethylfumarate reduces in vitro migration of mononuclear cells. Neurol Sci. 2014;35(7):1121–1125. doi: 10.1007/s10072-014-1663-2. [DOI] [PubMed] [Google Scholar]
- Gastpar R, et al. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol. 2004;172(2):972–980. doi: 10.4049/jimmunol.172.2.972. [DOI] [PubMed] [Google Scholar]
- Gastpar R, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65(12):5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermeeren M, Janssens S, Borgers M, Geysen J. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochemical and Biophysical Research Communications. 1997;234(1):19–23. doi: 10.1006/bbrc.1997.6570. [DOI] [PubMed] [Google Scholar]
- Rubant SA, et al. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. Journal of Investigative Dermatology. 2008;128(2):326–331. doi: 10.1038/sj.jid.5700996. [DOI] [PubMed] [Google Scholar]
- Hamann A, et al. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140(3):693–699. [PubMed] [Google Scholar]
- Shamri R, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6(5):497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- Didier N, et al. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem. 2003;86(1):246–254. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Dolman DE, Drndarski S, Fredriksson SM. An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. Methods Mol Biol. 2012;814:415–430. doi: 10.1007/978-1-61779-452-0_28. [DOI] [PubMed] [Google Scholar]
- Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Galla HJ, Beuckmann CT. An improved low-permeability in vitro-model of the blood-brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res. 1999;818(1):65–71. doi: 10.1016/s0006-8993(98)01282-7. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Dolman DE, Patabendige AK. Assays to predict drug permeation across the blood-brain barrier, and distribution to brain. Curr Drug Metab. 2008;9(9):901–910. doi: 10.2174/138920008786485182. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab. 2011;31(2):767–777. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB) Lab Chip. 2012;12(10):1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, et al. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubogu EE, Callahan MK, Tucky BH, Ransohoff RM. CCR5 expression on monocytes and T cells: modulation by transmigration across the blood-brain barrier in vitro. Cell Immunol. 2006;243(1):19–29. doi: 10.1016/j.cellimm.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, et al. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol. 2010;229(1-2):180–191. doi: 10.1016/j.jneuroim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Woolf E, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8(10):1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- Ando J, Nomura H, Kamiya A. The effect of fluid shear stress on the migration and proliferation of cultured endothelial cells. Microvasc Res. 1987;33(1):62–70. doi: 10.1016/0026-2862(87)90007-0. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Smith CW, Eskin SG, McIntire LV. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990;75(1):227–237. [PubMed] [Google Scholar]
- Wolff A, Antfolk M, Brodin B, Tenje M. In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J Pharm Sci. 2015;104(9):2727–2746. doi: 10.1002/jps.24329. [DOI] [PubMed] [Google Scholar]
- Cucullo L, et al. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48(3):505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]


