Abstract
Studies on skeletal muscle physiology face the technical challenge of appropriately processing the specimens to obtain sections with clearly visible cytoplasmic compartments. Another hurdle is the tight apposition of myofibers to the surrounding tissues. Because the process of tissue fixation and paraffin embedding leads to the shrinkage of muscle fibers, freezing is an optimal means of hardening muscle tissue for sectioning. However, a commonly encountered issue, the formation of ice crystals, occurs during the preparation of frozen sections because of the high water content of muscle. The protocol presented here first describes a simple and efficient method for properly freezing muscle tissues by immersing them in liquid nitrogen. The problem with using liquid nitrogen alone is that it causes the formation of a nitrogen gas barrier next to the tissue, which acts as an insulator and inhibits the cooling of the tissues. To avoid this "vapor blanket" effect, a new cryovial was designed to increase the speed of liquid flow around the tissue surface. This was achieved by punching a total of 14 inlet holes in the wall of the vial. According to bubble dynamics, a higher rate of liquid flow results in smaller bubbles and fewer chances to form a gas barrier. When liquid nitrogen flows into the cryovial through the inlet holes, the flow velocity around the tissue is fast enough to eliminate the gas barrier. Compared to the method of freezing muscle tissues using pre-chilled isopentane, this protocol is simpler and more efficient and can be used to freeze muscle in a throughput manner. Furthermore, this method is optimal for institutions that do not have access to isopentane, which is extremely flammable at room temperature.
Keywords: Physiology, Issue 122, muscle cryosection, freezing method, liquid nitrogen, new cryovial, vapor blanket.
Introduction
Skeletal muscle is the most valuable component of a meat-producing animal from the nutritional and processing point of view. In the meat industry, there are two especially critical aspects: the efficiency of muscle growth and the quality of the resulting meat. As a main component of the muscle, muscle fibers are directly related to growth performance and fresh meat quality in animals1. For example, the Total number of Fibers (TNF) and the Cross-sectional area of Fibers (CSAF) mostly determine muscle mass and meat quality; also, Fiber Type Composition (FTC) strongly affects fresh meat quality2. Therefore, the manipulation of muscle fiber characteristics in animals is a highly effective method to increase the core profitability and competitiveness of farms1.
To date, several intrinsic and extrinsic factors have been identified to manipulate muscle fiber characteristics1. This manipulation can be achieved through the targeted selection of animals with specific genes, such as the Myostatin gene in cattle3, the Callipyge gene in sheep4, and the RYR1 and IGF2 genes in pigs5. Also, diet control and treatments with specific hormones play an important role in muscle fiber characteristics6. Thus, an approach that combines genetic and nutritional factors might be able to improve lean meat content and meat quality. However, studies on muscle fibers are limited in the meat industry because the elucidation of the structure of muscle fibers still is a challenge.
Muscle fiber properties are identified using histochemical methods, such as the myosin adenosine triphosphatase (ATPase) assay. This method relies on the fact that enzymes located in thin (6-8 µm) frozen sections of muscle fibers can be chemically reacted with certain products. However, the water content of muscles is greater than 75% in pigs, rabbits, mice, and humans, regardless of the position (i.e., back, abdomen, or hindlimb)7. Such high moisture content in muscles causes a commonly encountered issue – freezing artifacts – during the preparation of cryosections, as previously described8,9. In most cases, it is almost impossible to appropriately freeze muscle tissues in a slaughterhouse production line, according to our experience.
The protocol presented here describes a simple and efficient method used in our laboratory to freeze muscle tissues for cryosectioning in a high-throughput manner. The highlight of this method is a new cryovial that is designed for flash-freezing muscle tissues in liquid nitrogen. The current workflow can concurrently facilitate tissue freezing and processing for an excellent muscle cryosection, with a clearly visible cytoplasmic compartment and the tight apposition of myofibers to the surrounding tissue. In addition, this protocol can be applied to a wide array of options for tissue analysis because liquid nitrogen does not mix with tissues.
Protocol
The current method has been established and validated to harvest and store more than 1,000 muscle samples for histological staining in our laboratory. All procedures involving animal care and use followed the guidelines established by the Ministry of Agriculture of China.
1. Equipment for Sample Collection
Label the sample identity on each cryogenic vial. Note: The cryogenic vial is specially designed to freeze fresh tissue samples for the cryosection procedure (Figure 1A). The vials are made of polypropylene in a mold factory (Figure 1C).
Obtain the following equipment: liquid nitrogen in an appropriate liquid nitrogen tank, safety glasses or a face shield, freezer gloves, 10 cm forceps, 25 cm tweezers, a Styrofoam cooler (inner dimensions: 20 cm long x 12 cm wide x 13.5 cm high; outer dimensions: 24 cm long x 16 cm wide x 15 cm high), plastic film (2.5 cm long x 0.8 cm wide x 0.1 mm thick), a scalpel, and A4 PVC binding covers (21 cm x 29.5 cm) (Figure 1B).
2. Preparation of Muscle Samples
Fill the Styrofoam cooler with liquid nitrogen 5 min ahead of sample collection. NOTE: The large column of liquid nitrogen is essential because part of the liquid nitrogen boils away and turns into gas when fresh samples touch it. In order to reduce the frequency of transfer of liquid nitrogen to replenish the loss, an appropriately sized Styrofoam cooler must be acquired in advance according to the number of samples.
Once a specimen is obtained, gently place it on a piece of A4 PVC binding cover and observe the direction of the muscle fibers. NOTE: Here, we used the cross-section of the longissimus doris muscle from the first to the last lumbar vertebra in pigs. The specimens are about 12 cm long, 8 cm wide, and 5 cm high.
Use a scalpel to make two parallel incisions through the specimen in the direction of the muscle fibers, approximately 3 cm in length and 0.6 cm apart. Trim the incised muscle into a rectangle approximately 0.6 cm wide x 0.6 cm high x 1.5 cm long. NOTE: The reliability of measuring the cross-sectional area of the muscle fibers mostly depends on the fiber orientation of the incised muscle.
Use 10 cm forceps to gently put the sample on a piece of plastic film, with the longitudinal axis of the incised muscle parallel to the long edge of the film (Figure 2A). NOTE: The plastic film has two functions: a holding function that helps in fixing the orientation of the muscle fibers and an adhesion function that prevents cracks in the sample due to rapid freezing.
Using forceps, place the muscle tissue into the lower middle of a labelled cryogenic vial, just between the inlet holes, and then tightly cap the vial (Figure 2B).
3. Freezing and Storing Muscle Samples
Using 25 cm tweezers, quickly submerge the whole cryogenic vial in liquid nitrogen in the pre-cooled Styrofoam cooler, waiting until the liquid nitrogen does not boil (approximately 10-20 s). NOTE: The cryovial will float when the liquid nitrogen is boiling, so carefully immerse the vial in the boiling liquid nitrogen for 10-20 s.
Transfer the muscle samples to a liquid nitrogen storage tank or a -80 °C freezer for long-term storage.
4. Slide Preparation
Precool a cryostat to between -20 °C and -22 °C.
Remove and discard the old microtome blade, wipe down the knife holder and anti-roll plate in the cryostat, install a new microtome blade in the cryostat, and set the cutting thickness to 8 µm.
Transfer the muscle sample in the cryogenic vial from the liquid nitrogen storage tank or -80 °C freezer to the cryostat, transporting it in liquid nitrogen or on dry ice.
Wait 30 min for the specimen to equilibrate with the temperature of the cryostat. NOTE: Any equipment that might touch the specimen must be pre-cooled, because a high temperature might cause the formation of ice crystals in the specimen.
Mount the sample in the specimen holder under the optimum cutting temperature formulation of water-soluble glycols and resins (OCT). Cut 8-µm sections. NOTE: Adjust the cutting angle perpendicularly to the orientation of the muscle fibers, because the CSAF is the cross-sectional area of muscle fibers.
Mount the sections towards the center of a room-temperature microslide. Immediately place the slide into a slide box in a -80 °C freezer. Do not allow the slide to dry at RT.
Representative Results
The cryovial illustration and the common laboratory equipment for freezing muscles during the preparation of frozen sections are shown in Figure 1. The cryovials are made of polypropylene in a mold factory. Each vial has a total of 14 inlet holes: one is on the cap; another is at the bottom; and the remaining 12 form four parallel lines, each with four holes at 90° to one another. These inlet holes can speed up the flow velocity of liquid nitrogen next to the tissue to avoid the "vapor blanket" effect when liquid nitrogen contacts the tissue. Consequently, very few ice crystals form in the frozen samples. Importantly, isopentane, the extremely volatile and flammable liquid with a boiling point at 28 °C, is not necessary in this procedure.
To further highlight the advantage of this method, we froze the longissimus dorsi muscle in pigs using five freezing methods and then observed their staining properties and histological details using a Hematoxylin/Eosin (HE) stain (Figure 3). A commonly encountered issue was the formation of ice crystals during the preparation of the muscles for cryosection. When the sample was placed directly in a -80 °C freezer (Figure 3A), large ice crystals formed and then caused a well-known "Swiss cheese" effect in the muscle cryosection. Furthermore, ice crystals also formed when the muscle tissue was immersed directly in liquid nitrogen (Figure 3B). Even though liquid nitrogen is a very cold liquid (-190 °C), its heat constant is extremely low. When any specimen contacts the liquid nitrogen, a vapor blanket can build up next to the tissue and insulate the penetration of cold into the tissue. Additionally, a routine procedure for freezing specimens in pathology laboratories is not yet applicable to muscle tissue. As shown in Figure 3C, many needle-like ice crystals formed in the cytoplasmic compartment of myofibers after the muscle tissue was dipped into OCT mounting medium in the proper orientation and then rapidly frozen in a slurry of isopentane (-160 °C). In conclusion, the appropriate freezing rate for muscle tissues must be strictly met in the operation.
The current protocol achieves excellent specimen integrity, with a clearly visible cytoplasmic compartment and the tight apposition of myofibers to the surrounding tissue (Figure 3D). It allows for excellent visualization of the metabolic properties of muscle fibers using the myosin ATPase assay (Figure 3E). For this assay, myosin ATPase activity was analyzed based on the fact that fast-contracting muscle fibers (mammalian type 2A and 2B) hydrolyze ATP faster than slow-contracting fibers (mammalian type 1). When given equivalent times, fast-contracting fibers appear light, with two degrees, and slow-contracting fibers appear black. Importantly, this method can be used efficiently in a high-throughput environment because it is easy to perform and saves time (Figure 4). Nowadays, a well-established freezing method is to submerge muscle tissues in a slurry of isopentane without contact with OCT mounting medium, as previously described8,9 (Figure 3F). In this procedure, it takes approximate 30 min for a solid-liquid slurry state of 240 mL of isopentane to be achieved by pre-cooling the isopentane in liquid nitrogen and stirring until small white precipitates appear at the bottom (Figure 4). There are three practical difficulties encountered by researchers while attempting to freeze muscle tissues in pre-chilled isopentane: (1) A solid-liquid slurry state of isopantane is difficult to maintain when the sampling time is more than 1 h. In our past attempts, freezing artifacts usually occurred in the samples that were done at the middle and late stages of the process, when dozens of muscle tissues were collected once. (2) As a dangerous liquid, isopantane is not allowed anywhere on public transport. (3) Isopentane is unavailable except in laboratories. Many meat science researchers usually freeze muscle tissues in a slaughterhouse that is far away from a laboratory and that is not allowed to have isopentane. In addition, diagnostic muscle biopsies are often sub-optimally processed due to the lack of isopentane at medical facilities. Thus, our protocol is an important development for freezing muscle specimens in situations where isopentane is unavailable.
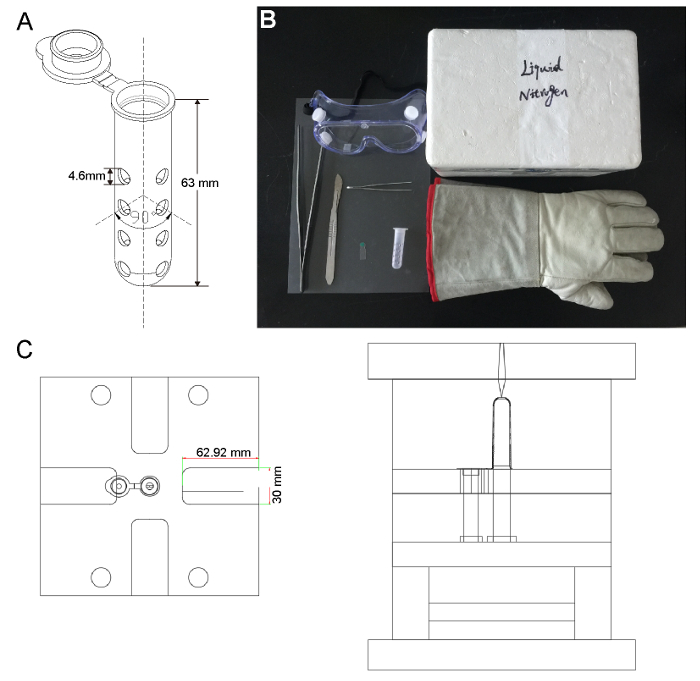 Figure 1: The Apparatus for Muscle Freezing. (A) The illustrations of the cryovial used in this study. 14 inlet holes are punched in the tube wall: one is on the cap; another is at the bottom; and the remaining 12 form four parallel lines, each with four holes at 90° to one another. (B) A photograph of the freezing apparatus. A safe and efficient means of freezing muscles in liquid nitrogen involves the use of a cryovial, plastic film, safety glasses, freezer gloves, 10 cm forceps, 25 cm tweezers, a Styrofoam cooler, a scalpel, and A4 PVC binding cover. (C) The illustrations of the cryovial mold provided by an engineer, Yonghua Wang, in the mold factory. Please click here to view a larger version of this figure.
Figure 1: The Apparatus for Muscle Freezing. (A) The illustrations of the cryovial used in this study. 14 inlet holes are punched in the tube wall: one is on the cap; another is at the bottom; and the remaining 12 form four parallel lines, each with four holes at 90° to one another. (B) A photograph of the freezing apparatus. A safe and efficient means of freezing muscles in liquid nitrogen involves the use of a cryovial, plastic film, safety glasses, freezer gloves, 10 cm forceps, 25 cm tweezers, a Styrofoam cooler, a scalpel, and A4 PVC binding cover. (C) The illustrations of the cryovial mold provided by an engineer, Yonghua Wang, in the mold factory. Please click here to view a larger version of this figure.
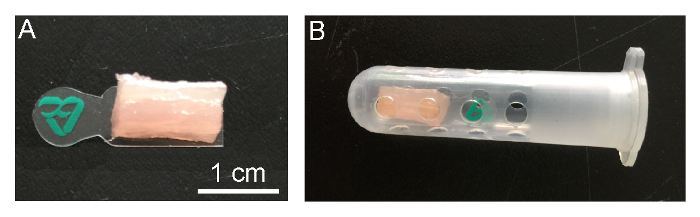 Figure 2: Preparation of Muscle Samples. (A) Place an incised muscle tissue on the plastic film.(B) The muscle tissue should be placed between the inlet holes in the cryogenic vial. Please click here to view a larger version of this figure.
Figure 2: Preparation of Muscle Samples. (A) Place an incised muscle tissue on the plastic film.(B) The muscle tissue should be placed between the inlet holes in the cryogenic vial. Please click here to view a larger version of this figure.
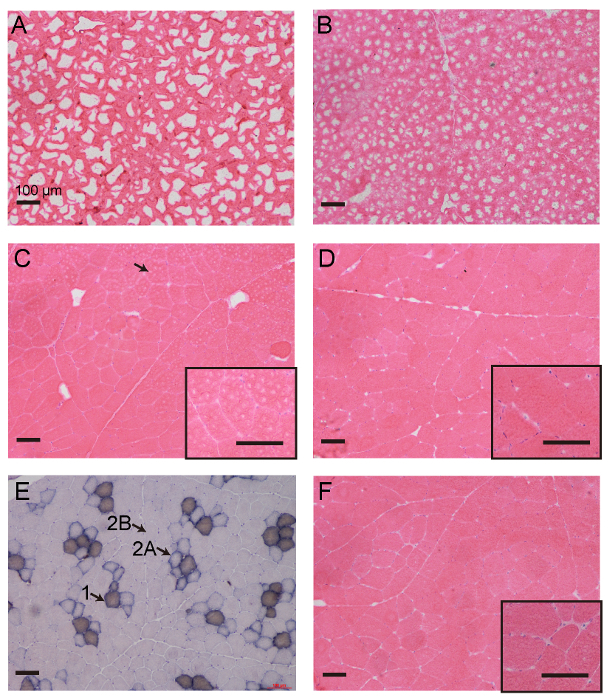 Figure 3: Representative Images of the Histological Evaluation of Pig Longissimus Dorsi Muscles after They are Frozen using Five Different Methods. Ice crystals destroy the readability of muscle cryosections imaged with HE staining when the muscles are frozen for histological analysis using inappropriate methods, as shown in (A-C). Considerable freezing artifacts occurred when muscle samples were directly placed in a 80°C freezer (A) or dipped in liquid nitrogen (B). Many needle-like ice crystals formed within individual muscle cells after the muscle tissue was dipped into OCT and then directly put into a slurry of isopentane (C). The protocol presented in this study can eliminate the formation of ice crystals when the muscle tissue is simply immersed in liquid nitrogen (D). This protocol is applicable to the analysis of enzyme activity in frozen muscle based on the myosin ATPase assay (E). The standard freezing method for muscle does not perform better; muscle tissue is dipped in a slurry of isopentane without direct contact with OCT mounting medium (F). All images are under a 10X objective, except for those with black edges (20X). Scale bars: 100 µm. The arrowhead in Figure 3C indicates small, needle-like ice crystals. The arrowhead and text in Figure 3E indicate the type of muscle fibers: type 1 represents the slow myosin heavy chain fibers, type 2A means intermediate myosin heavy chain fibers, and type 2B indicates fast myosin heavy chain fibers. Please click here to view a larger version of this figure.
Figure 3: Representative Images of the Histological Evaluation of Pig Longissimus Dorsi Muscles after They are Frozen using Five Different Methods. Ice crystals destroy the readability of muscle cryosections imaged with HE staining when the muscles are frozen for histological analysis using inappropriate methods, as shown in (A-C). Considerable freezing artifacts occurred when muscle samples were directly placed in a 80°C freezer (A) or dipped in liquid nitrogen (B). Many needle-like ice crystals formed within individual muscle cells after the muscle tissue was dipped into OCT and then directly put into a slurry of isopentane (C). The protocol presented in this study can eliminate the formation of ice crystals when the muscle tissue is simply immersed in liquid nitrogen (D). This protocol is applicable to the analysis of enzyme activity in frozen muscle based on the myosin ATPase assay (E). The standard freezing method for muscle does not perform better; muscle tissue is dipped in a slurry of isopentane without direct contact with OCT mounting medium (F). All images are under a 10X objective, except for those with black edges (20X). Scale bars: 100 µm. The arrowhead in Figure 3C indicates small, needle-like ice crystals. The arrowhead and text in Figure 3E indicate the type of muscle fibers: type 1 represents the slow myosin heavy chain fibers, type 2A means intermediate myosin heavy chain fibers, and type 2B indicates fast myosin heavy chain fibers. Please click here to view a larger version of this figure.
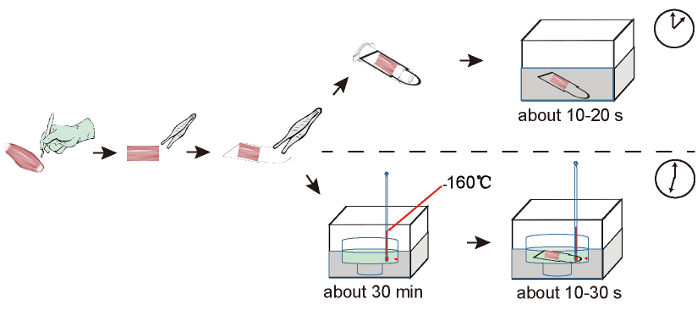 Figure 4: Comparison of Workflows for Liquid Nitrogen-based and Isopentane-based Freezing Procedures. In the liquid nitrogen-based protocol, the muscle tissue in the new cryovial is immersed in liquid nitrogen. In the isopentane-based protocol, a slurry state of isopentane must be achieved in advance by precooling the isopentane in liquid nitrogen and stirring until small, white precipitates appear at the bottom. Then, the muscle tissue is dipped into the precooled isopentane. Compared with 35 min in the isopentane-based procedure, as described in Figure 3F, it takes only 5 min to complete the freezing procedure presented in this protocol. Please click here to view a larger version of this figure.
Figure 4: Comparison of Workflows for Liquid Nitrogen-based and Isopentane-based Freezing Procedures. In the liquid nitrogen-based protocol, the muscle tissue in the new cryovial is immersed in liquid nitrogen. In the isopentane-based protocol, a slurry state of isopentane must be achieved in advance by precooling the isopentane in liquid nitrogen and stirring until small, white precipitates appear at the bottom. Then, the muscle tissue is dipped into the precooled isopentane. Compared with 35 min in the isopentane-based procedure, as described in Figure 3F, it takes only 5 min to complete the freezing procedure presented in this protocol. Please click here to view a larger version of this figure.
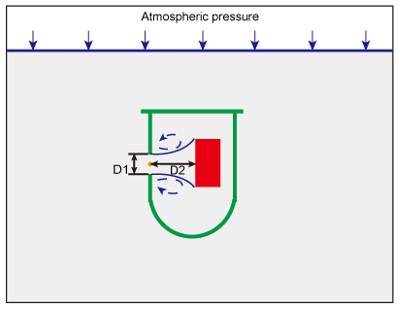 Figure 5: Liquid Nitrogen Flow through a Sharp-edged Orifice. When immersing the whole multi-hole cryovial in liquid nitrogen, atmospheric pressure forces liquid nitrogen into the tube through the 14 inlet holes. The presence of the holes makes the flow accelerate through them, thus increasing the velocity. A recirculating flow (in blue) develops immediately downstream of the orifice (in orange). D1 indicates the diameter of the orifice on the wall of the tube and D2 represents the distance between the orifice and the muscle sample (in red). Please click here to view a larger version of this figure.
Figure 5: Liquid Nitrogen Flow through a Sharp-edged Orifice. When immersing the whole multi-hole cryovial in liquid nitrogen, atmospheric pressure forces liquid nitrogen into the tube through the 14 inlet holes. The presence of the holes makes the flow accelerate through them, thus increasing the velocity. A recirculating flow (in blue) develops immediately downstream of the orifice (in orange). D1 indicates the diameter of the orifice on the wall of the tube and D2 represents the distance between the orifice and the muscle sample (in red). Please click here to view a larger version of this figure.
Discussion
Here, we describe a new, multi-hole cryovial for freezing and storing muscle tissues to perform histological assessments of muscle function. The critical modified step in this protocol is that the sample in the multi-hole cryovial is directly immersed in liquid nitrogen. To our knowledge, this is the simplest and rapidest way to obtain excellent frozen samples for muscle cryosectioning among the existing freezing methods (see the representative results).
The technical challenge faced by muscle researchers is that ice crystals are most likely to form when using freezing as the means to harden muscle tissue for sectioning. As noted in the representative results, three factors play significant roles in determining proper muscle freezing for pathological studies, including choosing an appropriate cold source, minimizing the moisture of the specimen, and increasing the percentage of the total surface in contact with the cold source. First, the rate of freezing is critical to avoid damage during the preparation of the frozen tissue for sectioning. When the freezing is slow, frozen muscles show the well-known "Swiss cheese" effect because large ice crystals are formed extracellularly. When the freezing is rapid but not sufficiently quick, large numbers of small, needle-like ice crystals are formed within individual muscle cells. These needle-like ice crystals cause little detectable structural damage, but they bring the following effects: mitochondria are damaged10, the size of the muscle fibers are artificially increased11, and the integrity of fibrous structures is destroyed11. Thus, a cold source at a sufficiently cold temperature and a sufficient freezing speed are essential to avoid the formation of ice crystals in muscle cryosections.
Ideally, the cold source is at extremely cold temperatures and is arranged to contact all surfaces. Both liquid nitrogen (-190 °C) and isopentane in a solid-liquid mixed state (-160 °C) satisfy this condition. However, the two freezing media have their own disadvantages. Liquid nitrogen has an extremely low specific heat constant. The result is that the contact between liquid nitrogen and the tissue causes a vapor barrier that builds up next to the tissue and greatly slows the penetration of cold into the tissue12. Thus, significant freezing artifacts occur inside the frozen muscle. The slurry state of isopentane is an ideal freezing medium that does not form vapor barriers, but the main problem is that isopentane must be used in conjunction with liquid nitrogen in order to achieve a liquid bath temperature of 160 °C. In addition, isopentane is an extremely volatile and flammable liquid at room temperature. Second, the muscle contains excessive water (>75%), so any way to introduce exogenous moisture into the specimen would cause freezing artifacts. For example, OCT mounting medium adds extra water to the muscle, so ice crystals are much more prominent in those muscle cryosections compared with ones without OCT mounting medium. Finally, the thickness of the muscle tissue is also important to appropriately freeze it because the rate of freezing partly depends on the percentage of the total surface in contact with the cold source (usually, the thickness <0.5 cm). Accordingly, the only existing way to properly freeze the tissue is to immerse muscle tissue with a thickness less than 0.5 cm into pre-cooled isopentane (160 °C), as previously described8.
This protocol first describes how to properly freeze muscle tissues by immersing them directly in liquid nitrogen. The problem with using liquid nitrogen alone is the formation of nitrogen gas over the tissue surface, acting as an insulator and inhibiting the cooling of the tissue13. Such a vapor blanket can form only when the merging of bubbles is so rapid that the nitrogen gas can separate the tissue from the liquid nitrogen. According to bubble dynamics, a quicker liquid flow will result in smaller bubbles, as it has fewer chances to merge14. Therefore, we punched 14 holes into the wall of the cryovial to increase the speed of liquid flow around the tissue and to help avoid the "vapor blanket" effect when liquid nitrogen contacts the tissue.
Considering that the one factor that determines experimental success is the speed of liquid flow around the tissue, three parameters of the cryovial are very important, including the position and number of holes, the diameter of the inlet holes, and the distance between the orifice and the sample. Liquid dynamics shows that the flow rate through an orifice is determined by the diameter of the holes and that a recirculating flow develops immediately downstream of the orifice (Figure 5)15,16. Correspondingly, the distance between the orifice and the sample mostly determines the contact area between the high-speed fluid flow and the sample, as shown in Figure 5. Consequently, the multi-hole cryovial in Figure 1A is suitable for a muscle sample that is 0.6 cm wide x 0.6 cm high x 1.5 cm long. For larger muscle fragments, it is worth trying this method with a larger tube and more holes. However, this protocol is not suitable for freezing muscle samples from small animals, such as in a murine model, because the inlet holes might be too large for smaller samples.
In addition, three critical steps must be strictly followed in the protocol. First, the liquid nitrogen should be adequate to immerse the whole cryovial, and the cryogenic vial must be entirely below the liquid level. Second, it is necessary to avoid accidental contact between frozen muscles and room-temperature containers or instruments. For example, without careful transportation, new ice crystals might form when transferring a frozen sample from the liquid nitrogen storage tank or -80 °C freezer to a cryostat. Third, there should not be too much OCT used when the specimens are mounted on the holder due to the high likelihood of ice crystal formation around the contact area between frozen muscles and OCT.
In the meat industry, a muscle cryosection procedure is indispensable because the process of formalin fixation and paraffin embedding causes shrinkage in muscle fibers, which impairs the evaluation of some muscle traits, such as TNF and CSAF. In addition, frozen muscle is required when evaluating FTC using the myosin ATPase assay. As shown in Figure 3E, this protocol can meet the requirements of both pathological analysis and histochemical stains in muscles. The preparation of a cryosection is a routine operation in clinical muscle pathology laboratories. As described by Meng et al.8, a standard operating procedure for freezing muscle tissues is to immerse autopsy specimens in pre-cooled isopentane. However, isopentane is unavailable to freeze autopsy specimens at institutions without clinical muscle pathology laboratories. Consequently, diagnostic muscle biopsies are often suboptimally processed at many medical facilities. Thus, our protocol could substantially improve the ability for small hospitals or labs to perform diagnostic muscle biopsies of adequate quality. Considering the high efficiency and simple operation of this protocol, it might be a promising alternative to the current isopentane-based freezing method and can be widely applied to histological studies in the future.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (NSFC): 31301950 and 31671288.
References
- Joo ST, Kim GD, Hwang YH, Ryu YC. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95(4):828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Lefaucheur L. A second look into fibre typing--relation to meat quality. Meat Sci. 2010;84(2):257–270. doi: 10.1016/j.meatsci.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Fiems LO. Double Muscling in Cattle: Genes, Husbandry, Carcasses and Meat. Animals (Basel) 2012;2(3):472–506. doi: 10.3390/ani2030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockett NE, et al. The callipyge mutation and other genes that affect muscle hypertrophy in sheep. Genet Sel Evol. 2005;37:65–81. doi: 10.1186/1297-9686-37-S1-S65. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinckens A, et al. The RYR1 g.1843C>T mutation is associated with the effect of the IGF2 intron3-g.3072G>A mutation on muscle hypertrophy. Anim Genet. 2007;38(1):67–71. doi: 10.1111/j.1365-2052.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- Brameld JM, Buttery PJ, Dawson JM, Harper JM. Nutritional and hormonal control of skeletal-muscle cell growth and differentiation. Proc Nutr Soc. 1998;57(2):207–217. doi: 10.1079/pns19980033. [DOI] [PubMed] [Google Scholar]
- Reinoso RF, Telfer BA, Rowland M. Tissue water content in rats measured by desiccation. J Pharmacol Toxicol Methods. 1997;38(2):87–92. doi: 10.1016/s1056-8719(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Meng H, et al. Tissue triage and freezing for models of skeletal muscle disease. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]
- Kumar A, Accorsi A, Rhee Y, Girgenrath M. Do's and don'ts in the preparation of muscle cryosections for histological analysis. J Vis Exp. 2015. p. e52793. [DOI] [PMC free article] [PubMed]
- Meijer AE, Vloedman AH. The histochemical characterization of the coupling state of skeletal muscle mitochondria. Histochemistry. 1980;69(3):217–232. doi: 10.1007/BF00489769. [DOI] [PubMed] [Google Scholar]
- Harnkarnsujarit N, Kawai K, Suzuki T. Effects of Freezing Temperature and Water Activity on Microstructure, Color, and Protein Conformation of Freeze-Dried Bluefin Tuna (Thunnus orientalis) Food Bioprocess Technol. 2015;8(4):916–925. [Google Scholar]
- Dubowitz V, Sewry C. Muscle Biopsy: A Practical Approach. Elsevier; 2007. pp. 407–422. Ch. 15. [Google Scholar]
- Suvarna SK, Layton C, Bancroft JD. Bancroft's Theory and Practice of Histological Techniques: Expert Consult: Online and Print, 7e. Elsevier; 2008. [Google Scholar]
- Sulaiman SA, Kamarudin NAZ. Bubbles Size Estimation in Liquid Flow Through a Vertical Pipe. J Appl Sci. 2012;12(23):2464–2468. [Google Scholar]
- Fukutomi K, et al. A Study of A Flow through Small Apertures : 2nd Report, Experiments on The Velocity Field. Nihon Kikai Gakkai Ronbunshu B Hen/transactions of the Japan Society of Mechanical Engineers Part B. 1987;53(496):3516–3521. [Google Scholar]
- Shah MS, Joshi JB, Kalsi AS, Prasad CSR, Shukla DS. Analysis of flow through an orifice meter: CFD simulation. Chem Eng Sci. 2012;71(9):300–309. [Google Scholar]


