Abstract
Cell function is mediated by interactions with the extracellular matrix (ECM), which has complex tissue-specific composition and architecture. The focus of this article is on the methods for fabricating ECM-derived porous foams and microcarriers for use as biologically-relevant substrates in advanced 3D in vitro cell culture models or as pro-regenerative scaffolds and cell delivery systems for tissue engineering and regenerative medicine. Using decellularized tissues or purified insoluble collagen as a starting material, the techniques can be applied to synthesize a broad array of tissue-specific bioscaffolds with customizable geometries. The approach involves mechanical processing and mild enzymatic digestion to yield an ECM suspension that is used to fabricate the three-dimensional foams or microcarriers through controlled freezing and lyophilization procedures. These pure ECM-derived scaffolds are highly porous, yet stable without the need for chemical crosslinking agents or other additives that may negatively impact cell function. The scaffold properties can be tuned to some extent by varying factors such as the ECM suspension concentration, mechanical processing methods, or synthesis conditions. In general, the scaffolds are robust and easy to handle, and can be processed as tissues for most standard biological assays, providing a versatile and user-friendly 3D cell culture platform that mimics the native ECM composition. Overall, these straightforward methods for fabricating customized ECM-derived foams and microcarriers may be of interest to both biologists and biomedical engineers as tissue-specific cell-instructive platforms for in vitro and in vivo applications.
Keywords: Bioengineering, Issue 122, extracellular matrix (ECM), collagen, scaffold, foam, microcarrier, decellularization, bioengineering, tissue-specific, microenvironment, instructive, cell culture, tissue engineering
Introduction
The extracellular matrix (ECM) is comprised of a complex 3D network of proteins, glycoproteins, and polysaccharides1. Once regarded as a predominantly structural framework, it is now well recognized that the ECM incorporates a diverse array of bioactive molecules with important functional roles2. Cell-ECM interactions can direct cell survival, adhesion, migration, proliferation, and differentiation3. While the major classes of ECM macromolecules are generally well conserved across tissues and species, each tissue has a unique matrix composition and architecture4. Overall, the tissue-specific ECM provides an instructive microenvironment that mediates function from the subcellular to the tissue/organ scale5.
Due to the critical role of the ECM in mediating cellular function, there has been increasing interest in the development of ECM-derived bioscaffolds for applications in tissue engineering and regenerative medicine. In particular, the method of decellularization has been extensively explored as a means of obtaining ECM from a wide range of tissues for use as a scaffolding material for tissue regeneration and cell delivery5,6,7. Decellularization typically involves a series of mechanical, chemical, and/or biological treatment stages targeted at removing cells and cellular components, while ideally causing minimal alterations to the 3D structure and composition of the ECM8. Through surveying the literature, various decellularization protocols can be identified for virtually every tissue in the body7.
While decellularized tissues can be used directly as implantable scaffolds or 3D cell culture substrates, cellular infiltration may be limited in tissues with a dense ECM structure9. Further, the natural heterogeneity in the ECM may cause variability in cell attachment and distribution within the decellularized matrices, which could potentially impact the cellular response10. Overall, while promising for some applications, applying decellularized tissues in their intact form offers limited versatility in terms of tuning scaffold properties including shape, porosity, and stiffness, as well as the mode of delivery for in vivo applications.
To circumvent these limitations, numerous research groups are applying further processing methods to generate customized scaffold formats using decellularized tissues as a base material. In the simplest form, this may involve cryomilling the decellularized tissues to generate injectable tissue-specific ECM particles11. These ECM particles may be incorporated as a cell-instructive component in composite scaffolds with other biomaterials, such as in situ crosslinking hydrogels12,13,14. In addition to mechanical processing, decellularized tissues can also be subjected to enzymatic digestion with proteolytic and/or glycolytic enzymes to fabricate ECM-derived hydrogels, foams, microcarriers, and coatings15,16,17, as well as to synthesize bioinks for 3D printing18.
In addition to tissue-engineering applications, ECM-derived bioscaffolds hold great potential for the generation of higher fidelity in vitro models for biological research. There is a significant need to develop 3D cell culture systems that better recapitulate the native cellular microenvironment19. Most in vitro cell culture studies to date are conducted on tissue culture polystyrene (TCPS), which has little correlation with the biologically complex and dynamic cellular milieu found within living tissues20. While convenient for studying cellular interactions within a controlled environment, culturing cells on these simplified rigid 2D substrates alters cell attachment and morphology, as well as both cell-cell and cell-ECM interactions21,22. The cellular adaptations observed on 2D TCPS can impact intracellular signaling pathways that regulate diverse cell functions including survival, proliferation, migration, and differentiation, raising questions of the relevance of 2D studies in modelling in vivo systems23. There has been increasing recognition that cellular behavior can vary greatly in 2D versus 3D systems24, and that biochemical and biomechanical signaling with the ECM are key mediators of cell function25. Many groups have attempted to overcome the limitations of established 2D systems by coating TCPS with ECM components such as collagen, laminin, and fibronectin. While these strategies can improve cell attachment and may alter cellular responses, these models remain limited by their 2D configuration that does not mimic the complex spatial organization or biochemistry of the native ECM26,27.
Our bioengineering laboratory has been interested in the development of ECM-derived bioscaffolds as substrates for 3-D cell culture and tissue-engineering applications. In particular, we have pioneered the use of decellularized adipose tissue (DAT) as a scaffolding platform for adipose regeneration28. Moreover, we have established methods for synthesizing 3D microcarriers and porous foams using DAT digested with the proteolytic enzyme pepsin or glycolytic enzyme α-amylase29,30,31. Notably, we have demonstrated across all of these scaffold formats that the adipose-derived ECM provides an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem/stromal cells (ASCs) in culture. More recently, we extended our fabrication methods to generate 3D porous foams from α-amylase-digested porcine decellularized left ventricle (DLV) (decellularization methods adapted from Wainwright et al.32), and showed that they provide a supportive platform for inducing early cardiomyogenic marker expression in human pericardial fat-derived ASCs31.
This article describes in detail the methods for synthesizing non-chemically crosslinked 3D porous foams and microcarriers derived purely from α-amylase-digested ECM for use as biologically complex 3D in vitro cell culture substrates and as biomaterials for tissue regeneration. In theory, any ECM source containing high molecular weight collagen may be used as the starting material for these techniques. To demonstrate the flexibility of this approach, the methods have been applied to generate tissue-specific bioscaffolds using human DAT, porcine decellularized dermal tissue (DDT)8, and porcine DLV as representative examples. Figure 1 provides a visual overview of the fabrication process for the ECM-derived foams and microcarriers.
 Figure 1. Overview of the Method for the Production of the Tissue-specific ECM-derived Foams and Microcarriers. 1. Decellularized tissues, prepared following established decellularization protocols, can be used for tissue-specific ECM-derived bioscaffold fabrication. Macroscopic images are shown of hydrated human DAT (prepared as described in Flynn 201028), porcine DDT (prepared as described in Reing, J. E., et al. 20108), and porcine DLV (prepared as described in Wainwright et al. 201032), as representative examples of ECM sources that can be used as starting materials. Scale bars represent 3 cm. 2. The decellularized tissues are lyophilized, and then 3. mechanically minced. Scale bars represent 1 cm. 4. The minced ECM can then be cryomilled, which is optional for foam fabrication, but required for microcarrier synthesis. Scale bar represents 3 mm. 5. The minced or cryomilled ECM is then digested with α-amylase and homogenized to create a homogenous ECM suspension. Scale bar represents 1 cm. 6a) For foam fabrication, the ECM suspension is transferred into a user-defined mold, frozen, and lyophilized to generate a porous 3D scaffold with a well-defined geometry. Scale bar represents 1 cm. 6b) For microcarrier fabrication, the cryomilled ECM suspension is electrosprayed to generate discrete spherical microcarriers. Scale bar represents 2 mm. 7. The foams and microcarriers can then be gradually rehydrated and seeded with cells. Representative images are shown of human ASCs (viable cells=green) seeded on a DAT foam (left) and DAT microcarrier (right). Scale bars represent 100 µm. Please click here to view a larger version of this figure.
Figure 1. Overview of the Method for the Production of the Tissue-specific ECM-derived Foams and Microcarriers. 1. Decellularized tissues, prepared following established decellularization protocols, can be used for tissue-specific ECM-derived bioscaffold fabrication. Macroscopic images are shown of hydrated human DAT (prepared as described in Flynn 201028), porcine DDT (prepared as described in Reing, J. E., et al. 20108), and porcine DLV (prepared as described in Wainwright et al. 201032), as representative examples of ECM sources that can be used as starting materials. Scale bars represent 3 cm. 2. The decellularized tissues are lyophilized, and then 3. mechanically minced. Scale bars represent 1 cm. 4. The minced ECM can then be cryomilled, which is optional for foam fabrication, but required for microcarrier synthesis. Scale bar represents 3 mm. 5. The minced or cryomilled ECM is then digested with α-amylase and homogenized to create a homogenous ECM suspension. Scale bar represents 1 cm. 6a) For foam fabrication, the ECM suspension is transferred into a user-defined mold, frozen, and lyophilized to generate a porous 3D scaffold with a well-defined geometry. Scale bar represents 1 cm. 6b) For microcarrier fabrication, the cryomilled ECM suspension is electrosprayed to generate discrete spherical microcarriers. Scale bar represents 2 mm. 7. The foams and microcarriers can then be gradually rehydrated and seeded with cells. Representative images are shown of human ASCs (viable cells=green) seeded on a DAT foam (left) and DAT microcarrier (right). Scale bars represent 100 µm. Please click here to view a larger version of this figure.
Protocol
1. Decellularized Tissue Processing
- Decellularization and Lyophilization
- Decellularize tissue(s) of interest following an established protocol. NOTE: Scaffolds in the current study have been prepared following published decellularization protocols for human DAT28, porcine DDT8, and porcine DLV32. Commercially-available, insoluble collagen can also be used to fabricate foams and microcarriers, such as collagen sourced from bovine tendon, which has been used successfully over a concentration range of 10 - 50 mg/mL. Other insoluble collagen sources may be utilized, but may require optimization to identify the concentration range that will yield stable scaffolds.
- Transfer the hydrated decellularized tissue or purified collagen into a 50 mL centrifuge tube with forceps and add sufficient double distilled water (ddH2O) to submerge the tissue, to a maximum total volume of 35 mL.
- Freeze the sample overnight at -80 °C, with the centrifuge tube positioned horizontally during freezing to maximize the surface area for sublimation during subsequent lyophilization.
- Remove the cap from the centrifuge tube and transfer the frozen sample into a lyophilization jar.
- Lyophilize the sample using a laboratory freeze dryer for 48 - 72 h or until fully dried. NOTE: Drying time may vary for different lyophilizers and decellularized tissues.
- Finely mince the lyophilized ECM into small pieces (~ 1 - 2 mm3) using sharp surgical scissors.
- Store the minced ECM in a desiccator until ready for further processing. NOTE: It is recommended that the minced ECM be used immediately to prevent moisture absorption from the environment.
- Cryomilling (optional for foam fabrication)
- Fill a 25 mL stainless steel milling chamber for a laboratory ball mill system with minced lyophilized ECM and add two 10 mm stainless steel milling balls.
- Close and completely submerge the loaded milling chamber in liquid nitrogen for 3 min.
- Mill the frozen sample for 3 min at 30 Hz (1,800 rpm).
- Repeat steps 1.2.2 and 1.2.3 until the ECM is milled into a fine powder. NOTE: The number of times these steps should be repeated may vary between ECM sources. For example, DAT typically requires 3 milling cycles to generate a fine powder.
- Transfer the powder using a scoopula into a glass vial, seal tightly, and store in a desiccator.
- ECM Suspension Preparation
- Weigh out 250 mg of either minced (for foams) or cryomilled (for foams or microcarriers) ECM and transfer it to a 15 mL centrifuge tube. Refer to step 1.1.6 and section 1.2 for preparation of minced ECM and cryomilled ECM, respectively. NOTE: This protocol is designed to prepare a 50 mg/mL ECM suspension, which is recommended for human DAT, porcine DDT, and porcine DLV. Depending on the specific ECM source, uniform suspensions may be generated at higher or lower starting concentrations.
- Add 5 mL of 0.22 M NaH2PO4 (pH 5.4) buffer to the centrifuge tube and mark the liquid level on the tube.
- Prepare an α-amylase stock solution by adding 7.5 mg of α-amylase to 1 mL 0.22 M NaH2PO4 (pH 5.4) buffer. Add 100 μL of the α-amylase stock solution (0.75 mg of α-amylase; 0.3% w/w of dry tissue) to the sample. Add 0.22 M NaH2PO4 to obtain a final volume of 10 mL.
- Agitate the suspension continuously at 300 rpm for 72 h at room temperature.
- Centrifuge the suspension at 1,500 x g for 10 min. Carefully collect and discard the supernatant without disturbing the digested ECM pellet. Resuspend the pelleted material in 10 mL of 5% NaCl diluted in ddH2O.
- Repeat step 1.3.5 for a total of two rinses with 5% NaCl in ddH2O.
- Discard the supernatant and resuspend the pelleted material in 10 mL of ddH2O.
- Agitate the suspension continuously at 300 rpm for 10 min at RT.
- Centrifuge the suspension at 1,500 x g for 10 min. Carefully collect and discard the supernatant without disturbing the digested ECM pellet.
- Add 0.2 M acetic acid to the 5 mL mark made in step 1.3.2.
- Agitate the suspension continuously at 120 rpm O/N at 37 °C.
- Homogenize the ECM suspension at room temperature in ten-second intervals using a hand-held homogenizer equipped with a saw tooth 10 mm wide probe until no visible fragments remain. Place the suspension in a beaker of cold water between the homogenization intervals to prevent overheating. NOTE: Depending on the ECM source, further dilution in 0.2 M acetic acid may be required to obtain a homogeneous suspension. Excessive cooling of the ECM suspension may result in an increase in viscosity that can interfere with efficient homogenization. If an increase in viscosity is noted, the sample can be rewarmed to 37 °C and subjected to further processing.
- Store at 4 °C for up to one month prior to foam or microcarrier fabrication.
2. ECM-derived Foam Fabrication
Incubate the ECM suspension from step 1.3.13 (minced or cryomilled) at 37 °C with continuous agitation at 120 rpm until the suspension is warm.
Dilute the ECM suspension in 0.2 M acetic acidic to the desired concentration. NOTE: Depending on the ECM source, stable foams may be prepared in the range of 10 - 100 mg/mL, with 15 - 50 mg/mL as the recommended range for DAT, DDT and DLV. Typically, foams fabricated at higher concentrations will be slightly less porous but more stable in culture and easier to handle.
Using a 3 mL syringe with an 18 G needle to prevent bubble formation in the ECM suspension, fill the desired mold with the ECM suspension. To fabricate the DAT, DDT, and DLV foams, dispense 400 μL of 35 mg/mL ECM suspension into a 48-well cell culture-treated plate. NOTE: The shape of the selected mold and the volume of ECM suspension will determine the geometry of the resultant foam.
Cover and freeze the molds overnight by placing them in a -20 °C or -80 °C freezer. NOTE: The freezing temperature may affect the porosity of the foams. Larger pores are expected when the samples are frozen at -20 °C as compared to -80 °C due to the formation of larger ice crystals33. To fabricate foams with a uniform porous structure, ensure that the mold is not in contact with a conductive surface to prevent directional cooling.
Place the molds containing the frozen samples into a lyophilizer flask. Connect the lyophilizer flask to the laboratory freeze dryer system and dry for 24 h. NOTE: It is important that the sample remains frozen prior to lyophilization.
Store the lyophilized foams in a desiccator until required.
3. ECM-derived Microcarrier Fabrication via Electrospraying
NOTE: An overview of the electrospraying set up is shown in Figure 2.
Incubate the cryomilled ECM suspension from step 1.3.13 at 37 °C with continuous agitation at 100 rpm O/N. NOTE: Cryomilled ECM is used to fabricate the ECM-derived microcarriers to ensure greater uniformity in the suspension and prevent clogging during electrospraying.
Load 3 mL of ECM suspension into a 3 mL Luer lock syringe and attach a winged infusion set onto the bore of the syringe. NOTE: A range of needle gauges can be used, recognizing that the selection may influence the size and shape of the resultant microcarriers. To fabricate the DAT, DDT, and DLV microcarriers, use a 25 G needle.
Secure the syringe within the syringe pump. Fasten the needle to a retort stand and position the needle tip vertically at a distance of 4 - 6 cm from the top of a low-form Dewar flask (250 - 500 mL size range recommended).
Attach an alligator clip electrode to the tip of the needle and connect it to the positive terminal of the high-voltage power supply.
Fold a strip of aluminum foil (12 x 5 cm) over the edge of the vacuum flask.
Attach a second alligator clip electrode to the outer edge of the foil and connect it to the ground source terminal of the power supply.
Fill the Dewar flask with liquid nitrogen to approximately 1 cm from the top, so that ¾ of the foil is submerged. NOTE: It is important to keep the Dewar flask filled with liquid nitrogen. The surface of the liquid nitrogen must be no less than 2 cm from the top of the flask during the electrospraying process. Continuous refilling of the liquid nitrogen is required to maintain a constant level during electrospraying.
Set the syringe pump to an infusion rate of 30 mL/h. NOTE: Varying the infusion rate can alter the microcarrier shape and diameter, but the recommended range is 25 - 35 mL/h. While it is highly dependent on the viscosity of the suspension, flow rates below 25 mL/h may result in the generation of larger microcarriers. At very low flow rates, the size and shape of the microcarriers may become less homogeneous. At infusion rates above 35 mL/h, the uniformity of the droplets generated via electrospraying may be disrupted, resulting in non-spherical microcarriers with a broader size distribution34.
Apply a voltage of 20 kV and turn on the syringe pump to initiate electrospraying. NOTE: The voltage may be varied to tune the size of the microcarriers and tends to have a greater impact on the diameter than the infusion rate. The recommended voltage range is 15 - 25 kV. A decrease in microcarrier diameter is expected with an increase in voltage35.
Once the infusion is complete, carefully pour off excess liquid nitrogen from the Dewar, leaving the microcarriers suspended in ~ 25 mL of liquid nitrogen to ensure they remain frozen.
Immediately transfer the microcarriers with the liquid nitrogen into a 50 mL centrifuge tube by pouring in one smooth motion. Collect any frozen microcarriers remaining in the Dewar with a scoopula and add them to the centrifuge tube. NOTE: Depending on the size of the Dewar, the microcarriers in liquid nitrogen can be initially poured into a mid-sized vessel, and then transferred immediately into the 50 mL centrifuge tube.
Cover the centrifuge tube containing the microcarriers in liquid nitrogen with aluminum foil perforated with small holes in preparation for lyophilization.
Place the covered centrifuge tubes into a lyophilizer flask. Immediately connect the flask to the lyophilizer and dry the samples O/N. NOTE: It is very important to keep the microcarriers suspended in liquid nitrogen to ensure they do not thaw before this step, as thawing may result in collapse and/or aggregation. DAT, DDT and DLV microcarriers fabricated at a concentration of 35 mg/mL using the electrospraying conditions described above will typically have a hydrated diameter ranging between 350 - 500 μm in size. The size distribution may vary depending on the ECM source.
Store the lyophilized microcarriers in a desiccator until required.
 Figure 2. Overview of the Electrospraying Apparatus used in Microcarrier Fabrication. A: Image showing the arrangement of the key electrospraying equipment including the syringe pump and high voltage power supply, as well as the positioning of the needle relative to the Dewar of liquid nitrogen. B: Electrospraying schematic, including the recommended ranges for the voltage, infusion rate, and distance. Please click here to view a larger version of this figure.
Figure 2. Overview of the Electrospraying Apparatus used in Microcarrier Fabrication. A: Image showing the arrangement of the key electrospraying equipment including the syringe pump and high voltage power supply, as well as the positioning of the needle relative to the Dewar of liquid nitrogen. B: Electrospraying schematic, including the recommended ranges for the voltage, infusion rate, and distance. Please click here to view a larger version of this figure.
4. Preparing Foams and Microcarriers for Cell Culture
- Rehydration
- Transfer the lyophilized foams with forceps into a 50 mL centrifuge tube containing excess absolute ethanol (~ 99.9%) (~ 5:1 ratio of ethanol to foams). Similarly, resuspend the lyophilized microcarriers in excess absolute ethanol within the original centrifuge tube used for collection. Using a serological pipette, filter the microcarriers through a stainless sieve with a defined mesh size into a new 50 mL centrifuge tube to remove any aggregates and select for desired size ranges. NOTE: If the foams are fabricated within TCPS plates, they can be rehydrated directly within these vessels.
- Incubate at 4 °C for 4 h or until the foams or microcarriers have gravity settled to the bottom of the centrifuge tube. Perform all subsequent steps in a laminar flow hood using sterile reagents and proper aseptic technique.
- Remove the absolute ethanol using a serological pipette and add excess (5:1 ratio) 95% ethanol diluted with sterile phosphate buffered saline (PBS) to the scaffolds. Incubate at 4 °C for 4 h or until the ECM-derived scaffolds have sunk to the bottom of the rehydration vessel.
- Gradually rehydrate the scaffolds through an ethanol series (90, 85, 80, 75, 70, 50, 25 and 0%, diluted with sterile PBS). At each step, incubate at 4 °C until the scaffolds have sunk to the bottom of the vessel before changing the solution. Degas the scaffolds under light vacuum if bubble formation within the scaffolds is observed.
- Replace the sterile PBS for an additional two rinses to remove residual ethanol.
- Store in 100% sterile PBS at 4 °C until ready for cell culture. Use the scaffolds within 1 - 2 weeks following rehydration.
- Preparation for Cell Seeding
- On the day prior to seeding, transfer the foams using forceps into cell culture treated well plates or transwell inserts and add sufficient cell culture medium (selected based on the cell type of interest) to completely submerge the scaffolds (e.g., 2.5 mL/scaffold in a 12-well plate). Similarly, allow the microcarriers to gravity settle within the centrifuge tube, carefully remove the PBS using a serological pipette without disturbing the microcarriers, and add cell culture medium to achieve a 5:1 ratio of medium to the microcarriers. NOTE: For seeding human ASCs, utilize Dulbecco's Modified Eagle's medium (DMEM):Ham's F12 nutrient mixture supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin.
- Replace the cell culture medium for a total of one rinse.
- Equilibrate the scaffolds overnight in cell culture medium at 37 °C. The scaffolds will be ready for cell seeding the next day. NOTE: The foams can be statically seeded in cell culture inserts or tissue culture well plates29, or dynamically seeded using a laboratory shaker36. Depending on the ECM source, processing methods and suspension concentration, dynamic seeding may enhance initial cell attachment, proliferation and infiltration. The microcarriers can be seeded dynamically using a spinner culture system11.
Representative Results
In the current study, we have fabricated ECM-derived foams and microcarriers using human DAT, porcine DDT, and porcine DLV as representative examples demonstrating that the techniques can be applied to generate tissue-specific bioscaffolds using a variety of decellularized tissues as ECM sources (Figure 1). For both foam and microcarrier fabrication, the Nishihara technique of collagen solubilization with the glycolytic enzyme α-amylase37 was adapted to generate a viscous ECM suspension from the decellularized tissue starting materials, which is used to synthesize the bioscaffolds through controlled freezing and lyophilization procedures.
To fabricate the foams, the decellularized tissues can be processed through either mechanical mincing or cryomilling to increase the surface area prior to enzymatic digestion. Following α-amylase treatment and homogenization, the resultant ECM suspension is dispensed into a user-defined mold, which is then frozen and lyophilized. The scaffolds can be stored stably in a dry state for an extended period of time. Prior to use in cell culture studies, the lyophilized scaffolds must be subjected to a controlled rehydration process that yields porous and highly-hydrated homogeneous foams derived from pure ECM (Figure 3A). If the foams are rehydrated too quickly, rapid swelling may damage delicate structural features, resulting in loss of integrity and structural collapse. In general, the foams will retain the shape defined by the original mold following rehydration and are stable without the need for chemical crosslinking. Figure 3Bshows representative scanning electron microscope (SEM) images of the DAT, DDT, and DLV foams fabricated with cryomilled ECM at a concentration of 35 mg/mL and a freezing temperature of -80 °C.
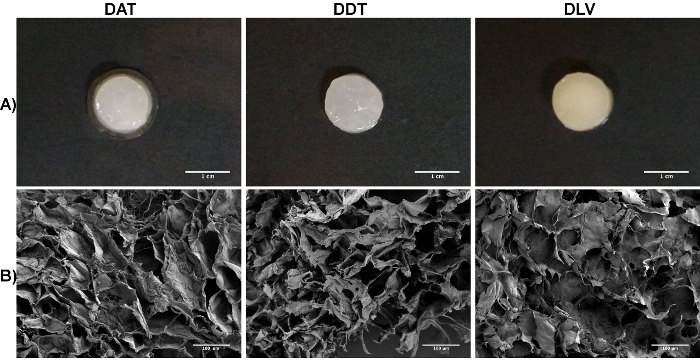 Figure 3. Representative Images of the DAT, DDT, and DLV Foams Fabricated with Cryomilled ECM Suspensions at a Concentration of 35 mg/mL and Frozen at -80 °C. A: Macroscopic view of the DAT, DDT, and DLV milled foams synthesized in a 48-well tissue culture plate mold following rehydration. Scale bars represent 1 cm. B: SEM images of the DAT, DDT, and DLV foams showing a homogeneous porous ultrastructure. Scale bars represent 100 µm. Please click here to view a larger version of this figure.
Figure 3. Representative Images of the DAT, DDT, and DLV Foams Fabricated with Cryomilled ECM Suspensions at a Concentration of 35 mg/mL and Frozen at -80 °C. A: Macroscopic view of the DAT, DDT, and DLV milled foams synthesized in a 48-well tissue culture plate mold following rehydration. Scale bars represent 1 cm. B: SEM images of the DAT, DDT, and DLV foams showing a homogeneous porous ultrastructure. Scale bars represent 100 µm. Please click here to view a larger version of this figure.
The cryomilled ECM suspensions can also be used to generate pure ECM-derived microcarriers via electrospraying techniques (Figure 2). For each ECM source, the suspension concentration, needle gauge, infusion rate, and voltage can be tuned to generate discrete spherical microcarriers ranging from 350 - 500 μm in diameter following controlled rehydration (Figure 4A). While the microcarriers can be stored in a lyophilized state, it is recommended for ease of handling that they are dispensed or sieved to a desired size range following resuspension in ethanol. SEM imaging suggests that the microcarrier ultrastructure can vary depending on the decellularized tissue source (Figure 4B).
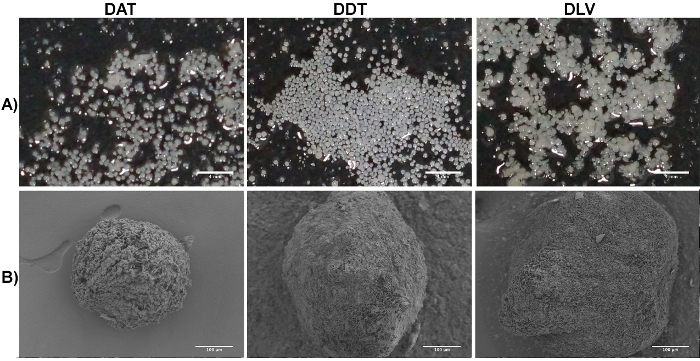 Figure 4. Representative Images of the DAT, DDT, and DLV Microcarriers Fabricated with Cryomilled ECM Suspensions at a Concentration of 35 mg/mL, an Infusion Rate of 0.5 mL/min, and an Applied Voltage of 20 kV. A: Macroscopic view of the hydrated DAT, DDT, and DLV microcarriers. Scale bars represent 4 mm. B: SEM images of the DAT, DDT, and DLV microcarriers showing that the ultrastructure and size can vary depending on the ECM source. Scale bars represent 100 µm. Please click here to view a larger version of this figure.
Figure 4. Representative Images of the DAT, DDT, and DLV Microcarriers Fabricated with Cryomilled ECM Suspensions at a Concentration of 35 mg/mL, an Infusion Rate of 0.5 mL/min, and an Applied Voltage of 20 kV. A: Macroscopic view of the hydrated DAT, DDT, and DLV microcarriers. Scale bars represent 4 mm. B: SEM images of the DAT, DDT, and DLV microcarriers showing that the ultrastructure and size can vary depending on the ECM source. Scale bars represent 100 µm. Please click here to view a larger version of this figure.
The mechanical properties of the scaffolds are dependent on the ECM source, the method of processing applied (i.e. mincing versus cryomilling), the ECM suspension concentration, and the freezing temperature. In general, the scaffolds are soft and compliant, with Young's moduli in the range of 1 - 5 kPa reported for the DAT (50 & 100 mg/mL)29 and DLV foams (20 - 50 mg/mL)31, and < 1 kPa for the microcarriers. The cryomilled foams are typically softer than the minced foams due to their more disrupted nature. Specialized mechanical testing systems equipped with highly-sensitive load cells are needed for accurate characterization of the compressive properties. Typically, the foams and microcarriers would have lower moduli than the native decellularized tissues due to the additional processing steps involved in fabrication including enzymatic digestion and homogenization, as well as the non-covalently crosslinked nature of the scaffolds. However, this can vary depending on the tissue of interest. For example, in previous work, we found that minced DAT foams fabricated at high ECM concentrations (100 mg/mL) had similar moduli to native adipose tissue29.
The rehydrated ECM-derived foams and microcarriers can be seeded with cells under static or dynamic culture conditions to provide a cell supportive platform for in vitro cell culture studies and/or in vivo cell delivery. While the scaffolds generally support cell attachment, the seeding efficiency will depend on the ECM source, the scaffold geometry and porosity, and the cell type of interest. As such, the seeding methods and densities will require optimization depending on the user-defined conditions. For the foams described above, we recommend a starting cell concentration in the range of 0.25 - 1 x 106 cells/scaffold, with dynamic seeding on an orbital shaker to enhance cell infiltration. For the microcarriers, we suggest an initial seeding density in the range of 25,000 - 50,000 cells/mg of microcarriers, with seeding performed under dynamic conditions in a spinner culture flask11. The images shown at the bottom of Figure 1 represent human ASCs seeded on a DAT foam (left) or DAT microcarrier (right, prelabeled with an amine-reactive dye and appearing blue13), as visualized by confocal microscopy using a fluorescent cell viability stain (live cells = green, dead cells = red; Scale bars represent 100 µm). Confocal microscopy can be used to visualize cells seeded on the surface of foams up to 2 mm in thickness, but visualization into the central regions of the scaffolds is limited by their opaque nature. However, the foams and microcarriers can be treated as tissues and paraffin-embedded or cryo-sectioned for histological and immunohistochemical analyses to visualize cell distribution and marker expression.
Discussion
In general, bioscaffolds derived from decellularized tissues can more closely approximate the complex 3D composition and structure of the ECM in the native cellular microenvironment as compared to synthetic scaffolds or standard culture models based on 2D TCPS. As previously discussed, cell-ECM interactions are critically important in mediating cellular behavior both in culture and in the body1. Recognizing that the biochemical, biophysical, and biomechanical properties of the ECM are unique to each tissue, there is increasing evidence to support the rationale for applying tissue-specific approaches in the design of biomaterials for tissue engineering, as well as in the development of more physiologically relevant culture models for in vitro experiments20. Utilizing decellularized tissues as a starting material, our methods can incorporate the complex composition of the tissue-specific ECM within more customizable scaffold formats. While the mechanical and enzymatic processing steps will result in a loss of the native ECM ultrastructure, previous studies with DAT have demonstrated that the instructive effects of the adipose-derived ECM are conserved in these scaffold formats, suggesting that the bioscaffold composition is a key mediator of cell function11,29. A significant advantage to using the ECM-derived foams and microcarriers as cell culture substrates as compared to the intact decellularized tissues is that they are more homogeneous, which can improve uniformity in cell distribution and cell-cell/cell-ECM interactions.
The methods described here can be utilized to generate a broad array of tissue-specific bioscaffolds for use in cell culture and tissue-engineering applications. For example, in addition to the DAT, DDT, and DLV, our lab has successfully applied these techniques to generate 3D porous foams using decellularized bone, cartilage, nucleus pulposus, and annulus fibrosis, as well as commercially-available, insoluble collagen derived from bovine tendon. From an in vitro perspective, these bioscaffolds could be used as a basis for higher-fidelity 3D culture models for investigating cellular biology, physiology or disease pathology38, as bioactive substrates in high-throughput drug screening platforms39, or as instructive matrices for stem cell differentiation40,41. DAT, DDT and DLV foams fabricated at concentrations of 25 - 50 mg/mL are stable in long-term in vitro culture (tested up to 28 days). Further, all three types of microcarriers can support cell attachment and proliferation under dynamic conditions in a low-shear spinner culture system (10 - 15 rpm) for at least 2 weeks. For in vivo applications, the biocompatible and biodegradable ECM-derived foams and microcarriers hold promise as off-the-shelf products to stimulate constructive tissue remodeling and regeneration11,29. Further, the cell-adhesive scaffolds could be used as cell therapy delivery systems42,43. As an example, DAT foams were shown to promote angiogenesis and adipogenesis when seeded with allogeneic ASCs and implanted subcutaneously in an immunocompetent rat model29. Relative to the intact DAT, the more highly processed DAT foams degraded much more rapidly, with a 50% reduction in volume noted at 3 weeks as they became integrated with the host tissues, and almost complete resorption by 12 weeks. However, the foams also induced a more potent angiogenic response, suggesting that the enzyme-digested ECM had unique pro-regenerative effects. Similarly, the ECM-derived microcarriers could be used as in vitro cell culture substrates within dynamic culture systems and as injectable cell delivery vehicles11,30,44. More specifically, the small diameter and large surface area of the microcarriers could enable the delivery of a large quantity of cells in a small volume, while providing a matrix that may help to support cell viability and increase cell retention at the site of injection30. Prior to use in any living system, it is critical to ensure that the source ECM is substantially devoid of antigenic cellular components and/or potentially cytotoxic decellularization reagents that could trigger a negative host response7.
The proteolytic enzyme pepsin is commonly used in the preparation of ECM-derived hydrogels15. Pepsin is a non-specific protease that will digest collagen and other ECM proteins into small fragments45. While hydrogels fabricated from pepsin-digested ECM have been reported to have cell-instructive effects, a limitation is that these materials tend to be extremely mechanically weak46. In our initial development of the DAT microcarriers, we utilized a composite approach in which pepsin-digested DAT was combined with alginate and added dropwise into CaCl2 to form spherical beads30. The beads were subsequently photo-crosslinked and the alginate was extracted using sodium citrate. In addition to the requirement for chemical crosslinking, a key limitation was that the microcarriers fabricated with this approach had poor stability below a size range of 900 - 950 µm30. In place of pepsin, the methods presented here utilize a more mild digestion of the ECM with the glycolytic enzyme α-amylase, which is postulated to cleave carbohydrate groups from the telopeptide regions of collagen, thereby increasing solubility in acetic acid37. This approach enables the isolation of highly polymerized collagen that can be used to generate pure ECM-derived foams and microcarriers without the need for chemical crosslinking or other additives. These bioscaffolds are stabilized through physical interactions and hydrogen bonding between well-preserved collagen fibrils, similar to the collagen in the native ECM microenvironment.
The foams are a highly flexible platform that can be fabricated in a wide range of geometries depending on the specific mold selected. For cell culture studies, the foams may be cast directly in TCPS well plates, to form coatings or 3D scaffolds of varying thickness. To fabricate 3D foams with very uniform surfaces, it is recommended that a custom mold is designed that can be sealed on both sides with plastic or glass slides. Either minced or cryomilled ECM can be used to synthesize the foams. In general, we have found that the cryomilled foams tend to be macroscopically softer and have a more disrupted ultrastructure at lower concentrations31,36. Depending on the tissue source, the additional mechanical processing steps may cause alterations in the ECM composition that could impact cell function. For example, in our previous work, laminin was detected in minced DLV foams, but not cryomilled DLV foams31. In contrast, collagen I, collagen IV, laminin, and fibronectin were detected in both minced and cryomilled DAT foams36. In addition to the mechanical processing steps, the porosity and pore size of the foams can be tuned to some extent by varying the ECM suspension concentration and the freezing temperature47. In general, lower concentration foams (~ 10 - 15 mg/mL) are qualitatively more porous, but may contract rapidly and have poor stability in long-term culture31,36. Similarly, a slower freezing rate, typically achieved by a higher freezing temperature, can result in larger pores in the foams due to the size of the ice crystals formed during fabrication29. All of these parameters may influence cell interactions with the materials, including attachment, infiltration, and remodeling. For example, cell growth on foams that are fabricated with higher ECM concentrations may be limited to the surface regions, particularly with minced ECM sources and under static culture conditions36.
For the microcarriers, the key parameters that can be tuned are the ECM suspension concentration, needle gauge, and applied voltage, with higher concentrations typically yielding microcarriers that are more stable under long-term dynamic culture. Following the initiation of electrospraying, the ECM suspension droplets should quickly fall into the center of the flask, towards the direction of the aluminum foil collector. To prevent aggregation, it is important that the beads contact the liquid nitrogen prior to the foil. The distance between the needle and the surface of the liquid nitrogen can be adjusted to meet these requirements. It is important to note that optimization may be required depending on the properties of each specific ECM source, in particular in selecting the concentration range that will generate stable bioscaffolds. Another key factor is the decellularization protocol that is used to generate the starting materials, as decellularization methods that degrade the ECM or the presence of residual reagents (e.g., surfactants) may negatively impact the stability of the resultant foams and microcarriers. If challenges are encountered with bioscaffold stability, options that can be investigated include using a more gradual rehydration process, increasing the ECM suspension concentration, and exploring minced versus cryomilled ECM. Should all of these options fail to resolve the issue, it may be necessary to explore alternative decellularization protocols or ECM sources.
To ensure reproducibility during scaffold production, special care must be taken at certain steps in the protocol. When cryomilling the decellularized tissues, it is recommended that milling be conducted immediately after lyophilization in a dry environment to reduce the likelihood of particle aggregation due to the absorption of moisture from the environment. During microcarrier fabrication, it is suggested that the suspension is electrosprayed in small batches, with a maximum volume of 3 mL, to avoid issues with sample cooling that can result in clogging of the needle. Further, it is essential that the microcarriers are not permitted to thaw after the electrospraying process. To maintain their spherical geometry and mechanical stability, the microcarriers should be collected from the liquid nitrogen, transported in a liquid nitrogen-filled container, and immediately lyophilized. Finally, for both the foams and microcarriers, it is critical that the rehydration steps are performed slowly over a period of multiple days. Rapid rehydration can result in structural collapse on the macro- and/or micro-scale. Further, rehydration must occur slowly to prevent the formation of small air bubbles within the scaffold, which can require a significant amount of time to degas under light vacuum.
In conclusion, the methods presented in this paper can be used to fabricate a diverse array of tissue-specific foams and microcarriers comprised of pure, non-chemically crosslinked ECM. An advantage for biological researchers is that the bioscaffolds are easy to handle and can be processed similarly to tissues when performing analyses with techniques such as histology, immunohistochemistry, or gene and protein expression assays. In addition, the ECM-derived scaffolds can be enzymatically degraded to extract seeded cell populations or can be used directly as biodegradable and biocompatible cell delivery vehicles. Overall, this flexible platform technology holds great utility for numerous applications including for 3D cell culture studies investigating cell function, as cell expansion substrates, and as pro-regenerative bioscaffolds.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The Natural Sciences and Engineering Research Council (NSERC) of Canada and the Canadian Institutes of Health Research (CIHR) have provided funding for this work. The authors would like to acknowledge Dr. Amin Rizkalla for the use of his electrospraying system, the Nanofabrication Facility at Western University for use of SEM imaging equipment, the Mount Brydges Abattoir for the provision of porcine tissue samples, and Drs. Aaron Grant, Brian Evans, and Robert Richards for their clinical collaborations in support of this research.
References
- Eweida AM, Marei MK. Naturally Occurring Extracellular Matrix Scaffolds for Dermal Regeneration: Do They Really Need Cells? Biomed Res Int. 2015. pp. 1–9. [DOI] [PMC free article] [PubMed]
- Badylak SF, Taylor D, Uygun K. Whole Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso F, Giordano A, Barbarisi M, Barbarisi A. From Cell-ECM Interactions to Tissue Engineering. J. Cell. Phys. 2004;199(2):174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- Du J, et al. Extracellular Matrix Stiffness Dictates Wnt Expression Through Integrin Pathway. Sci. Rep. 2016;6:4195–4200. doi: 10.1038/srep20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak SF. The Extracellular Matrix as a Scaffold for Tissue Reconstruction. Cell & Dev. Biol. 2002;13(2):377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Sellaro TL, Badylak SF. Decellularization of Tissues and Organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Crapo PM, Gilbert TW, Badylak SF. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reing JE, et al. The Effects of Processing Methods Upon Mechanical and Biologic Properties of Porcine Dermal Extracellular Matrix Scaffolds. Biomaterials. 2010;31(33):8626–8633. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, et al. Morphological Appearance, Content of Extracellular Matrix and Vascular Density of Lung Metastases Predicts Permissiveness to Infiltration by Adoptively Transferred Natural Killer and T Cells. Cancer Immun. Immunother. 2006;55(6):699–707. doi: 10.1007/s00262-005-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle E, Ghaedi M, Sundaram S, Sivarapatna A, Tseng MK, Niklason LE. Strategies for Whole Lung Tissue Engineering. IEEE Trans. Biomed. Eng. 2014;61(5):1482–1496. doi: 10.1109/TBME.2014.2314261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AEB, Yu C, Bianco J, Watkins JF, Flynn LE. The Performance of Decellularized Adipose Tissue Microcarriers as an Inductive Substrate for Human Adipose-Derived Stem Cells. Biomaterials. 2012;33(18):4490–4499. doi: 10.1016/j.biomaterials.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Brown CFC, Yan J, Han TTY, Marecak DM, Amsden BG, Flynn LE. Effect of Decellularized Adipose Tissue Particle Size and Cell Density on Adipose-Derived Stem Cell Proliferation and Adipogenic Differentiation in Composite Methacrylated Chondroitin Sulphate. Biomed. Mater. 2015;10(4):1–12. doi: 10.1088/1748-6041/10/4/045010. [DOI] [PubMed] [Google Scholar]
- Cheung HK, Han TTY, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite Hydrogel Scaffolds Incorporating Decellularized Adipose Tissue for Soft Tissue Engineering with Adipose-Derived Stem Cells. Biomaterials. 2014;35(6):1914–1923. doi: 10.1016/j.biomaterials.2013.11.067. [DOI] [PubMed] [Google Scholar]
- Almeida HV, Eswaramoorthy R, Cunniffe GM, Buckley CT, O'Brien FJ, Kelly DJ. Fibrin Hydrogels Functionalized with Cartilage Extracellular Matrix and Incorporating Freshly Isolated Stromal Cells as an Injectable for Cartilage Regeneration. Acta Biomat. 2016;36:55–62. doi: 10.1016/j.actbio.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Wassenaar JW, Braden RL, Osborn KG, Christman KL. Modulating in vivo Degradation Rate of Injectable Extracellular Matrix Hydrogels. J. Mater. Chem. B. 2016;4(16):2794–2802. doi: 10.1039/C5TB02564H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugerleider JL, et al. Extracellular Matrix Hydrogel Promotes Tissue Remodeling, Arteriogenesis, and Perfusion in a Rat Hindlimb Ischemia Model. JACC Basic Transl. Sci. 2015;1(1-2):32–44. doi: 10.1016/j.jacbts.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao RJ, et al. Decellularized Human Kidney Cortex Hydrogels Enhance Kidney Microvascular Endothelial Cell Maturation and Quiescence. Tissue Eng. Part A. 2016;22(19-20):1140–1150. doi: 10.1089/ten.tea.2016.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati F, et al. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular. Matrix Bioink. Nat. Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD. 3D Cell Culture Systems - Advantages and Applications. J. Cell. Phys. 2015;230(1):16–26. doi: 10.1002/jcp.24683. [DOI] [PubMed] [Google Scholar]
- Lee J, Cuddihy MJ, Kotov N. Three-dimensional Cell Culture Matrices: State of the Art. Tissue Eng Part B, Rev. 2008;14(1):61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG, Stelzer EHK. The Third Dimension Bridges the Gap Between Cell Culture and Live Tissue. Nature Rev. Mol. Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Bouet G, Marchat D, Cruel M, Malaval L, Vico L. In Vitro Three-Dimensional Bone Tissue Models: From Cells to Controlled and Dynamic Environment. Tissue Eng. Part B Rev. 2015;21(1):133–156. doi: 10.1089/ten.TEB.2013.0682. [DOI] [PubMed] [Google Scholar]
- Birgersdotter A, Sandberg R, Ernberg I. Gene Expression Perturbation in vitro - A Growing Case for Three-Dimensional (3D) Culture Systems. Sem. Cancer Biol. 2005;15(5):405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bonnier F, et al. Cell Viability Assessment Using the Alamar Blue Assay: A Comparison of 2D and 3D Cell Culture Models. Toxicology in vitro. 2015;29(1):124–131. doi: 10.1016/j.tiv.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The Extracellular Matrix at a Glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberio MS, Sadowski MC, Soekmadji C, Davis RA, Nelson CC. Differential Effects of Tissue Culture Coating Substrates on Prostate Cancer Cell Adherence, Morphology and Behavior. PLoS ONE. 2014;9(11):e112122. doi: 10.1371/journal.pone.0112122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee C, Perez-Cruet M, Chavez F, Chaudhry GR. Simplified Three-Dimensional Culture System for Long-Term Expansion of Embryonic Stem Cells. World J. Stem Cells. 2015;7(7):1064–1077. doi: 10.4252/wjsc.v7.i7.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn LE. The Use of Decellularized Adipose Tissue to Provide an Inductive Microenvironment for the Adipogenic Differentiation of Human Adipose-Derived Stem Cells. Biomaterials. 2010;31(17):4715–4724. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Yu C, et al. Porous Decellularized Adipose Tissue Foams for Soft Tissue Regeneration. Biomaterials. 2013;34(13):3290–3302. doi: 10.1016/j.biomaterials.2013.01.056. [DOI] [PubMed] [Google Scholar]
- Turner AEB, Flynn LE. Design and Characterization of Tissue-Specific Extracellular Matrix-Derived Microcarriers. Tissue Eng. Part C: Methods. 2012;18(3):186–197. doi: 10.1089/ten.TEC.2011.0246. [DOI] [PubMed] [Google Scholar]
- Russo V, Omidi E, Samani A, Hamilton A, Flynn LE. Porous, Ventricular Extracellular Matrix-Derived Foams as a Platform for Cardiac Cell Culture. Biores Open Access. 2015;4(1):374–388. doi: 10.1089/biores.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright JM, et al. Preparation of Cardiac Extracellular Matrix from an Intact Porcine Heart. Tissue Eng. Part C, Methods. 2010;16(3):525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SC, Fisher SA, Tam RY, Nimmo CM, Shoichet MS. Hyaluronic Acid Click Hydrogels Emulate the Extracellular Matrix. Langmuir. 2013;29(24):7393–7400. doi: 10.1021/la305000w. [DOI] [PubMed] [Google Scholar]
- Zargham S, Bazgir S, Tavakoli A, Rashidi AS, Damerchely R. The Effect of Flow Rate on Morphology and Deposition Area of Electrospun Nylon 6 Nanofiber. J. Eng. Fibers Fabr. 2012;7(4):42–49. [Google Scholar]
- Gryshkov O, Pogozhykh D, Zernetsch H, Hofmann N, Mueller T, Glasmacher B. Process Engineering of High Voltage Alginate Encapsulation of Mesenchymal Stem Cells. Mater. Sci. Eng. C Biol. Appl. 2014;36:77–83. doi: 10.1016/j.msec.2013.11.048. [DOI] [PubMed] [Google Scholar]
- Turco B. Characterization and Cell-Seeding of Decellularized Adipose Tissue Foams for Wound Healing. Kingston, Ontario, Canada: Queen's University; 2014. [Google Scholar]
- Steven FS. The Nishihara Technique for the Solubilization of Collagen. Application To the Preparation of Soluble Collagens From Normal and Rheumatoid Connective Tissue. Ann. Rheum. Dis. 1964;23:300–301. doi: 10.1136/ard.23.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen NUB, Genovese F, Leeming DJ, Karsdal MA. The Importance of Extracellular Matrix for Cell Function and in vivo Likeness. Exp. Mol. Pathol. 2015;98(2):286–294. doi: 10.1016/j.yexmp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Justice BA, Badr NA, Felder RA. 3D Cell Culture Opens New Dimensions in Cell-Based Assays. Drug Discov. Today. 2009;14(1-2):102–107. doi: 10.1016/j.drudis.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Cortiella J, et al. Influence of Acellular Natural Lung Matrix on Murine Embryonic Stem Cell Differentiation and Tissue Formation. Tissue Eng. Part A. 2010;16(8):2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- Liao J, Guo X, Grande-Allen KJ, Kasper FK, Mikos AG. Bioactive Polymer/Extracellular Matrix Scaffolds Fabricated with a Flow Perfusion Bioreactor for Cartilage Tissue Engineering. Biomaterials. 2010;31(34):8911–8920. doi: 10.1016/j.biomaterials.2010.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YC, Choi JS, Woo CH, Cho YW. Stem Cell Delivery Systems Inspired by Tissue-Specific Niches. J. Control. Release. 2014;193:42–50. doi: 10.1016/j.jconrel.2014.06.032. [DOI] [PubMed] [Google Scholar]
- Han TTY, Toutounji S, Amsden BG, Flynn LE. Adipose-Derived Stromal Cells Mediate in vivo Adipogenesis , Angiogenesis and Inflammation in Decellularized Adipose Tissue Bioscaffolds. Biomaterials. 2015;72:125–137. doi: 10.1016/j.biomaterials.2015.08.053. [DOI] [PubMed] [Google Scholar]
- Yu C, Kornmuller A, Flynn LE. Porous Decellularized Extracellular Matrix Microcarriers for Tissue-Specific Cell Expansion and Delivery. Front. Bioeng. Biotechnol. 2016.
- Qian J, et al. Kinetic Analysis of the Digestion of Bovine Type I Collagen Telopeptides with Porcine Pepsin. J. Food Sci. 2016;81(1):C27–C34. doi: 10.1111/1750-3841.13179. [DOI] [PubMed] [Google Scholar]
- Lin H, Yang G, Tan J, Tuan RS. Influence of Decellularized Matrix Derived from Human Mesenchymal Stem Cells on their Proliferation, Migration and Multi-Lineage Differentiation Potential. Biomaterials. 2012;33(18):4480–4489. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Fonte P, Reis S, Sarmento B. Facts and Evidences on the Lyophilization of Polymeric Nanoparticles for Drug Delivery. J. Control. Release. 2016;225:75–86. doi: 10.1016/j.jconrel.2016.01.034. [DOI] [PubMed] [Google Scholar]


