Abstract
Childhood adversity plays an important role for development of major depressive disorder (MDD). There are differences in subcortical brain structures between patients with MDD and healthy controls, but the specific impact of childhood adversity on such structures in MDD remains unclear. Thus, aim of the present study was to investigate whether childhood adversity is associated with subcortical volumes and how it interacts with a diagnosis of MDD and sex. Within the ENIGMA-MDD network, nine university partner sites, which assessed childhood adversity and magnetic resonance imaging in patients with MDD and controls, took part in the current joint mega-analysis. In this largest effort world-wide to identify subcortical brain structure differences related to childhood adversity, 3036 participants were analyzed for subcortical brain volumes using FreeSurfer. A significant interaction was evident between childhood adversity, MDD diagnosis, sex, and region. Increased exposure to childhood adversity was associated with smaller caudate volumes in females independent of MDD. All subcategories of childhood adversity were negatively associated with caudate volumes in females - in particular emotional neglect and physical neglect (independently from age, ICV, imaging site and MDD diagnosis). There was no interaction effect between childhood adversity and MDD diagnosis on subcortical brain volumes. Childhood adversity is one of the contributors to brain structural abnormalities. It is associated with subcortical brain abnormalities that are relevant to psychiatric disorders such as depression.
Keywords: Depression, Childhood adversity, MRI, Caudate, Hippocampus, ENIGMA
1. Introduction
Exposure to neglect and abuse in childhood – here briefly called childhood adversity - plays a crucial role in the development of major depressive disorder (MDD) (Frodl and O’Keane, 2013; Nusslock and Miller, 2016; Trotta et al., 2015). The relationship between childhood adversity and depression is mediated by sex, genetic risk, parental psychopathology, stressful life events during adulthood, and social support (Pagliaccio and Barch, 2015). Childhood adversity as well as the above mentioned factors may contribute to treatment resistance in MDD (Pagliaccio and Barch, 2015; Tunnard et al., 2014). As such, understanding the role of childhood adversity is important to improve assessment and treatment of depression (Teicher and Samson, 2013).
As described in our recent review, childhood adversity also was suggested as a key factor associated with structural brain abnormalities in subjects who developed psychiatric disorders (Frodl and O’Keane, 2013). It has been demonstrated that childhood adversity and MDD are associated with structural brain changes (Dannlowski et al., 2012; Frodl et al., 2010; Gerritsen et al., 2015). Experimentally, exposure to severe chronic stressors may induce glucocorticoid-mediated pyramidal dendrite retraction in the hippocampus, and changes in dendrite arborization in the prefrontal cortex (PFC) in vulnerable individuals (Kole et al., 2004; Magarinos et al., 1996; Wellman, 2001; Woolley et al., 1990). Moreover, stress or cortisol administration may lead to neuronal atrophy in the hippocampus and to states that share features with depression (Duman, 2002).
Patients with MDD showed consistently reduced subcortical brain volumes compared to healthy controls. A recent meta-analysis, by the ENIGMA-MDD consortium, investigated subcortical volume differences between 1728 MDD patients and 7199 controls from 15 research samples worldwide. On average, the hippocampus was significantly smaller in patients compared with controls, especially in patients with early-onset or recurrent MDD (Schmaal et al., 2015). Interestingly, sample characteristics such as mean age, the proportion of antidepressant users or proportion of remitted patients and methodological characteristics did not significantly moderate these alterations of brain volumes in MDD (Schmaal et al., 2015). Previous meta-analyses also confirmed smaller hippocampal volumes (Arnone et al., 2016; Campbell et al., 2004; McKinnon et al., 2009; Videbech and Ravnkilde, 2004) and structural alterations in the hippocampus, basal ganglia, orbitofrontal cortex and the rectal gyrus (Kempton et al., 2011) in patients with MDD compared to healthy controls.
Our primary aim was to identify associations of childhood adversity and a life-time diagnosis of MDD on subcortical volumes in a large multi-center sample. Moreover, an additional goal was to assess subcortical volumes using a standardized segmentation protocol to avoid effects due to different processing and analysis techniques. Furthermore, a third aim was to consider current antidepressant treatment and to test whether this might affect subcortical volumes in the framework of the childhood adversity analyses. Using this approach, also prior methodological limitations including age and sex influences were addressed. We initiated the childhood adversity subproject within the Major Depressive Disorder (MDD) Working Group of the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) consortium (http://enigma.ini.usc.edu/ongoing/enigma-mdd-working-group/). Nine partners in this network had addressed childhood adversity in their studies using the childhood trauma questionnaire (CTQ). Their subcortical volume measures were included in our ENIGMA-MDD analysis of childhood adversity.
2. Materials and methods
2.1. Samples
At the time when this subproject was proposed, the ENIGMA-MDD Childhood Adversity Working Subgroup included nine international samples with neuroimaging, childhood adversity, and clinical data from MDD patients and healthy controls. All of the nine research groups agreed to participate in the subgroup analysis. For future projects, new research groups around the world are continuously encouraged to join the ongoing ENIGMA work, to increase sample size and thereby increase statistical power and evaluate the generalizability of our results on MDD. Detailed demographics for each sample are found in Table S1 and clinical characteristics in Table S2. Exclusion criteria for study enrollment in each sample are given in Table S3. In total, we analyzed data from 3036 people, including 958 MDD patients and 2078 healthy controls. All participating sites obtained approval from local institutional review boards and ethics committees. All study participants provided written, informed consent at their local institution.
2.2. Assessments
The Childhood Trauma Questionnaire Short Form (CTQ-SF) (Bernstein et al., 1994) was used to investigate childhood adversity in all sites. The CTQ-SF is a standardized self-report instrument with 28 items including five subscales of childhood maltreatment: emotional, physical, and sexual abuse, and emotional and physical neglect that sum up to a total score. CTQ subscale scores and CTQ total score were firstly used as continuous variables. A categorical variable was assessed coding for either no childhood adversity or mild to severe childhood adversity. Participants with at least one mild expression of sexual abuse (subscale score > 7), emotional abuse (subscale score > 12) or physical abuse (subscale score > 9), emotional neglect (subscale score > 14) or physical neglect (sub-scale score > 9) were categorized as having had a history of childhood adversity. Reliability and validity of the CTQ has been established, including measures of convergent and discriminative validity from structured interviews, stability over time, and corroboration (Bernstein et al., 2003).
2.3. Image processing and analysis
Structural T1-weighted MRI brain scans were acquired at each site and analyzed locally using the fully-automated and validated segmentation software FreeSurfer (version 5.0 and higher) (Fischl et al., 2002). Image acquisition parameters and software descriptions for each sample are given in Table S4. FreeSurfer provides segmentations and volume quantifications for seven subcortical grey matter regions (nucleus accumbens, amygdala, caudate nucleus, hippocampus, pallidum, putamen, thalamus), lateral ventricles, and total intracranial volume. The derived volumes were visually inspected for accuracy following standardized protocols designed to facilitate harmonized image analysis across multiple sites (http://enigma.ini.usc.edu/protocols/imaging-protocols/). Further details on image exclusion criteria and quality control can be found in SI1.
2.4. Statistical framework of mega-analysis
Statistical analyses were conducted using SPSS Statistics version 22 (IBM Analytics). In this mega-analysis, subcortical volumes, demographics, and childhood adversity scores were obtained in anonymized form from each partner site. Data were merged and analyzed at one site. After testing for normal distribution of the data using a Kolmogorov-Smirnov test, we ran a generalized estimating equations (GEE) model with subcortical volumes as dependent variables. A linear scale response model (GEE) was defined, since normal distributions for the dependent variables were found. Our model comprised the following independent between-subject variables: childhood adversity (continuous: CTQ total score), MDD lifetime diagnosis (factor: 0 = controls, 1 = patients) and sex (factor: 0 = males, 1 = females) as well as the following within-subject factors: brain region and hemisphere (left, right). Age (continuous), neuroimaging site (factor), and total intracranial volume (continuous) were used as between-subjects covariates. FreeSurfer version and scanner type are comprised in the factor neuroimaging site. The alpha threshold for the main GEE model was 0.05. Interaction effects for variables of interest were calculated for the 4-way interaction sex x diagnosis x childhood adversity x region and for all possible 2 and 3 way interactions between these 4 variables.
Moreover, an additional and similar GEE analysis was performed using 3 groups (controls, MDD patients without antidepressant medication, MDD patients with antidepressant medication) instead of just 2 groups (MDD patients, controls) to study differences in medicated and currently unmedicated MDD patients in the above described analysis and in particular interaction with childhood adversity. The alpha threshold for post-hoc tests was reduced to 0.007 after considering 7 different regions. The analyses were also redone using childhood adversity as categorical variable (yes, no).
After locating regions showing a significant predictive effect of CTQ, we analyzed whether the subscales demonstrated additional effects, independent from the confounders intracranial volume (ICV), age, imaging site and MDD diagnosis by running Spearman correlations on the residual values obtained from GEE model with ICV, age, MDD and imaging site as covariates. We used Steiger’s Z test (Steiger, 1980) in order to compare the magnitudes of correlation coefficients.
3. Results
Patients with MDD were significantly younger (mean difference: 3 years), more often female, and had significantly higher scores on the childhood trauma questionnaire (total score and all five subscales; p < 0.001, Table 1) than healthy controls. Thus, as described in the methods section, age, ICV and imaging site were used as covariates and sex as between-subjects variable in the analyses.
Table 1.
Demographic and clinical data. CTQ: childhood adversity; BDI: Beck Depression Index: given from studies with available BDI scores, some studies did not include depression severity for healthy controls.
| Patients (N = 958) | Controls (N = 2078) | df | Diagnosis effect | |
|---|---|---|---|---|
| Age | 42.4 (14.3) | 46.3 (15.2) | 3034 | t = 6.7, p < 0.001 |
| Sex (Female/Male) | (614/344) | (994/1084) | 3036 | Chi = 69.6, p < 0.001a |
| BDI | 18.2 (12.3) | 5.2 (4.3) | 2030 | p < 0.001a |
| CTQ total | 42.0 (15.7) | 32.4 (8.1) | 3034 | p < 0.001a |
| Physical abuse | 6.9 (3.5) | 5.6 (1.7) | 3034 | p < 0.001a |
| Emotional abuse | 9.4 (4.9) | 6.3 (2.3) | 3034 | p < 0.001a |
| Sexual abuse | 6.0 (3.2) | 5.2 (1.1) | 3034 | p < 0.001a |
| Physical neglect | 7.8 (3.0) | 6.6 (2.2) | 3034 | p < 0.001a |
| Emotional neglect | 12.0 (5.5) | 8.6 (3.8) | 3034 | p < 0.001a |
| Age of onset | 30.7 (14.1) | |||
| Antidepressants users | 37.9% |
Mann Whitney-U Test.
3.1. Effect of childhood adversity and MDD diagnosis on subcortical volumes
The GEE model detected significant main effects of region, hemisphere and of the covariates age, sex, imaging site, and ICV. No significant main effects were found for childhood adversity or MDD diagnosis separately in the full GEE model. There was a significant 4-way interaction between MDD diagnosis, childhood adversity, sex, and region (F = 13.5, p = 0.035). Since all significant 3-way interactions included the factor region (Table 2), we further explored this interaction effect by running the analysis separately for each region.
Table 2.
GEE results with subcortical volumes as dependent variables and factors age, sex, neuroimaging site, Intracranial volume (ICV), childhood adversity (CTQ), MDD diagnosis and interactions.
| Source | Wald Chi-square | df | p-value |
|---|---|---|---|
| Neuroimaging site | 513.9 | 7 | <0.001 |
| Hemisphere | 281.6 | 1 | <0.001 |
| ICV | 1458.5 | 1 | <0.001 |
| Age | 1317.2 | 1 | <0.001 |
| MDD diagnosis | 0.89 | 1 | 0.35 |
| Sex | 4.0 | 1 | 0.045 |
| Region | 27478.5 | 6 | <0.001 |
| CTQ | 0.3 | 1 | 0.61 |
| Diagnosis × Sex | 0.05 | 1 | 0.83 |
| CTQ × Sex | 4.4 | 1 | 0.036 |
| Diagnosis × Region | 6.0 | 6 | 0.43 |
| Diagnosis × CTQ | 5.4 | 1 | 0.02 |
| CTQ × Region | 37.1 | 6 | <0.001 |
| Sex × Region | 72.8 | 6 | <0.001 |
| Diagnosis × Sex × Region | 6.5 | 6 | 0.37 |
| Diagnosis × Sex × CTQ | 0.65 | 1 | 0.42 |
| Sex × CTQ × Region | 19.6 | 6 | 0.003 |
| Diagnosis × Region × CTQ | 14.1 | 6 | 0.028 |
| Diagnosis × Sex × Region × CTQ | 13.5 | 6 | 0.035 |
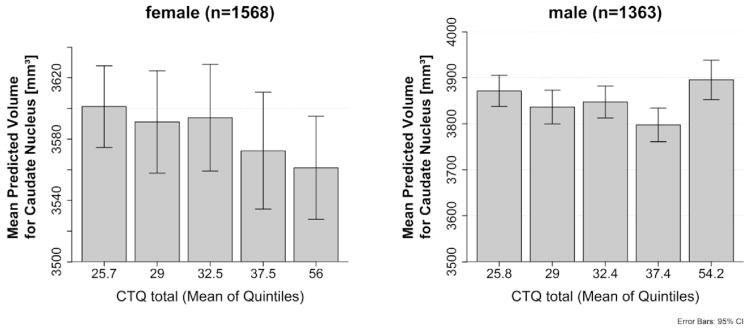
Post hoc analysis revealed a significant interaction between childhood adversity and sex on caudate volumes (F = 14.1, p < 0.001) that survived Bonferroni correction. There was a significant effect in females on right caudate (F = 10.7, p = 0.001, Bonferroni correction: pcorr = 0.007) and on left caudate volumes (F = 13.4, p < 0.001, pcorr <0.005) (Fig. 1). No significant effects were found in males on either right (F = 3.5, p = 0.06) or left (F = 2.8, p = 0.09) caudate volumes, although this can be considered a trend in agreement with the results in females. Moreover, effects were observed for the 2-way interaction between childhood adversity and sex on putamen volumes (F = 4.9, p = 0.027, pcorr = 0.19), and for the 3-way interaction between childhood adversity, sex and MDD diagnosis on thalamus volumes (F = 4.0, p = 0.044, pcorr = 0.31) in the analyses, but these did not resist Bonferroni correction. Using childhood adversity as categorical variable (yes/ no) did not lead to different results.
Fig. 1.
Association between childhood adversity and caudate volumes (mean of left and right) in females and males. Shown are quintiles (quintiles of the population, with mean of quintiles labeled) for the different severities of childhood adversity and predicted values after correction for covariates, age, ICV, imaging site as well as the standard error.
3.2. Childhood adversity subscales
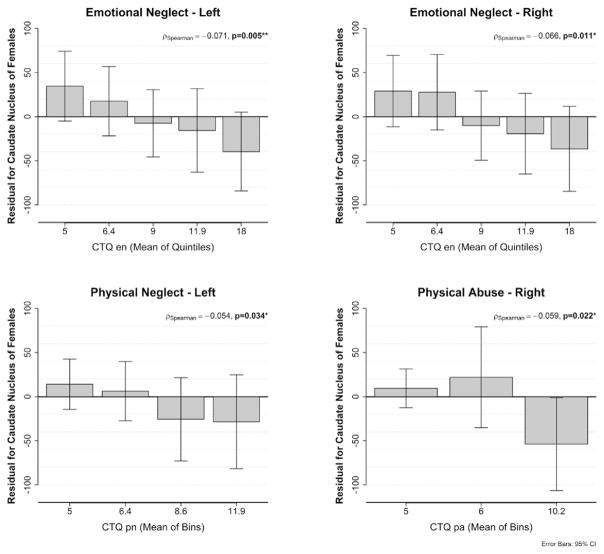
Significant negative correlations were seen for the association of childhood adversity subscales physical neglect, emotional neglect and physical abuse and caudate volumes independent of confounders like age, ICV, imaging site and MDD diagnosis (Fig. 2, Table 3). Using Steiger’s Z test no significant differences in magnitude of correlations were observed between the childhood adversity subscales.
Fig. 2.
Association between emotional neglect, physical neglect and physical abuse and subcortical volumes of nucleus caudate in females. Shown are quintiles/quantiles (with mean of quintiles/quantiles/tertiles labeled) for the different severities of childhood adversity and residual values after correction for covariates, age, ICV, imaging site as well as the standard error. Left for association with left caudate, Right for association with right caudate. The distribution of physical neglect was obtained in quantiles).
Table 3.
Spearman correlation coefficients for the correlations between subcategories of childhood adversity and those subcortical volumes showing significant association with childhood adversity (residual values).
| Left caudate | Right caudate | |
|---|---|---|
| Physical abuse | r = −0.033, p = 0.19 | r = −0.059, p = 0.022 |
| Emotional abuse | r = −0.0.02, p = 0.95 | r = −0.016, p = 0.53 |
| Sexual abuse | r = −0.018, p = 0.47 | r = −0.030, p = 0.25 |
| Emotional neglect | r = −0.071, p = 0.005 | r = −0.066, p = 0.011 |
| Physical neglect | r = −0.054, p = 0.034 | r = −0.047, p = 0.069 |
3.3. Effects of antidepressant medication
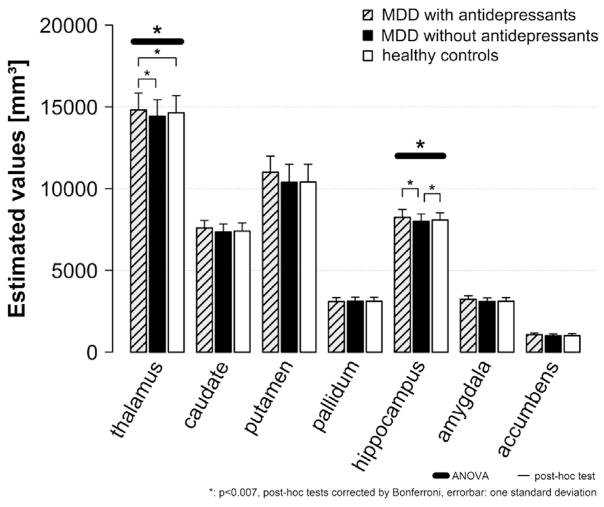
Using current antidepressant medication as classification criteria for the group difference (controls, MDD patients without antidepressant medication, MDD patients with antidepressant medication) in the analysis, childhood adversity was still associated with caudate volume. Significant main effects of antidepressant group were revealed for the following subcortical volumes: thalamus (F (2/2826) = 10.5, p < 0.001), hippocampus (F (2/2850) = 6.5, p = 0.001), amygdala (F (2/2844) = 4.1, p = 0.017) and putamen (F (2/2739) = 4.2, p = 0.016), although the latter two were not significant after Bonferroni correction (Fig. 3).
Fig. 3.
Bar diagram shows means and standard deviations (estimated values using age, sex, field strength and site as covariates) for MDD patients currently taking antidepressants compared to those MDD patients currently unmedicated and compared to controls. Patients currently taking antidepressants had significantly larger thalamus volumes compared to controls and patients not taking antidepressants. Patients not taking antidepressants had significantly smaller hippocampal volumes compared to controls and patients taking antidepressants.
Post hoc analyses revealed that patients without current anti-depressant medication had significantly smaller hippocampal volumes compared to controls (mean difference (d) = −78.8, p = 0.001) and patients on current antidepressant medication (d = −154.7, p < 0.001). With regard to thalamus volumes, patients on current antidepressant medication had significantly larger volumes compared to patients without antidepressant medication (d = 70.3, p < 0.001) and controls (d = 64.2, p < 0.001).
4. Discussion
This multicenter study included 958 patients with MDD and 2078 healthy controls. It offers the largest data for analysing the effects of severity of childhood adversity on subcortical brain structures in relation to MDD.
Severity of childhood adversity was significantly associated with lower left and right caudate nucleus volumes in females, whereby no significant effects were found in males. Our findings of distinct effects in females are interesting since a recent epidemiological study showed that those participants reporting childhood adversity were more likely to be female than male (Curran et al., 2016). The caudate nuclei as part of the basal ganglia integrate information from large cortical regions and feed back to cortical areas involved in motor planning, procedural learning, but also in cognitive, behavioural and emotional processes (Alexander et al., 1986; Bonelli and Cummings, 2007). Therefore, the caudate nuclei play an important role in feedback regulation of cognitive and emotional processes that are required for adaptation to adverse environments. So they might undergo structural re-organisation in case of exposure to childhood adversity.
An interaction between sex and childhood adversity was also found in a recent voxel based morphometry study. In this study, women with childhood adversity had less gray matter in the visual posterior precuneal region than controls (Everaerd et al., 2016). Our result of subcortical volume reductions in subjects who experienced childhood adversity corroborates also previous results in smaller samples and extends these findings to be sex specific. A prior study of 265 healthy Australian subjects found that participants with more than two adverse events during childhood had smaller anterior cingulate cortex and caudate nuclei than those without adverse events during childhood. Moreover, a significant association was detected between volumes of these structures and the total number of adverse events during childhood (Cohen et al., 2006). Van Hermelen et al. reported that a group of subjects with a history of emotional abuse and/or emotional neglect showed significantly smaller medial prefrontal cortex volumes compared to subjects without abuse and/or neglect (van Harmelen et al., 2010). Edmiston et al. found that total CTQ scores were negatively correlated (p < 0.005) with grey matter volume of prefrontal cortex, striatum, amygdala, sensory association cortices, and cerebellum (Edmiston et al., 2011). Recently, the NESDA study showed a significant interaction between MDD diagnosis and childhood adversity on hippocampal volume. In this study patients with MDD and a history of childhood adversity had reduced hippocampal volumes compared to controls with childhood adversity (Gerritsen et al., 2015).
Previous studies examining the striatum reported reduced volumes in depressed youth compared to controls (MacMillan et al., 2003). However, in our database, there was no significant association between MDD diagnosis and reduced subcortical volumes when childhood adversity was considered in addition to the covariates age, sex, imaging site, and ICV. Therefore, these results suggest that effects previously reported as associated with a diagnosis of depression may partly be associated with childhood adversity. Childhood adversity seems an important factor in interpreting brain structural differences and thus should be considered in future psychiatric imaging studies.
When analysing the subscales of childhood adversity, we found that in females the effects of childhood adversity on caudate volumes were present for all subscales. Correlations between caudate volume and childhood adversity were present independently from confounders such as age, ICV, MDD diagnosis and imaging site. These negative associations between caudate volume and the subscales of childhood neglect were most pronounced for emotional neglect and physical neglect and thus the findings are in line with a recent voxel based morphometry MRI study, showing an association between deprivation and gray matter volumes (Everaerd et al., 2016).
Similar to our study, prior work did not find significant associations between childhood adversity and hippocampal volumes (Cohen et al., 2006; Edmiston et al., 2011). This is surprising as the hippocampus was found to be associated with childhood adversity in previous studies (Bremner et al., 1997; Frodl and O’Keane, 2013). Interestingly, the hippocampus was also found to be significantly altered in depressed individuals in our recent ENIGMA MDD meta-analysis (Schmaal et al., 2015) and in studies of both depressed children and adults (McKinnon et al., 2009). Differences between the previous ENIGMA MDD meta-analysis and the present mega-analysis include sample size and confounders: the overall sample for the subcortical meta-analysis was larger than the sample for the current analysis and there was also a difference in the composition of the cohorts evaluated. In this previous meta-analysis the samples from Munster and South Africa have not yet been used, whereas other samples used in the meta-analysis did not assess the childhood trauma questionnaire and thus were not included in the present study.
An influential factor turned out to be current antidepressant medication use: patients currently undergoing treatment had larger hippocampus and thalamus volumes compared to currently untreated patients, and compared to controls for thalamus volumes. Nonetheless, it is important to take into account that only information about current antidepressant medication use at time of scanning and not the full history of antidepressant treatment (type, dose, duration) was available. This is an important fact, since therapy with antidepressants may reverse neural changes induced by chronic stress (Santarelli et al., 2003). Moreover, in a longitudinal voxel-based morphometry study hippocampal volumes of MDD patients with continuous antidepressant medication use increased significantly during the 3 year follow-up period (Frodl et al., 2008a). On the other hand a negative clinical outcome (more relapses and a chronic course during a 3 year follow-up) was associated with volume decline in the hippocampus, amygdala, anterior cingulate cortex, and dorsomedial prefrontal cortex (Frodl et al., 2008b). These results are in line with effects found in other disorders, such as PTSD, where increased hippocampal volumes have been observed after treatment with SSRI’s (Vermetten et al., 2003). In an 11-year-follow-up study, differences in brain structures between patients with MDD and controls present at baseline were no longer detectable when the patients were in remission (Ahdidan et al., 2011). Therefore, these longitudinal studies provide some initial evidence that treatment and remission from depression might be associated with volumetric increases in subcortical brain structures, which is corroborated by the results of the present study.
Despite several strengths of this mega-analysis, including sample size, harmonization of image processing and quality control, our study also has some limitations. Since different sites used different imaging techniques (different field strength, sequence parameters and spatial resolution) and applied different inclusion and exclusion criteria, imaging site was used as a covariate in the analyses. But this practice cannot completely rule out remaining effects from these methodological differences that might have influenced our results. To overcome these issues further studies should recruit all subjects in one site or apply matched imaging procedures between multiple centres to maintain the advantage of large sample sizes. Further investigations of cortical measures in subjects with more pronounced childhood adversity, for example childhood trauma, might be an important next step to clarify effects of childhood adversity on brain volume and structures. To increase our understanding on how enduring effects of childhood adversity differ from acute depression-related effects, the underlying morphological, histological, and molecular mechanisms of volume change after childhood adversity deserve more attention. Furthermore, the CTQ does not assess crucial time windows to adverse experiences during development (Cowell et al., 2015) and might be influenced by recall bias, which might have affected our results. Since age and antidepressant medication use strongly affected subcortical volumes, future studies will need to carefully control for these confounders.
In conclusion, the present study emphasizes that childhood adversity is associated with alterations in caudate volumes and thus may be an important factor involved in caudate development. This mechanism seems to be more prominent in females. Interestingly, neither an interaction effect of childhood adversity and MDD diagnosis nor a more pronounced effect of childhood adversity on brain volumes in patients with MDD was detected.
Supplementary Material
Acknowledgments
Role of funding
Funding was obtained as acknowledged to carry out research in the universities. There is no conflict of interest.
The ENIGMA-Major Depressive Disorder working group gratefully acknowledges support from the NIH BD2K award, U54EB020403.
NESDA: The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by participating universities (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen) and mental health care organizations, see www.nesda.nl. Lianne Schmaal is supported by The Netherlands Brain Foundation Grant number F2014(1)-24. Marie-José van Tol is supported by VENI grant (NWO grant number 016.156.077).
QTIM: Australian National Health and Medical Research Council (Project Grants No. 496682 and 1009064 to MJ Wright and Fellowship No. 464914 to IB Hickie), US National Institute of Child Health and Human Development (RO1HD050735 to MJ Wright), and US National Institute on Drug Abuse (R00DA023549 to NA Gillespie). Baptiste Couvy-Duchesne is supported by a University of Queensland International PhD scholarship. We are grateful to the twins for their generosity of time and willingness to participate in our studies. We thank research assistants Marlene Grace, Ann Eldridge, Richard Parker, Lenore Sullivan, Lorelle Nunn, Kerrie Mcaloney, Kori Johnson, Aaron Quiggle, and Natalie Garden, radiographers Matthew Meredith, Peter Hobden, Kate Borg, Aiman Al Najjar, and Anita Burns for acquisition of the scans, and David Smyth, Anthony Conciotrorre, Daniel Park, and David Butler for IT support.
CODE: The CODE cohort was collected from studies funded by Lundbeck and the German Research Foundation (WA 1539/4-1, SCHN 1204/3-1). Elizabeth Schramm is supported by the Grant of the Deutsche Forschungsgemeinschaft/German Research Association (SCHR443/11-1).
MPIP: The MPIP Munich Morphometry Sample comprises images acquired as part of the Munich Antidepressant Response Signature Study and the Recurrent Unipolar Depression (RUD) Case-Control study performed at the MPIP, and control subjects acquired at the Ludwig-Maximilians-University, Munich, Department of Psychiatry. We wish to acknowledge Anna Olynyik and radiographers Rosa Schirmer, Elke Schreiter, Reinhold Borschke and Ines Eidner for image acquisition and data preparation. We thank Dorothee P. Auer for local study management in the initial phase of the RUD study. The study is supported by a grant of the Exzellenz-Stiftung of the Max Planck Society. This work has also been funded by the Federal Ministry of Education and Research (BMBF) in the framework of the National Genome Research Network (NGFN), FKZ 01GS0481.
SHIP: The Study of Health in Pomerania (SHIP) is supported by the German Federal Ministry of Education and Research (grants 01ZZ9603, 01ZZ0103 and 01ZZ0403) the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. MRI scans were supported by Siemens Health-care, Erlangen, Germany. SHIP-LEGEND was supported by the German Research Foundation (GR1912/5-1).
Rotterdam study: Netherlands Organisation for Scientific Research (NWO – ZonMW, VIDI grantnumber 017.106.370 to Henning Tiemeier. Dr. Meike W. Vernooij is supported by an Erasmus MC fellowship and a ZonMW clinical fellowship (90700435). We thank Daniel Bos for his contribution to this work.
South Africa: Dan Stein is supported by the Medical Research Council of South Africa.
Dublin: Thomas Frodl and Leonardo Tozzi are supported by European Union Marie Curie International Training Grant (r’Birth). Moreover, Thomas Frodl is supported by Science Foundation Ireland (SFI) through a SFI-Stokes Professorship Grant and Health Research Board Ireland (HRB).
Münster: The study was supported by grants from the German Research Foundation (DFG; grant FOR 2107; DA1151/5-1 to UD), and Innovative Medizinische Forschung (IMF) of the Medical Faculty of Münster (DA120903 to UD, DA111107 to UD, and DA211012 to UD).
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jpsychires.2016.11.010.
Footnotes
Conflicts of interest
All authors have no conflicts of interest related to this study. Dr. Stein has received research grants and/or consultancy honoraria from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun.
Professor Ian Hickie is a Commissioner in Australia’s new National Mental Health Commission from 2012. He is the Co-Director, Health and Policy at the Brain and Mind Centre which operates two early-intervention youth services under contract to headspace. Professor Hickie has led a range of community-based and pharmaceutical industry-supported depression awareness and education and training programs. He has led projects for health professionals and the community supported by governmental, community agency and pharmaceutical industry partners (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) for the identification and management of depression and anxiety. He has received honoraria for presentations of his own work at educational seminars supported by a number of non-government organisations and the pharmaceutical industry (including Servier, Pfizer, AstraZeneca, and Eli Lilly). He is a member of the Medical Advisory Panel for Medibank Private and also a Board Member of Psychosis Australia Trust. He leads an investigator-initiated study of the effects of agomelatine on circadian parameters (supported in part by Servier) and has participated in a multicentre clinical trial of the effects of agomelatine on sleep architecture in depression and a Servier-supported study of major depression and sleep disturbance in primary care settings.
Carsten Konrad received fees for an educational program from Aristo Pharma, Janssen-Cilag, Lilly, MagVenture, Servier, and Trommsdorff as well as travel support and speakers honoraria from Aristo Pharma, Janssen, Lundbeck and Servier.
Professor Thomas Frodl received fees for presentations and scientific organisation of a conference from Janssen-Cilag, Lund-beck and Servier.
Dr. Van Erp has a contract with Otsuka Pharmaceutical, Inc.
Author contributions
Protocol design, quality testing, and mega-analysis: L.S., D.P.H., T.G.M.V., N.J., T.F.
Data collection, processing, analysis and funding: T.F., D.J. L.S., L.T., D.J.S., D.J.V., K.W., T.G.M.V., N.J. A.B., E.L., K.H., H.V., J.L., S.N.H., I.B.H., E.M.F., A.C., S.J.B., A.U., D.V. I.M.V., H.W., K.S., B.W.J.J.B., N.J.A.W. P.M.T., D.P.H., U.D., H.J.G.
Manuscript preparation: T.F., D.J., D.J.S.
All authors contributed revised the manuscript critically, edited it and approved the final version to be published.
References
- Ahdidan J, Hviid LB, Chakravarty MM, Ravnkilde B, Rosenberg R, Rodell A, Stodkilde-Jorgensen H, Videbech P. Longitudinal MR study of brain structure and hippocampus volume in major depressive disorder. Acta Psychiatr Scand. 2011;123(3):211–219. doi: 10.1111/j.1600-0447.2010.01644.x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arnone D, Job D, Selvaraj S, Abe O, Amico F, Cheng Y, Colloby SJ, O’Brien JT, Frodl T, Gotlib IH, Ham BJ, Kim MJ, Koolschijn PC, Perico CA, Salvadore G, Thomas AJ, Van Tol MJ, van der Wee NJ, Veltman DJ, Wagner G, McIntosh AM. Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp. 2016;37(4):1393–1404. doi: 10.1002/hbm.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9(2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse–a preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, Toth SL. Childhood maltreatment and its effect on neurocognitive functioning: timing and chronicity matter. Dev Psychopathol. 2015;27(2):521–533. doi: 10.1017/S0954579415000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran E, Adamson G, Stringer M, Rosato M, Leavey G. Severity of mental illness as a result of multiple childhood adversities: US National Epidemiologic Survey. Soc Psychiatry Psychiatric Epidemiol. 2016;51(5):647–657. doi: 10.1007/s00127-016-1198-3. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17(Suppl 3):306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D, Klumpers F, Zwiers M, Guadalupe T, Franke B, van Oostrom I, Schene A, Fernandez G, Tendolkar I. Childhood abuse and deprivation are associated with distinct sex-dependent differences in brain morphology. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41(7):1716–1723. doi: 10.1038/npp.2015.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, Reiser M, Moller HJ, Meisenzahl EM. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008a;33(5):423–430. [PMC free article] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, Moller HJ, Meisenzahl EM. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35(6):1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, Reiser M, Möller HJ, Meisenzahl EM. Depression-related variation in brain morphology over 3 years: effects of stress? Archives General Psychiatry. 2008b;65(10):1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, van Velzen L, Schmaal L, van der Graaf Y, van der Wee N, van Tol MJ, Penninx B, Geerlings M. Childhood maltreatment modifies the relationship of depression with hippocampal volume. Psychol Med. 2015;45(16):3517–3526. doi: 10.1017/S0033291715001415. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kole MH, Czeh B, Fuchs E. Homeostatic maintenance in excitability of tree shrew hippocampal CA3 pyramidal neurons after chronic stress. Hippocampus. 2004;14(6):742–751. doi: 10.1002/hipo.10212. [DOI] [PubMed] [Google Scholar]
- MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J, Banerjee SP, Rosenberg DR. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13(1):65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Barch DM. Early Life Adversity and Risk for Depression: Alterations in Cortisol and Brain Structure and Function as Mediating Mechanisms. Elsevier; Amsterdam: 2015. [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen WJ, Vernooij MW, Ikram MA, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Volzke H, Hoehn D, Czisch M, Lagopoulos J, Hatton SN, Hickie IB, Goya-Maldonado R, Kramer B, Gruber O, Couvy-Duchesne B, Renteria ME, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Wright MJ, Hall GB, MacQueen GM, Frey EM, Carballedo A, van Velzen LS, van Tol MJ, van der Wee NJ, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Sussmann JE, Godlewska BR, Cowen PJ, Fischer FH, Rose M, Penninx BW, Thompson PM, Hibar DP. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Testing pattern hypotheses on correlation matrices: alternative Statistics and some empirical results. Multivar Behav Res. 1980;15(3):335–352. doi: 10.1207/s15327906mbr1503_7. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A, Murray RM, Fisher HL. The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychol Med. 2015;45(12):2481–2498. doi: 10.1017/S0033291715000574. [DOI] [PubMed] [Google Scholar]
- Tunnard C, Rane LJ, Wooderson SC, Markopoulou K, Poon L, Fekadu A, Juruena M, Cleare AJ. The impact of childhood adversity on suicidality and clinical course in treatment-resistant depression. J Affect Disord. 2014;152–154:122–130. doi: 10.1016/j.jad.2013.06.037. [DOI] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BW, Elzinga BM. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry. 2010;68(9):832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.