Summary
Background
As the prevalence of artemisinin-resistant Plasmodium falciparum malaria increases in the Greater Mekong Subregion (GMS), emerging resistance to partner drugs in artemisinin combination therapies (ACTs) seriously threatens global efforts to treat and eliminate this disease. Molecular markers for ACT failure are urgently needed to monitor the spread of partner drug resistance, and to recommend alternative treatments in Southeast Asia and beyond.
Methods
We performed a genome-wide association study (GWAS) of 297 P. falciparum isolates from Cambodia to investigate the relationship of 11,630 exonic single-nucleotide polymorphisms (SNPs) and 43 copy number variations (CNVs) with in-vitro piperaquine 50% inhibitory concentrations (IC50s), and tested whether these genetic variants are markers of dihydroartemisinin-piperaquine failures. We then performed a survival analysis of 133 patients to determine whether candidate molecular markers predicted parasite recrudescence following dihydroartemisinin-piperaquine treatment.
Findings
Piperaquine IC50s increased significantly from 2011 to 2013 in 3 Cambodian provinces. Genome-wide analysis of SNPs identified a chromosome 13 region that associates with elevated piperaquine IC50s. A nonsynonymous SNP (encoding a Glu415Gly substitution) in this region, within a gene encoding an exonuclease, associates with parasite recrudescence following dihydroartemisinin-piperaquine treatment. Genome-wide analysis of CNVs revealed that a single copy of the mdr1 gene on chromosome 5 and a novel amplification of the plasmepsin II and plasmepsin III genes on chromosome 14 also associate with elevated piperaquine IC50s. After adjusting for covariates, both exo-E415G and plasmepsin II-III markers significantly associate with decreased treatment efficacy (0.38 and 0.41 survival rates, respectively).
Interpretation
The exo-E415G SNP and plasmepsin II-III amplification are markers of piperaquine resistance and dihydroartemisinin-piperaquine failures in Cambodia, and can help monitor the spread of these phenotypes into GMS countries, and elucidate the mechanism of piperaquine resistance. Since plasmepsins are involved in the parasite’s haemoglobin-to-haemozoin conversion pathway, targeted by related antimalarials, plasmepsin II-III amplification likely mediates piperaquine resistance.
Funding
Intramural Research Program of the US National Institute of Allergy and Infectious Diseases, National Institutes of Health; Wellcome Trust; Bill and Melinda Gates Foundation; Medical Research Council; and UK Department for International Development.
Introduction
Artemisinin combination therapy (ACT) – the use of a short-acting artemisinin derivative and a long-acting partner drug – is recommended worldwide for the treatment of Plasmodium falciparum malaria.1 Dihydroartemisinin-piperaquine, a current frontline ACT in Cambodia, Vietnam, Thailand, Myanmar, China, and Indonesia is now failing in Cambodian provinces where artemisinin resistance2 has emerged and spread.3–12 This situation likely arose because the survival of parasites after artemisinin exposure increases the chance that they develop spontaneous genetic resistance to piperaquine, survive declining piperaquine plasma concentrations, and propagate via mosquitoes to other humans. Through such processes, artemisinin resistance, which has evolved across the Greater Mekong Subregion (GMS),7,8,13 threatens to compromise the efficacy of dihydroartemisinin-piperaquine and other ACTs in the global treatment and elimination of malaria.14 The natural selection of low-frequency, pre-existing resistance to piperaquine may also be occurring and further contributing to this problem.
Recent increases in dihydroartemisinin-piperaquine failures4,9,11,12 and piperaquine 50% inhibitory concentrations (IC50s)15 suggest that piperaquine resistance has emerged in Cambodia. These findings, and the discovery that piperaquine-resistant parasites are sensitive to the former ACT partner drug mefloquine,4 have recently led Cambodia’s national malaria control program and the World Health Organisation to recommend artesunate-mefloquine as the first-line ACT in 10 Cambodian provinces, including Pursat and Preah Vihear. Molecular markers are urgently needed for large-scale surveillance programs to predict dihydroartemisinin-piperaquine failures in Cambodia and other GMS countries, and investigate the molecular mechanism of piperaquine resistance.
Several genetic variations have been associated with decreased piperaquine susceptibility: a single copy of the mdr1 gene has been associated with dihydroartemisinin-piperaquine failures in Cambodian patients,4 while amplification of a region downstream of mdr1 and the crt SNP C101F have been associated with elevated piperaquine IC50s in vitro.16 These genetic variants are not useful as molecular markers, however, as the first is wild-type, the second is very uncommon, and the third has not yet been observed in MalariaGEN P. falciparum Community Project’s global catalogue of variation in clinical samples (https://www.malariagen.net/projects/p-falciparum-community-project). Another study11 found that a triple mutation (kelch13-C580Y, MAL10:688956 SNP, and MAL13:1718319 SNP) linked with slow parasite clearance is associated with a 5.4-fold greater risk of dihydroartemisinin-piperaquine failure in Cambodia’s Oddar Meancheay Province; however, these SNPs were not linked to piperaquine resistance.
The purpose of this genome-wide association study (GWAS) was to discover molecular markers of dihydroartemisinin-piperaquine failures in Cambodia. In designing it, we reasoned that such markers would associate with elevated piperaquine IC50s, increase in prevalence over time in areas where artemisinin resistance is common, and be uncommon where dihydroartemisinin-piperaquine has not been used. We therefore sequenced the genomes and measured the piperaquine IC50s of parasites collected in 2010-2013 from patients with P. falciparum malaria in Pursat, Preah Vihear, and Ratanakiri, where the prevalences of kelch13 mutations – genetic markers for artemisinin resistance6,7,17 – were 77%, 34%, and 11%,4 and where the prevalences of dihydroartemisinin-piperaquine failures were 46%, 16%, and 2%,4 respectively, in 2012-2013. GWAS analysis identified 2 molecular markers of dihydroartemisinin-piperaquine failures: an exonuclease SNP encoding a Glu415Gly substitution, and amplification of the plasmepsin II and plasmepsin III genes.
Methods
Study design and participants
To obtain samples for this GWAS study, we enrolled patients with uncomplicated P. falciparum malaria into parasite clearance rate3,7 and drug efficacy4 studies in 2010-2013 in 3 provinces where piperaquine resistance is common (Pursat), emerging (Preah Vihear), or uncommon (Ratanakiri). Written informed consent was given by adult patients, or a parent or guardian of child patients. Protocols were approved by the Cambodian National Ethics Committee for Health Research and the National Institute of Allergy and Infectious Diseases Institutional Review Board, and are registered with Clinicaltrials.gov, numbers NCT00341003, NCT01240603, and NCT01736319.
Using parasitized blood samples from these studies, we measured piperaquine IC50s ex vivo or after short-term culture in vitro, and obtained whole-genome parasite sequence data, whenever possible. The GWAS was designed to identify genetic markers of elevated piperaquine IC50s, while a dihydroartemisinin-piperaquine efficacy study4 was used to test for association between GWAS candidate markers and parasite recrudescence. PCR genotyping using msp1, msp2, and glurp microsatellites as genetic markers distinguished recrudescences from newly acquired infections.18 A summary of samples, according to province of origin and year of collection, is shown in supplementary table 1.
GWAS and survival analyses
The preparation, sequencing, genotyping, and phenotyping of samples is described in the appendix (supplementary methods 1, p.16). The GWAS and correction for population structure were performed using a linear mixed model, implemented in FaST-LMM19 v2.06.17 We tested 11,630 SNPs with minor allele frequency (MAF)>0.033, using genotypes encoded as the number of non-reference alleles (0 or 1), and excluding heterozygous calls to minimize confounding effects of mixed infections. Piperaquine IC50 was used as the continuous dependent variable. When whole-genome sequence or phenotype data were available for both initial and recrudescent samples, we only analysed data from the recrudescent samples. Different approaches (using initial sample data only, using the phenotype and genotype of the same sample, averaging initial and recrudescent IC50s) did not alter results. A relationship matrix was calculated using a subset of 6678 unlinked SNPs with MAF>0.03, extracted using PLINK20 v1.07 (options: --indep-pairwise 100 10 0.3 --maf 0.03 –geno 0.25). In estimating the relationship matrix, we found that excluding proximal SNPs (within 10 Kb or 100 Kb from the tested variant) did not significantly affect results. Given the number of independent SNPs used, we applied Bonferroni correction to define a “significance” threshold of p≤8.6×10-7 for GWAS analyses. We also defined a “suggestive” threshold of p≤10-4 to identify relatively high-ranking loci.
To adjust for potential confounder effects, we treated the geographical origin of the sample, the presence of mdr1 amplification, and the presence of kelch13 resistance alleles collectively as covariates in the linear mixed-model. These covariates reduced the genomic inflation factor λGC from 3.455 to 1.964 (supplementary figure 1, p.6). We also treated genetic similarity across samples as a random effect to correct for confounding effects of population structure, which further reduced λGC to 1.106. While this value is still slightly above 1.0, this is unlikely due to un-accounted population stratification. In fact, when we confined the estimation of λGC to only un-linked SNPs, its value dropped to 1.013, suggesting that residual inflation is due to extended homozygosity haplotypes in the samples and thus have little impact on the GWAS.
We performed a survival analysis using the R package “survival”. We fitted a Cox proportional hazard regression model, where treatment success (recrudescent vs non-recrudescent infection) represented the survival status, and we added the age of the patient (in years), parasitaemia on day 0 (log-scaled), and dose of piperaquine given (mg/kg, in 5-unit increments starting at 35) as covariates.21 We then included the 2 markers (exo-E415G and plasmepsin II-III) as covariates, and estimated adjusted hazard ratios (aHRs) and adjusted survival curves.
Copy number variations
We subsequently tested the association of copy number variations (CNVs) with piperaquine IC50s using the same method and parameters just described. We tested 43 CNVs present in ≥5 samples; genotypes represented the presence or absence (encoded as 0 or 1) of the CNV. In this analysis, mdr1 amplification was not included as a covariate in the linear mixed model. To call CNVs across the genome, we modified a procedure used previously.22,23 Briefly, we first divided the genome into 300-bp non-overlapping bins and calculated for each sample the number of reads whose alignment started within each bin. We then normalised these binned read counts by dividing by the median read count of the core regions of chromosome 9. We excluded bins where GC content was <20% due to coverage bias in most samples. Copy number state for each bin was predicted in each sample by fitting a Gaussian hidden Markov model to the normalised coverage data.
After determining the most likely state for each window (Viterbi algorithm), in each sample independently we merged adjacent windows having the same state prediction and allowed a maximum gap of 5000 bp between them. We then further removed windows <700 bp or not supported by face-away reads, and data for 43 samples with excessive variation in read coverage. This led to the identification of a median 6 CNVs per sample. Finally, we merged all the CNV calls across the samples, by considering any partial overlap. In total, we identified 134 regions containing CNVs across the core genome; 43 of these CNVs were present in ≥5 samples and were tested for association with the phenotype, as described above. For the 133 samples in the clinical study used for the survival analysis, we manually inspected each sample individually and scanned them for the presence of reads spanning the breakpoints.
Role of the funding source
The sponsors of the study had no role in study design; data collection, analysis, and interpretation; or report writing. The corresponding authors had full access to all data in the study and final responsibility for the decision to submit for publication.
Results
We analysed 486 P. falciparum clinical isolates collected between July 9, 2010 and Dec 31, 2013 in 3 Cambodian provinces where artemisinin resistance is entrenched (Pursat), emerging (Preah Vihear), and uncommon (Ratanakiri) (supplementary table 1, p.2).3,4,7 To monitor the evolution of piperaquine resistance, we measured piperaquine IC50s for 297 P. falciparum clinical isolates obtained directly from patients (ex vivo, 275/297, 93%) or following short-term culture (in vitro, 22/297, 7%) in 2011, 2012, and 2013. In all 3 provinces, IC50s increased significantly over time, especially when comparing province-stratified data from 2011 and 2013 (supplementary figure 2, p.7; Kruskal-Wallis test, all p≤10-3). Despite parasites having comparable IC50s at all sites in 2011 (medians 20.0, 19.3, and 19.6 nM for Ratanakiri, Preah Vihear, and Pursat, respectively), they were remarkably differentiated at all sites by 2013, showing approximately 2-, 3-, and 4-fold increases in IC50s (medians 39.2, 66.2, and 81.1 nM, respectively). These regional differences are consistent with the relative prevalences of artemisinin-resistant parasites and recrudescent parasitemias following dihydroartemisinin-piperaquine therapy.4,7
To investigate the genetic basis of piperaquine resistance in vitro, we performed GWAS analysis on 297 samples (supplementary table 1, p.2) for which we had both piperaquine IC50s and whole-genome sequences (see Methods). We tested 11,630 SNPs that were well covered in this set of samples, and where 1 of the 2 alleles was present in ≥10 samples. IC50s were treated as a continuous dependent variable in a linear mixed-model algorithm, and we treated the province of sample origin, presence of mdr1 amplification, and presence of kelch13 mutations as covariates; to correct for the confounding effect of population structure, we treated genetic similarity across samples as a random effect (supplementary figure 1, p.6).
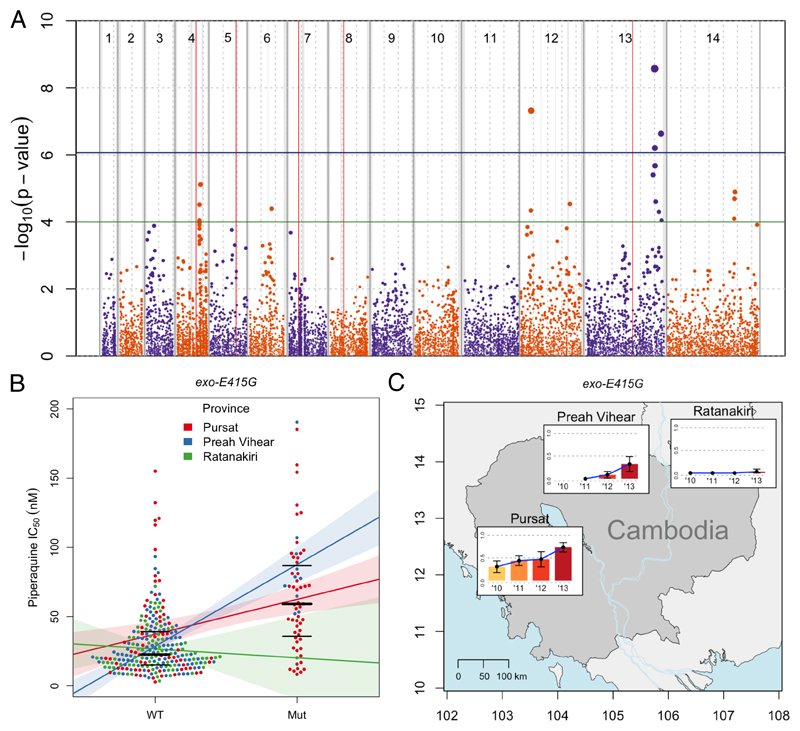
GWAS analysis identified 1 major locus on chromosome 13, containing 2 nonsynonymous SNPs, that significantly associated with elevated IC50s (p≤8.6×10−7; figure 1A, table 1). The strongest signal (2.55-fold increase in IC50 after adjustment, p=2.7×10−9) is from a nonsynonymous SNP (referred to as exo-E415G) producing a Glu415Gly substitution in a putative exonuclease. The second-strongest signal (p=2.3×10−7) from a nonsynonymous variant is a SNP (referred to as mcp-N252D) within the same locus, producing an Asn252Asp substitution in a putative mitochondrial carrier protein 1. A locus on chromosome 12 also reaches genome-wide significance, but only with a single synonymous SNP. To exclude the possibility of a batch effect, we repeated the GWAS analysis by including the year of sample collection as a covariate in the linear mixed-model (supplementary figure 3, p.8). This analysis confirmed the locus on chromosome 13 as the only one significantly associated with the phenotype. When also correcting for year, mcp-N252D is the only SNP to reach genome-wide significance (p=7.3×10−7), although exo-E415G is just below the threshold (p=1.3×10−6). These data indicate that the chromosome 13 locus (referred to as L13) shows multiple significant GWAS hits that are robust to multiple corrections.
Figure 1. Manhattan plot showing the statistical significance level of SNP associations in the GWAS (A), piperaquine IC50s (B), and exo-E415G frequency distribution (C).
(A) Each point represents 1 of the 11,630 SNPs with MAF>0.033 in the set of 297 P. falciparum clinical isolates, coloured according to chromosome. Genomic location is shown on the x-axis. The p value for each SNP's association, calculated using a linear mixed model, is shown on the y-axis; point size is proportional to significance level. Province of sample origin, status of kelch13 (mutant vs wild-type), presence or absence of mdr1 amplification, and a genetic relatedness matrix were added as fixed effects to the analysis. 4 SNPs reached the Bonferroni-corrected, genome-wide significance level of p≤8.6×10−7 (above horizontal blue line). Loci containing these significant SNPs, plus those containing suggestive SNPs reaching the significance level of p≤10−4 (above horizontal green line), are listed in Table 1. Vertical red lines mark known drug resistance loci: dhfr, mdr1, crt, dhps, and kelch13 on chromosomes 4, 5, 7, 8, and 13, respectively. Dashed grey vertical lines are plotted every 500 Kbp from the beginning of each chromosome. Solid grey vertical boxes mark telomeric, sub-telomeric, and internal hypervariable regions. (B) Each point represents the piperaquine IC50 for a P. falciparum clinical isolate carrying the wild-type ‘A’ allele (WT) or mutant ‘G’ allele (Mut) allele of exo-E415G (Pf3D7_13_v3:2504560). Bold and thin horizontal lines indicate the median and interquartile range of each distribution, respectively. Samples are divided into 3 coloured groups depending on their geographical origin. Coloured lines represent the least-squares linear regression of the phenotype on the 2 genotypes, calculated in each group separately. Shaded areas represent 95%CIs of the regression. (C) Coloured bars indicate the mutant allele frequencies in each of the 3 provinces over time (no samples were available from Preah Vihear in 2010). Error bars indicate 95% confidence intervals of the estimation. Geographical coordinates are shown on the axes.
Table 1. Genome-wide SNPs most strongly associated with piperaquine IC50s.
| Locus | Chr. | Position | Gene ID | Gene description | N/S | Alteration | p value |
|---|---|---|---|---|---|---|---|
| L13 | 13 | 2,504,560 | PF3D7_1362500 | exonuclease, putative | N | p.Glu415Gly | 2.69 × 10−9 |
| L12-01 | 12 | 418,346 | PF3D7_1208900 | conserved Plasmodium protein, unknown function | S | p.981Pro | 4.80 × 10−8 |
| L13 | 13 | 2,728,402 | PF3D7_1368700 | mitochondrial carrier protein, putative | N | p.Asn252Asp | 2.33 × 10−7 |
| L13 | 13 | 2,512,415 | PF3D7_1362700 | conserved Plasmodium protein, unknown function | S | p.687Asn | 6.25 × 10−7 |
| L13 | 13 | 2,519,091 | PF3D7_1362800 | conserved Plasmodium protein, unknown function | N | p.Gly202Asp | 2.12 × 10−6 |
| L13 | 13 | 2,447,146 | PF3D7_1361000 | arginine methyltransferase 5, putative (PRMT5) | N | p.Asn40Ser | 3.96 × 10−6 |
| L04-01 | 4 | 904,088 | PF3D7_0420000 | zinc finger protein, putative | S | p.213Leu | 7.67 × 10−6 |
| L14-01 | 14 | 2,411,942 | PF3D7_1458700 | conserved Plasmodium protein, unknown function | N | p.Arg25Lys | 1.27 × 10−5 |
| L14-01 | 14 | 2,395,752 | PF3D7_1458300 | conserved Plasmodium protein, unknown function | N | p.Ile301Phe | 2.03 × 10−5 |
| L13 | 13 | 2,542,366 | PF3D7_1363300 | mitochondrial ribosomal protein L9 precursor, putative | N | p.Thr28Ile | 2.48 × 10−5 |
| L12-02 | 12 | 1,787,279 | PF3D7_1242000 | conserved Plasmodium protein, unknown function | N | p.Ser518Asn | 2.90 × 10−5 |
| L04-01 | 4 | 865,807 | PF3D7_0419400 | conserved Plasmodium protein, unknown function | S | p.2423Ile | 3.05 × 10−5 |
| L06-01 | 6 | 866,478 | PF3D7_0621100 | conserved Plasmodium protein, unknown function | N | p.Asp1144Glu | 4.04 × 10−5 |
| L12-01 | 12 | 407,838 | PF3D7_1208800 | zinc finger protein, putative | N | p.Ser438Asn | 4.56 × 10−5 |
| L13 | 13 | 2,656,558 | PF3D7_1366400 | rhoptry protein (Rhop148) | N | p.Lys836Asn | 5.02 × 10−5 |
| L14-01 | 14 | 2,385,550 | PF3D7_1457900 | conserved Plasmodium protein, unknown function | S | p.3324Thr | 8.00 × 10−5 |
| L04-01 | 4 | 865,709 | PF3D7_0419400 | conserved Plasmodium protein, unknown function | N | p.Leu2391Ile | 8.96 × 10−5 |
| L13 | 13 | 2,746,936 | PF3D7_1369100 | conserved Plasmodium protein, unknown function | N | p.Leu430Arg | 8.98 × 10−5 |
Significant SNPs (Bonferroni-corrected p≤8.6×10−7, boldface) and suggestive SNPs (p≤10−4) associated with piperaquine IC50s, according to increasing p value, are shown. For each SNP, we list the locus name; chromosome number (Chr.); nucleotide position; gene ID; gene description; whether the SNP is nonsynonymous (N) or synonymous (S); encoded amino-acid alteration; and association p value.
To prioritise the multiple GWAS hits, we analysed their geographical spread, on the assumption that piperaquine resistance is emerging in Cambodia and neighbouring countries where dihydroartemisinin-piperaquine therapy is used (supplementary note 1, p.14; supplementary figure 4, p.9). Taken together, the results of the GWAS and global allele frequency survey identify exo-E415G as the best candidate SNP marker of piperaquine resistance.
To gain a better picture of its frequency distribution in Cambodia, we used Sanger sequencing of PCR-amplified DNA fragments to genotype this SNP in 168 additional samples – for which whole-genome sequencing data were not available – from the same initial cohort of 241 patients.4 As expected, given the low p value of this SNP in the GWAS, the phenotype distribution differs significantly between the wild-type and the mutant alleles. When stratified by province and year, the association seems to be particularly strong in Pursat and Preah Vihear (figure 1B; supplementary table 2, p.3), and from 2012 onwards (supplementary figure 5, p.10; supplementary table 2, p.3). Consistent with these results, the frequency of exo-E415G significantly increases in Pursat and Preah Vihear over time (figure 1C, table 2). In summary, exo-E415G is the best predictor of elevated IC50s in the current dataset, although this finding alone is insufficient to infer a causal role for it in mediating piperaquine resistance.
Table 2. Joint distribution of the 2 piperaquine resistance markers in Cambodia.
| plasmepsin II-III | CN1 | CN2+ | |||
|---|---|---|---|---|---|
| exo-E415G | WT | Mut | WT | Mut | |
| Ratanakiri | |||||
| 2010 | 49 | ||||
| 2011 | 71 | ||||
| 2012 | 23 | ||||
| 2013 | 11 | 1 | 1 | ||
| Preah Vihear | |||||
| 2010 | - | - | - | - | |
| 2011 | 66 | ||||
| 2012 | 30 | 3 | 1 | ||
| 2013 | 12 | 1 | 7 | ||
| Pursat | |||||
| 2010 | 31 | 3 | 9 | 6 | |
| 2011 | 33 | 13 | 1 | 35 | |
| 2012 | 6 | 1 | 10 | ||
| 2013 | 2 | 5 | 5 | 26 | |
The number of samples that are wild-type (WT) or mutant (Mut) for the exonuclease E415G mutation (exo-E415G) and have 1 (CN1) or multiple (CN2+) copies of plasmepsin II-III (plasmepsin II-III), according to province and year of collection, are shown. Samples where data for 1 of the 2 markers were not available or the marker was present in a mixed infection were excluded.
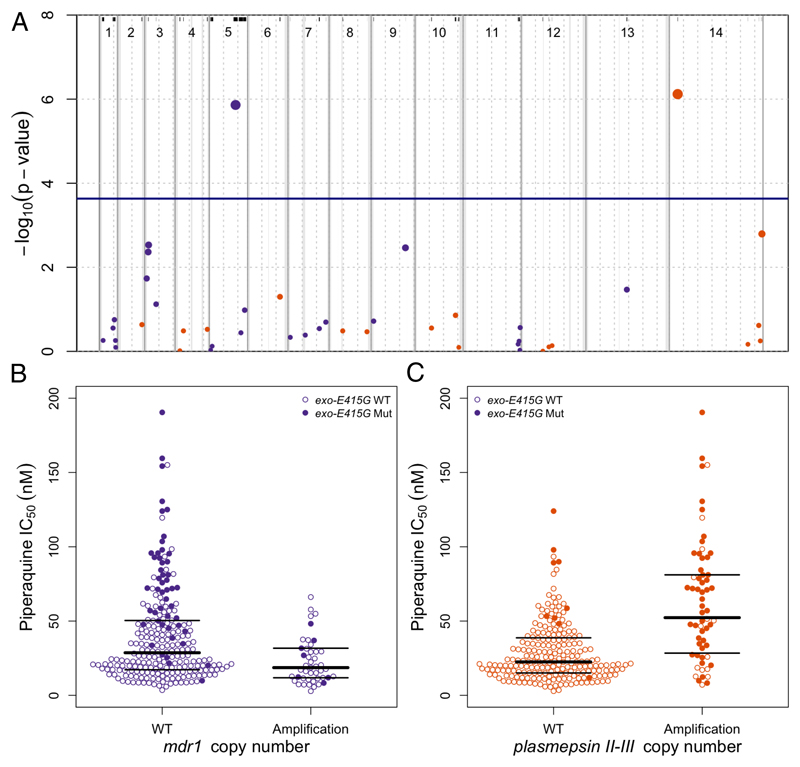
Since gene CNVs have also been associated with drug resistance in P. falciparum,24 we investigated the association of piperaquine IC50s with 43 genomic regions exhibiting CNVs in ≥5 samples. We conducted a GWAS using the same method we used in the SNP GWAS, except that genotypes were the presence or absence of the copy number variant in each sample. We found that 2 CNVs strongly associated with IC50s (p≤2.3×10−4; figure 2A; supplementary table 3, p.4). As expected,4 mdr1 amplification was associated (p=1.4×10−6) with low IC50s. However, the most significant association (p=7.6×10−7) was found for a novel amplification on chromosome 14, encompassing 2 of the 10 plasmepsin genes in the P. falciparum genome – plasmepsin II and plasmepsin III, referred to as “plasmepsin CNV” – that encode aspartic proteases involved in the haemoglobin-to-haemozoin conversion pathway targeted by quinolines.25
Figure 2. Manhattan plot showing the statistical significance level of CNV associations in the GWAS (A) and piperaquine IC50s according to mdr1 and plasmepsin II-III copy number (B).
(A) Each point represents 1 of the 43 CNVs present in ≥5 samples, coloured according to chromosome. Genomic location is shown on the x-axis. The p value for each CNV's association, calculated using a linear mixed model, is shown on the y-axis; point size is proportional to significance level. The province of sample origin, status of kelch13 (mutant vs wild-type), and a genetic relatedness matrix were added as fixed effects to the analysis. 2 CNVs reached the Bonferroni-corrected, genome-wide significance level of p≤2.3×10−4 (above the horizontal blue line), 1 including plasmepsin II and plasmepsin III, and 1 including mdr1. All 43 CNVs are marked by black lines at the top and are listed in supplementary table 3. Dashed grey vertical lines are plotted every 500 Kbp from the beginning of each chromosome. Solid grey vertical boxes mark telomeric, sub-telomeric, and internal hypervariable regions. (B, C) Each point represents the piperaquine IC50 for a P. falciparum clinical isolate carrying wild-type (WT) or amplified (Amplification) mdr1 (B) or plasmepsin II-III (C) genes. Bold and thin horizontal lines indicate the median and interquartile range of each distribution, respectively. Filled circles identify samples also carrying exo-E415G.
A detailed analysis of sequencing reads aligned within the plasmepsin CNV locus revealed that the amplification boundaries were identical in all samples. The putative breakpoints lie near the 3’ end of both plasmepsin I and III, so that each amplification creates an intact extra copy of plasmepsin II together with a new chimeric version of plasmepsin III, with its 3’ end replaced by the 3’ end of plasmepsin I (supplementary figure 6, p.11). Due to the degree of homology between plasmepsin I and plasmepsin III, the amino acid sequence of the chimeric plasmepsin III protein is identical to that of plasmepsin III, except that an asparagine residue is replaced by 2 consecutive lysines.
The prevalence of plasmepsin CNV in our cohort shows a steady increase over time in Pursat and Preah Vihear compared to Ratanakiri (supplementary table 2, p.3), and reflects our observations of increasing piperaquine IC50s and rising prevalence of exo-E415G in these 2 provinces (table 2; supplementary table 2, p.3). Despite being on different chromosomes, exo-E415G and plasmepsin CNV are in significant LD (r2=0.56, empirical p=1.6×10-5, supplementary figure 7, p.12), and have a very similar allele frequency distribution in these Cambodian data. In particular, of the 462 samples where both markers were reliably typed, 72% (n=334) have neither marker, 19% (n=86) have both markers, and only 9% (n=42) have 1 of the 2 markers. While the co-occurrence of the 2 markers in the population is interesting and surprising, the few samples where the 2 markers are found separately makes any conclusion regarding their relative effect on the phenotype difficult to support statistically (supplementary figure 8, p.13).
To further investigate whether the exo-E415G and plasmepsin CNV markers segregate with a newly described piperaquine resistance phenotype, we subjected a subset of 12 clinical isolates in triplicate to the in-vitro piperaquine survival assay (PSA).26 In this assay, the survival of early ring-stage parasites following exposure to piperaquine for 48 hours is assessed relative to non-exposed parasites tested in parallel. For these experiments, we selected 2 groups of parasites (supplementary table 4, p.5). In the first group, parasites did not recrudesce after dihydroartemisinin-piperaquine treatment, had low piperaquine IC50s ex vivo and after cultivation in vitro, were wildtype for kelch13 and exonuclease, and carried single copies of mdr1 and plasmepsins II-III. In the second group, parasites recrudesced after dihydroartemisinin-piperaquine treatment, had high piperaquine IC50s ex vivo and after cultivation in vitro, carried single copies of mdr1, and harboured the following mutations: kelch13-C580Y, exo-E415G, and plasmepsin CNV. This second group of parasites showed significantly higher PSA survival rates (median 61.6 nM, interquartile range 56.8-67.0, n=6) than the first (2.4 nM, 1.6-2.9, n=6; p=0.002). The relationship between elevated PSA survival rate and the presence of either exo-E415G or plasmepsin CNV were fully concordant.
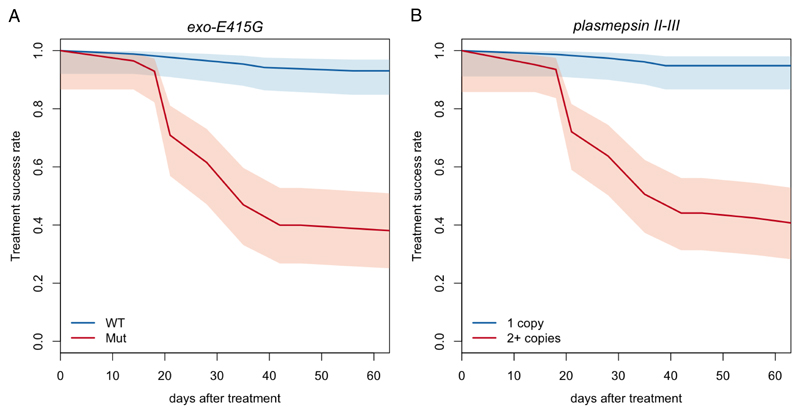
Given that exo-E415G and plasmepsin CNV are associated with elevated IC50s and PSA survival rates, which, in turn, have been associated with parasite recrudescence,4,26 we directly tested their association with dihydroartemisinin-piperaquine failures. Of the 241 samples with clinical outcome data, we analysed a subset of 133 samples for which we had complete genetic and clinical information (supplementary note 2, p.15). Both exo-E415G and plasmepsin CNV mutants showed a highly significant enrichment in recrudescent samples (supplementary table 2, p.3; p=1.6×10-8 and 1.8×10-11, respectively, Fisher’s exact test), with an adjusted hazard ratios (aHRs) of recrudescence of 13.4 and 16.7 (95%CIs 5.3-33.5 and 5.8-48.1), respectively. Furthermore, the aHR for plasmepsin CNV is still significant (p=8.6×10-3, aHR=5.2, 95%CI 1.5-17.7) when only kelch13 mutants were considered, suggesting that while artemisinin resistance is certainly a risk factor, plasmepsin CNV potentially has an additional, independent effect on piperaquine resistance and, ultimately, dihydroartemisinin-piperaquine treatment failure. To explicitly clarify the effect of the 2 markers on treatment success, we analysed the survival rate in samples with or without the 2 markers. We found that the adjusted survival rates at 63 days in the presence or absence of exo-E415G were 0.38 and 0.93 (95%CIs 0.25-0.51 and 0.85-0.97; figure 3A), respectively. In turn, samples with or without the plasmepsin CNV had survival rates of 0.41 and 0.95 (95%CIs 0.28-0.53 and 0.87-0.98; figure 3B), respectively. Considering that non-recrudescent infections are not necessarily indicators of drug sensitivity, these values are likely underestimated. Together, these data identify exo-E415G and plasmepsin CNV as strongly predictive markers of dihydroartemisinin-piperaquine failures in Cambodia.
Figure 3. Treatment success rate stratified by exo-E415G (A) and plasmepsin II-III copy number (B).
Adjusted survival curves showing the proportion of recrudescent cases following dihydroartemisinin-piperaquine treatment, according to the presence of the exo-E415G SNP (A) and plasmepsin II-III amplification (B).
Discussion
In GWAS analyses of piperaquine IC50 phenotypes, we identified 2 genetic markers of piperaquine resistance in vitro and of dihydroartemisinin-piperaquine failures in patients: a nonsynonymous SNP encoding a Glu415Gly mutation in a putative exonuclease (exo-E415G), and amplification of the plasmepsin II and plasmepsin III genes (plasmepsin CNV). The prevalence of these 2 markers has increased substantially in recent years in Pursat and Preah Vihear, where artemisinin resistance is prevalent and where dihydroartemisinin-piperaquine has been the frontline ACT since 2008 and 2010, respectively. In a global dataset of SNP allele frequencies, the exo-E415G allele is observed only in eastern mainland southeast Asia, where dihydroartemisinin-piperaquine is used, and completely absent where this ACT has not been used.
Of the associated variations found in this study, amplification of plasmepsin II and plasmepsin III is the strongest candidate for mediating piperaquine resistance, because of the role of plasmepsins in haemozoin synthesis pathways in the parasite food vacuole. Since piperaquine, like chloroquine, is believed to inhibit the conversion of toxic haem moieties to nontoxic haemozoin crystals during haemoglobin digestion, it is possible that piperaquine targets plasmepsin II, plasmepsin III, or both, and that amplification of these plasmepsins counteracts the drug’s action. The SNP markers – exo-E415G and mcp-N252D – do not lend themselves to an equally simple explanation and, until functional studies are carried out, it will not be possible to determine whether either is directly involved in mediating piperaquine resistance, whether their role is compensatory for lost fitness in piperaquine-resistant mutants, or whether they are associated with piperaquine resistance simply because of their strong linkage to plasmepsin CNV or some other functional mutation in the Cambodian parasite populations.
Assigning a role to the single copy variant of mdr1, which is associated with lower sensitivity to piperaquine, is equally problematic. While it is possible that mdr1 plays a functional role in piperaquine resistance (e.g., by restricting the amount of drug that enters the parasite’s food vacuole), we highlight that recent changes in Cambodia’s antimalarial drug policy have caused a decline in mefloquine pressure, which may have promoted the loss of mdr1 amplifications in the parasite populations. In view of the pronounced population structure following the emergence of artemisinin resistance in Cambodia,27 it is possible that the association with a single copy of mdr1 is the result of piperaquine resistance having emerged in specific artemisinin-resistant, mefloquine-sensitive populations.
Taken together, these findings identify plasmepsin CNV as the most likely candidate to be a causal variant of piperaquine resistance. In the samples studied here, it is strongly linked to exo-E415G, which is on a different chromosome and might represent some other functional component of the resistance phenotype. In practice, this means that the exo-E415G SNP can be presently used as a predictive marker of piperaquine resistance in Cambodia; however, this may not be the case elsewhere. Therefore, plasmepsin CNV should be monitored in areas where dihydroartemisinin-piperaquine is used, although it is somewhat more laborious to genotype. Despite unresolved questions about causal mechanism, we now have tools to identify areas where dihydroartemisinin-piperaquine failures are likely to occur, and thereby to empower national malaria control programs to make informed decisions about whether to switch to alternative ACTs for first-line treatment of P. falciparum malaria.
Supplementary Material
Panel: Research in context
We searched PubMed using the terms “artemisinin,” “piperaquine,” “resistance,” and “Cambodia” without any date or language restrictions. This search identified 26 articles, 5 of which reported original clinical trials that documented dihydroartemisinin-piperaquine failures in the treatment of uncomplicated Plasmodium falciparum malaria in Cambodia. Collectively, these studies reported 11-54% dihydroartemisinin-piperaquine failure rates in Pailin, Pursat, Oddar Meancheay, and Preah Vihear Provinces in 2010-2013, and associated an increased risk of failure with several parasite genotypes and phenotypes: the presence of kelch13 gene mutations that confer artemisinin resistance; the presence of 2 mutations (MAL10:688956 and MAL13:1718319) linked to slow parasite clearance after artesunate treatment; the absence of mdr1 gene amplification, a molecular marker of mefloquine resistance; and decreased piperaquine susceptibility in vitro. These studies did not identify molecular markers of both dihydroartemisinin-piperaquine failure and piperaquine resistance.
Added value of this study
This study, which investigated the whole-genome sequences and piperaquine susceptibilities of 297 P. falciparum clinical isolates from Cambodia, provides 2 new molecular markers of piperaquine resistance and dihydroartemisinin-piperaquine failures. The first marker is a single-nucleotide polymorphism, which encodes a glutamic acid-to-glycine amino acid substitution, in a putative exonuclease gene. This “exonuclease” marker is associated with decreased piperaquine susceptibility in vitro, and a low rate (0.38) of dihydroartemisinin-piperaquine treatment efficacy. This marker is found predominantly in Cambodia but not in other areas of the world where dihydroartemisinin-piperaquine has not been used. The second marker is an amplification of the plasmepsin II and plasmepsin III genes. This “plasmepsin” marker is also associated with decreased piperaquine susceptibility in vitro, and a low rate (0.41) of dihydroartemisinin-piperaquine treatment success. This marker is a strong candidate causal mutation because plasmepsins encode aspartic proteases, which are involved in the parasite’s haemoglobin degradation pathway targeted by related antimalarial drugs such as chloroquine. These exonuclease and plasmepsin markers are commonly found together, along with kelch13 gene mutations, which confer artemisinin resistance, and a single copy of the mdr1 gene, which is associated with decreased piperaquine, but increased mefloquine, susceptibility in vitro.
Implications of all the available evidence
In Cambodia, emerging dihydroartemisinin-piperaquine failure is associated with the presence of plasmepsin and exonuclease markers. The plasmepsin marker is a strong candidate for a functional polymorphism and can be used to monitor the spread of dihydroartemisinin-piperaquine failures in Cambodia and neighbouring countries in mainland Southeast Asia, where dihydroartemisinin-piperaquine is a first-line treatment for malaria. Mapping the extent of this marker will enable national malaria control programs to immediately recommend alternative therapies to improve the treatment of patients and drive the elimination of malaria.
Acknowledgments
This work was funded by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health; Bill & Melinda Gates Foundation (OPP1040463, OPP11188166); Medical Research Council (G0600718); and UK Department for International Development (M006212). This publication uses data from the MalariaGEN Plasmodium falciparum Community Project (www.malariagen.net/projects/parasite/pf).28 Genome sequencing was performed by the Wellcome Trust Sanger Institute and the Community Project was coordinated by the MalariaGEN Resource Centre with funding from the Wellcome Trust (098051, 090770). We thank all patients for participating in this study; Marcus Lee for helpful discussions; and Robert Gwadz and Thomas Wellems for their efforts in support of this work.
Footnotes
Conflicts of interest
We declare that we have no conflicts of interest.
Contributors
RA, PL, OM, DPK, and RMF designed the study. RA, PL, CA, DD, ATN, SSr, SSu, and ED collected data. RA, PL, OM, RDP, JA-G, DJ, JS, DPK, and RMF analysed and interpreted data. RA, PL, DPK, and RMF wrote the manuscript.
References
- 1.http://www.who.int/malaria/publications/atoz/9789241547925/en/
- 2.Fairhurst RM. Understanding artemisinin-resistant malaria: what a difference a year makes. Current opinion in infectious diseases. 2015;28(5):417–25. doi: 10.1097/QCO.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaratunga C, Sreng S, Suon S, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. The Lancet Infectious diseases. 2012;12(11):851–8. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaratunga C, Lim P, Suon S, et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. The Lancet Infectious diseases. 2016;16(3):357–65. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. The New England journal of medicine. 2009;361(5):455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–5. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. The New England journal of medicine. 2014;371(5):411–23. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takala-Harrison S, Jacob CG, Arze C, et al. Independent Emergence of Artemisinin Resistance Mutations Among Plasmodium falciparum in Southeast Asia. The Journal of infectious diseases. 2014 doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leang R, Barrette A, Bouth DM, et al. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrobial agents and chemotherapy. 2013;57(2):818–26. doi: 10.1128/AAC.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders DL, Vanachayangkul P, Lon C, et al. Dihydroartemisinin-piperaquine failure in Cambodia. The New England journal of medicine. 2014;371(5):484–5. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 11.Spring MD, Lin JT, Manning JE, et al. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. The Lancet Infectious diseases. 2015 doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 12.Leang R, Taylor WR, Bouth DM, et al. Evidence of Plasmodium falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment. Antimicrobial agents and chemotherapy. 2015;59(8):4719–26. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tun KM, Imwong M, Lwin KM, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. The Lancet Infectious diseases. 2015 doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dondorp AM, Fairhurst RM, Slutsker L, et al. The threat of artemisinin-resistant malaria. The New England journal of medicine. 2011;365(12):1073–5. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaorattanakawee S, Saunders DL, Sea D, et al. Ex Vivo Drug Susceptibility Testing and Molecular Profiling of Clinical Plasmodium falciparum Isolates from Cambodia from 2008 to 2013 Suggest Emerging Piperaquine Resistance. Antimicrobial agents and chemotherapy. 2015;59(8):4631–43. doi: 10.1128/AAC.00366-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eastman RT, Dharia NV, Winzeler EA, Fidock DA. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrobial agents and chemotherapy. 2011;55(8):3908–16. doi: 10.1128/AAC.01793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miotto O, Amato R, Ashley EA, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nature genetics. 2015 doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snounou G, Beck HP. The use of PCR genotyping in the assessment of recrudescence or reinfection after antimalarial drug treatment. Parasitology today. 1998;14(11):462–7. doi: 10.1016/s0169-4758(98)01340-4. [DOI] [PubMed] [Google Scholar]
- 19.Lippert C, Listgarten J, Liu Y, Kadie CM, Davidson RI, Heckerman D. FaST linear mixed models for genome-wide association studies. Nature methods. 2011;8(10):833–5. doi: 10.1038/nmeth.1681. [DOI] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WorldWide Antimalarial Resistance Network DPSG. The effect of dosing regimens on the antimalarial efficacy of dihydroartemisinin-piperaquine: a pooled analysis of individual patient data. PLoS medicine. 2013;10(12):e1001564. doi: 10.1371/journal.pmed.1001564. discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.http://dx.doi.org/10.1101/024182
- 23.Pearson RD, Amato R, Auburn S, et al. Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nature genetics. 2016;48(8):959–64. doi: 10.1038/ng.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price RN, Uhlemann AC, Brockman A, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364(9432):438–47. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chugh M, Sundararaman V, Kumar S, et al. Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(14):5392–7. doi: 10.1073/pnas.1218412110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duru V, Khim N, Leang R, et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC medicine. 2015;13:305. doi: 10.1186/s12916-015-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miotto O, Almagro-Garcia J, Manske M, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nature genetics. 2013;45(6):648–55. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malaria GENPfCP. Genomic epidemiology of artemisinin resistant malaria. eLife. 2016;5 doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.