Abstract
The objective of this study was to evaluate the steady-state pharmacokinetics of metoprolol during pregnancy and lactation. Serial plasma, urine, and breast milk concentrations of metoprolol and its metabolite, α-hydroxymetoprolol, were measured over 1 dosing interval in women treated with metoprolol (25–750 mg/day) during early pregnancy (n = 4), mid-pregnancy (n = 14), and late pregnancy (n = 15), as well as postpartum (n = 9) with (n = 4) and without (n = 5) lactation. Subjects were genotyped for CYP2D6 loss-of-function allelic variants. Using paired analysis, mean metoprolol apparent oral clearance was significantly higher in mid-pregnancy (361 ± 223 L/h, n = 5, P < .05) and late pregnancy (568 ± 273 L/h, n = 8, P < .05) compared with ≥3 months postpartum (200 ± 131 and 192 ± 98 L/h, respectively). When the comparison was limited to extensive metabolizers (EMs), metoprolol apparent oral clearance was significantly higher during both mid- and late pregnancy (P < .05). Relative infant exposure to metoprolol through breast milk was <1.0% of maternal weight-adjusted dose (n = 3). Because of the large, pregnancy-induced changes in metoprolol pharmacokinetics, if inadequate clinical responses are encountered, clinicians who prescribe metoprolol during pregnancy should be prepared to make aggressive changes in dosage (dose and frequency) or consider using an alternate beta-blocker.
Keywords: hypertension, metoprolol, pharmacokinetics, pregnancy, CYP2D6, breast milk
Metoprolol is a cardioselective β-adrenergic receptor antagonist used to treat pregnant women with cardiovascular diseases including hypertension, cardiomyopathy, ischemic heart disease, and arrhythmias. In the currently utilized dosage range, metoprolol does not appear to pose a major teratogenic risk; however, there is limited information available on metoprolol pharmacokinetics (PK) during pregnancy to inform dosage.1–3 In healthy volunteers, metoprolol bioavailability is ~50%.4 In nonpregnant subjects, cytochrome P450 2D6 (CYP2D6) mediates 70%–80% of metoprolol metabolism by catalyzing the oxidation of metoprolol to α-hydroxymetoprolol and O-desmethylmetoprolol.5 CYP2D6 polymorphism results in phenotypic variation in metoprolol PK and pharmacdynamics.6
Small single-dose studies (Högstedt et al,2 n = 5; Högstedt et al,3 n = 5; Högstedt et al,7 n = 8) have reported substantially lower metoprolol plasma concentrations during late pregnancy than postpartum.2,3,7 Intravenous clearance of metoprolol was not significantly changed, but apparent oral clearance increased 4-fold (P<.05).2,3 Furthermore, changes in dextromethorphan metabolism have been shown to occur across gestation, with approximately 25%, 35%, and 50% increase in CYP2D6 activity during early, mid-, and late pregnancy, respectively. We hypothesized that pregnancy alters the PK of metoprolol in a gestational age and genotypic manner. Our objective was to study steady-state PK of metoprolol and its metabolite, α-hydroxymetoprolol, across gestation and postpartum and to assess the relationship between genotype and metoprolol apparent oral clearance during pregnancy.
Methods
Subjects
The study was approved by the Institutional Review Boards at the University of Washington, University of Texas Medical Branch in Galveston, and the University of Pittsburgh and conducted in accordance with their guidelines. All subjects gave written informed consent. We examined steady-state PK of oral metoprolol in 22 pregnant women 21–40 years of age receiving metoprolol for therapeutic purposes. Women were excluded if their hematocrit was <28%. Blood and urine samples were collected during early pregnancy (10 to 14 weeks gestation), mid-pregnancy (22 to 26 weeks gestation), and late pregnancy (34 to 38 weeks gestation), as well as ≥3 months postpartum, depending on subject availability. Our study was designed to provide distinct representations of early, mid-, and late pregnancy compared with postpartum. Our 4-week study windows during pregnancy limited gestational changes during each phase. The 8-week period between windows allowed for separation of effects. Subjects were eligible to participate in up to 3 pregnancy studies (early, mid-, and late pregnancy) and 2 postpartum studies (lactating and nonlactating).
Maternal Dosing Regimen
Metoprolol was dosed as clinically indicated without regard to the study. Total doses ranged from 25 to 750 mg/day (in divided doses). Subjects either received immediate-release (IR) metoprolol tablets (Caraco Pharmaceutical Laboratories, Detroit, Michigan) or extended-release (ER) metoprolol tablets (Toprol-XL; AstraZeneca Pharmaceuticals, Wilmington, Delaware). The study provided metoprolol for the 3 days prior to each PK visit. Each subject used the same formulation on all the study days. Subjects completed dosing calendars for documentation of administration times, and pill counts were used for verification. PK studies followed a 5-hour fast (except for clear liquids). Subjects avoided caffeine for 24 hours prior to each study day and throughout the sampling period.
Sample Collection
On each study day, serial blood samples were collected at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours following dosing and truncated to the dosing interval. Urine was collected over 1 dosing interval for evaluation of metoprolol and α-hydroxymetoprolol excretion as well as creatinine clearance. Maternal and umbilical cord (arterial and venous) blood samples were collected immediately after delivery when possible. Breast milk was collected every 2–3 hours over 1 dosing interval using the Medela Classic double electric breast pump from those participating in the lactation study. Both breasts were completely emptied of milk during each collection, and breastfeeding was not allowed during the study day. Buccal swabs were collected from each subject for genotyping. Samples were stored at −80°C until analysis.
Metoprolol and α-Hydroxymetoprolol Concentrations
Plasma, breast milk, and urine concentrations of metoprolol and α-hydroxymetoprolol were assayed according to a modified version of a previously published method using high-performance liquid chromatography coupled with tandem mass spectrometry.8 The limit of detection for both metoprolol and α-hydroxymetoprolol in plasma and breast milk was 0.5 ng/mL, and the limit of quantification for both compounds was 1.0 ng/mL. The intra- and interday coefficients of variation for metoprolol in both plasma and breast milk were 1.3%–6.4% and 2.3%–−5.8% and of α-hydroxymetoprolol were 2.1%–7.2% and 4.4%–6.1%, respectively. The limit of detection for metoprolol and α-hydroxymetoprolol in urine was 1.6 and 6.8 ng/mL, respectively; the corresponding limit of quantification was 6.9 and 24.8 ng/mL. The intra- and interday coefficients of variation for metoprolol in urine were 0.9%–1.6% and 10.0%–10.3% and of α-hydroxymetoprolol were 1.4%–1.9% and 7.0%–8.2%, respectively. More detailed metoprolol and α-hydroxymetoprolol assay descriptions in plasma, urine, and breast milk can be found in the Supplemental Methods.
Pharmacokinetic Analysis
Metoprolol steady-state PK parameters were estimated using standard noncompartmental techniques as previously described.9 The total area under the plasma concentration–time curve over a steady-state dosage interval (AUCτSS) was estimated using the linear trapezoidal rule. Metoprolol apparent oral clearance (CL/F) was estimated as CL/F = dose/AUCτSS. Metoprolol/α-hydroxymetoprolol metabolic ratio (MR) in plasma was estimated by plasma AUCτSS(metoprolol)/AUCτSS(α-hydroxymetoprolol), corrected for the difference in molecular weight between parent drug and metabolite. Metoprolol/α-hydroxymetoprolol MR in urine was determined by AeSS(metoprolol)/AeSS(α-hydroxymetoprolol), corrected for molecular weight difference, where AeSS(metoprolol) was the amount of unchanged metoprolol and AeSS(α-hydroxymetoprolol) was the amount of α-hydroxymetoprolol excreted in the urine over a steady-state dosing interval. Metoprolol renal clearance was calculated as CLrenal = AeSS(metoprolol)/AUCτSS(metoprolol). The percentage of metoprolol dose excreted in urine was calculated as (AeSS[metoprolol]/metoprolol dose) × 100. Actual body weights were used for weight-adjusted parameters.
The amount of metoprolol excreted in breast milk for each collection interval was summed over the full dosing interval (breast milk volume for each interval × concentration). Metoprolol breast milk/plasma ratio was determined by (breast milk AUCτSS)/(maternal plasma AUCτSS). The percent of maternal metoprolol dose excreted unchanged in breast milk was determined as: (amount of metoprolol excreted in the breast milk over a steady-state dosing interval/maternal metoprolol dose) × 100. Infant daily exposure to metoprolol via breast milk was calculated as: (amount of metoprolol excreted in breast milk over a steady-state dosing interval × number of metoprolol doses per day)/body weight of an age-matched 50th percentile infant girl.10 Relative infant dose was calculated as: infant daily exposure/(maternal daily dose/maternal actual body weight) × 100.
Genotyping
Buccal cell DNA was isolated using a Puregene buccal cell kit (Gentra Systems, Minneapolis, Minnesota)11 except in 1 subject, for whom we used a Qiagen QIAamp DNABlood Mini Kit (Germantown, Maryland) following the manufacturer’s established protocol. Subjects were genotyped for the CYP2D6*3 (2549delA), CYP2D6*4 (1846G>A), CYP2D6*5 (CYP2D6 deleted), CYP2D6*6 (1707delT), CYP2D6*9 (2613 delAGA), CYP2D6*10 (100C>T), CYP2D6*17 (1023C>T), CYP2D6*35 (−1584 C>G and 31G>A), and CYP2D6*41 (2988 G>A) alleles. CYP2D6*3, *4, *5, and *6 account for 97% of the alleles causing the PM phenotype in white populations, whereas *10 and *17 are prevalent in Asians and Africans, respectively.12 Alleles were determined using validated TaqMan allelic discrimination assays from Applied Biosystems (Foster City, California). PCR amplification conditions and allelic discrimination were determined as previously described.11 For subjects 11–18, single-nucleotide polymorphisms (SNPs) CYP2D6*3, *9, *10, *17, *35, and *41 could not be determined by TaqMan assays and were genotyped by a 4 oligo-based PCR method developed in our lab. For subject 22, TaqMan SNP Genotyping Assays (Applied Biosystems, Inc.) were run in duplicate on 96.96 Dynamic Genotyping Arrays (Fluidigm) according to the manufacturer’s established protocol for BioMark 96.96 Genotyping. More detailed descriptions of the assays used for genotyping can be found in the Supplemental Methods. CYP2D6 copy number was assessed using a Taqman Copy Number assay from Applied Biosystems (Hs00010001_cn; Foster City, California), according to the manufacturer’s recommendations and its Copy Caller program. One copy and more than 2 copy controls were identified through sequencing analysis and inclusion of validated samples of genomic DNA (*5 gene deletion and gene duplication). Alleles for which no targeted sequence variations were detected were defaulted to *1 status.
Subjects were classified as extensive metabolizers (EMs) if they had 2 fully functional CYP2D6 alleles (*1 and *35) or 2 reduced-activity alleles (*9, *10, *17, or *41) or 1 fully functional allele together with 1 reduced-activity allele; intermediate metabolizers (IMs) if they had 1 nonfunctional CYP2D6 allele (*3, *4, *5, or *6) with either 1 functional allele or 1 reduced-activity allele; and poor metabolizers (PMs) if they carried 2 nonfunctional CYP2D6 alleles.
Statistical Analysis
Statistical comparisons were made using the Wilcoxon signed rank test for paired data and the Mann-Whitney test for comparisons between metabolizer status and formulations. Pearson’s correlation was used for variable correlations. The Skillings-Mack test was used to analyze the full data set across pregnancy and postpartum. Results are reported as mean ± SD, with P < .05 considered significant.
Results
Twenty-two subjects (15 white, 4 black, 1 Native American/white, 1 Hispanic/white, and 1 Alaskan Native) participated in the study. On study day 1, average age and height were 30 ± 5 years and 167 ± 8 cm, respectively (Table 1). Subjects were taking metoprolol for hypertension (n = 10), migraine (n = 1), heart disease/myocardial infarction (n = 1), cardiac hypertrophy (n = 2), tachycardia (n = 2), arrhythmia (n = 4), and dilated aorta (n = 2). For the 3 days prior to each study day, subjects took their metoprolol dose within 30 minutes of the designated dosing times. No carriers of CYP2D6 allele duplications were identified. Fourteen subjects were EMs (CYP2D6 genotypes *1/*1 in 7, *1/*9 in 1, *1/*10 in 1, *1/*35 in 2, *1/*41 in 2, and *17/*35 in 1), 6 were IMs (*1/*5 in 2, *4/*10 in 2, *6/*9 in 1, and a carrier of *4, *10, and *41), and 1 subject was a PM (*4/*4). One subject did not have sufficient DNA sample to be analyzed fully and was excluded from the genotype comparisons. The 1 PM and another subject who was treated with fluoxetine (20 mg daily), a potent CYP2D6 inhibitor,13 were excluded from our pharmacokinetic comparisons. Three subjects were treated with sertraline, a weak CYP2D6 inhibitor, during their studies.14 Subject 9 received sertraline during pregnancy and postpartum (50 and 75 mg daily, respectively), subject 5 only postpartum (75 mg daily), and subject 13 only during pregnancy (25 mg daily). In addition, subject 21 was treated with hydroxychloroquine (200 mg daily), a weak CYP2D6 inhibitor.15 Given the low doses of sertraline and hydroxychloroquine that our subjects received and that their PK parameters were within the range of the other subjects, these subjects’ PK parameters were included in our analysis. Urine collection was incomplete for subjects 19 and 21; therefore, related PK parameters were not estimated. Of the 22 subjects, 12 received metoprolol ER during their study day(s). Because the CL/F, CLrenal, metabolic ratios, and percent excreted unchanged in the urine did not differ between subjects who received metoprolol ER and IR formulations, we report them together. Postpartum PK parameters were averaged for subjects 14 and 18, who participated in 2 postpartum studies (lactating and nonlactating). Venous (n = 7) and arterial (n = 6) umbilical cord plasma samples were collected at the time of delivery.
Table 1.
Characteristics of Study Subjects During Early, Mid-, and Late Pregnancy as well as Postpartum
| Characteristics | Early Pregnancy (10–14 Week) (n = 4) |
Mid-Pregnancy (22–26 Weeks) (n = 15) |
Late Pregnancy (34–38 Weeks) (n = 17) |
Postpartum (≥ 3 Months) (n = 9) |
|---|---|---|---|---|
| Actual body weight (kg) | 73 ± 8 | 89 ± 25 | 98 ± 26 | 81 ± 27 |
| Median metoprolol dose (mg/day) | 87.5 (25–750) | 75 (25–750) | 100 (25–300) | 75 (25–250) |
Results are reported as mean ± SD or median (range).
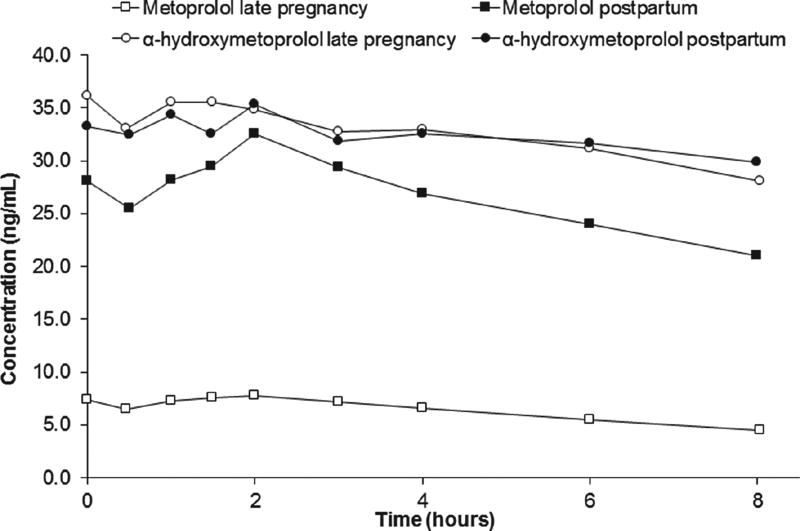
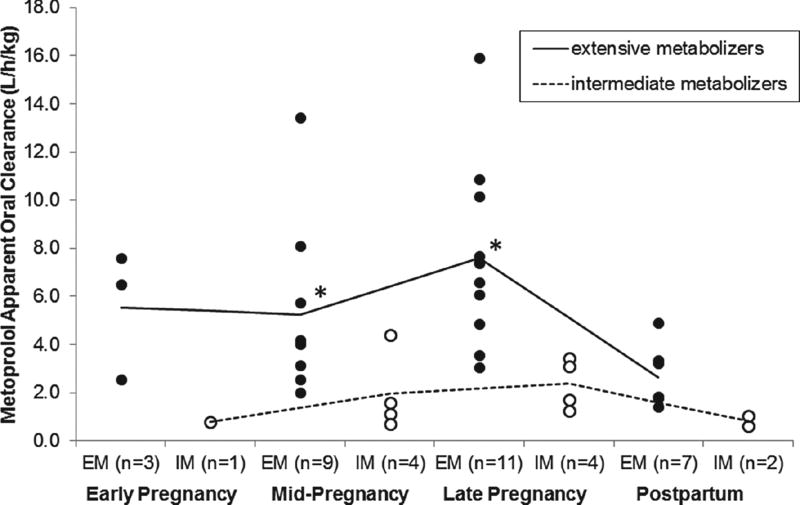
Figure 1 is a representative concentration–time profile for metoprolol and α-hydroxymetoprolol during late pregnancy and postpartum in an EM (subject 5; CYP2D6*1/*1). Using the same dosage regimen (metoprolol ER 25 mg orally every 8 hours), metoprolol concentrations were lower during late pregnancy compared with postpartum. Table 2 summarizes the paired estimated metoprolol PK parameters comparing mid-pregnancy and late pregnancy to postpartum. The mean metoprolol weight-adjusted CL/F was on average 1.7-fold (NS) and 2.6-fold (P < .05) higher during mid- and late pregnancy, respectively, compared with postpartum. In the EMs, metoprolol CL/F was significantly increased during mid-pregnancy (432 ± 181 L/h; P < .05) and late pregnancy (629 ± 228 L/h; P < .05) compared with postpartum (238 ± 113 L/h, n = 4; 209 ± 92 L/h, n = 7, respectively). Mean metoprolol CL/F in the IMs fell below EMs across all gestational ages and postpartum (Figure 2). Comparison between gestational periods was not possible for IMs because of the limited number of subjects. In 1 IM subject (CYP2D6*4/*10) who participated in both pregnancy and postpartum studies, metoprolol CL/F was 0.79 L/h/kg during early pregnancy, 1.11 L/h/kg mid-pregnancy, and 0.63 L/h/kg postpartum. In the only PM subject, metoprolol CL/F was 0.37 L/h/kg during mid-pregnancy and 0.41 L/h/kg during late pregnancy. Both values were lower than those found in EM and IM subjects. In our PM subject in both mid- and late pregnancy study days, α-hydroxymetoprolol concentrations were undetectable. Postpartum PK parameters were not available in our PM subject for comparison. Including all subjects except the PM, terminal half-life in pregnancy with metoprolol ER was 13.2 ± 12.1 hours (n = 11) compared with metoprolol IR 3.7 ± 3.0 hours (n = 6), P < .05.
Figure 1.
Metoprolol and α-hydroxymetoprolol plasma concentration–time profiles during late pregnancy and postpartum in an extensive metabolizer (CYP2D6*1/*1). The subject was treated during pregnancy and postpartum with oral metoprolol ER 25 mg every 8 hours.
Table 2.
Paired Estimated Metoprolol Pharmacokinetic Parameters in Extensive (EM) and Intermediate Metabolizers (IM) During Mid-Pregnancy (22–26 Weeks) and Late (34–38 weeks) Pregnancy Compared to Postpartum (≥ 3 Months)
| Parameter EM and IM | Mid-Pregnancy (n = 5) | Postpartum (n = 5) | Late Pregnancy (n = 8) | Postpartum (n = 8) |
|
| ||||
| CL/F (L/h) | 361 ± 223a | 200 ± 131 | 568 ± 273a | 192 ± 98 |
| CL/F (L/h/kg) | 4.02 ± 2.62a | 2.39 ± 1.68 | 6.37 ± 3.20a | 2.43 ± 1.29 |
| CLrenal (ml/min) | 112 ± 33 | 108 ± 25 | 139 ± 51a | 92 ± 31 |
| MR in plasma | 4.28 ± 8.53 | 2.39 ± 3.79 | 0.47 ± 0.46a | 1.07 ± 0.95 |
| MR in urine | 2.35 ± 4.88 | 1.31 ± 1.96 | 0.21 ± 0.17 | 0.53 ± 0.35 |
| % dose recovered in urine as metoprolol | 2.75 ± 2.38a | 4.86 ± 3.27 | 1.82 ± 1.21 | 3.45 ± 1.87 |
| Creatinine clearance (mL/min) | 165 ± 40a | 100 ± 12 | 157 ± 29a | 119 ± 33 |
|
| ||||
| EM only | Mid-Pregnancy (n = 4) | Postpartum (n = 4) | Late Pregnancy (n = 7) | Postpartum (n = 7) |
|
| ||||
| CL/F (L/h) | 432 ± 181a | 238 ± 113 | 629 ± 228a | 209 ± 92 |
| CL/F (L/h/kg) | 4.74 ± 2.37 | 2.83 ± 1.57 | 7.04 ± 2.79a | 2.62 ± 1.25 |
| CLrenal (mL/min) | 117 ± 36 | 117 ± 16 | 143 ± 53 | 97 ± 30 |
| MR in plasma | 0.46 ± 0.18 | 0.71 ± 0.40 | 0.32 ± 0.14a | 0.75 ± 0.34 |
| MR in urine | 0.17 ± 0.05 | 0.44 ± 0.26 | 0.15 ± 0.05a | 0.42 ± 0.20 |
| % dose recovered in urine as metoprolol | 1.70 ± 0.42 | 3.73 ± 2.42 | 1.42 ± 0.46a | 3.20 ± 1.86 |
CL/F, apparent oral metoprolol clearance; CLrenal, metoprolol renal clearance; MR, metoprolol/α-hydroxymetoprolol metabolic ratio.
Significantly different than postpartum (P < 0.05). Results reported in means ± SD.
Figure 2.
Metoprolol apparent oral clearance of extensive and intermediate metabolizers during pregnancy and postpartum. Subjects were classified as extensive metabolizers (EMs) if they had 2 fully functional CYP2D6 alleles (*1 and *35) or 2 reduced-activity alleles (*9, *10, *17, or *41) or 1 fully functional allele together with 1 reduced-activity allele; intermediate metabolizers (IMs) if they had 1 nonfunctional CYP2D6 allele (*3, *4, *5, or *6) with either 1 functional allele or 1 reduced-activity allele; and poor metabolizers (PMs) if they carried 2 nonfunctional CYP2D6 alleles. The lines indicate mean values; closed circles indicate EMs; and open circles indicate IMs. Statistical significance (P < .05) when compared with postpartum is indicated by an asterisk.
As expected, creatinine clearance was much higher during mid-pregnancy (165 ± 40 mL/min; P < .05) and late pregnancy (157 ± 29 mL/min; P < .05) compared with postpartum (100 ± 12 mL/min, n = 5; 116 ± 32 mL/min, n = 8, respectively); see Table 2. A similar change was observed in early pregnancy, but the small number of subjects did not allow for significance testing. Although there was a trend toward higher mean metoprolol CLrenal in all pregnancy phases, significance was only achieved in late pregnancy as compared with postpartum (141 ± 52 versus 90 ± 30 mL/min, respectively; P < .05; Table 2). Metoprolol CLrenal was found to weakly correlate with creatinine clearance (r = 0.38; P < .05) and with urine pH (r = −0.39, P < .05), data not shown. Of note, in 2 subjects (CYP2D6*1/*1) who participated in 2 postpartum studies, metoprolol CLrenal was 25%–36% higher during the postpartum study with lactation (12 and 15.3 weeks postpartum) than without lactation (6 months and 1 year postpartum).
Metoprolol/α-hydroxymetoprolol MR in plasma (0.47 ± 0.46, P < .05) was significantly lower during late pregnancy compared with postpartum (1.07 ± 0.95); see Table 2. The MR in plasma and urine was significantly lower for EM subjects in late pregnancy compared with postpartum (Table 2). Across all subjects and study days, metoprolol/α-hydroxymetoprolol MR in plasma correlated well with the MR in urine (r = 0.98, P < .05, data not shown). In addition, the percent of dose collected in urine as unchanged metoprolol was lower in mid-pregnancy than postpartum (2.75% ± 2.38% versus 4.86% ± 3.27%, P < .05).
The Skillings-Mack test confirmed significant differences in metoprolol CL/F, MR in plasma, and percent dose excreted as metoprolol in urine during pregnancy compared with postpartum (P < .05) in EMs alone and with EMs and IMs combined. However, MR in urine was only significant for EMs, and metoprolol CLrenal did not reach significance in pregnancy.
Metoprolol was present in all umbilical cord blood samples collected at delivery except in 2 subjects who took their last metoprolol dose 33 and 39.8 hours prior to sample collection. The mean umbilical cord plasma-to-maternal concentration ratios, excluding 1 subject without cord sample, for metoprolol (n = 5) were 1.0 ± 0.2 (venous) and 1.1 ± 0.3 (arterial) and for α-hydroxymetoprolol (n = 5) were 0.9 ± 0.1 (venous) and 0.9 ± 0.2 (arterial). The ratios remained fairly constant between 1 and 5 hours after the last dose. There was a significant correlation between maternal venous blood concentrations and umbilical venous cord concentrations for metoprolol (r = 0.97, P < .05) and α-hydroxymetoprolol (r = 0.98, P < .05); data not shown. The mean maternal plasma metoprolol concentration (n = 6) was 18.9 ± 20.0 ng/mL (range, below limit of quantification to 55.3 ng/mL) and for α-hydroxymetoprolol was 32.3 ± 22.3 ng/mL (range, below limit of quantification to 64.2 ng/mL). The mean venous and arterial umbilical cord metoprolol concentrations at the time of delivery were 10.6 ± 8.6 ng/mL (range, 1.9–20.6 ng/mL) and 11.5 ± 9.0 ng/mL(range, 2.9–21.7 ng/mL), respectively, and for α-hydroxymetoprolol were 30.0 ± 21.1 ng/mL (range, 5.8–57.7 ng/mL) and 30.6 ± 22.1 (range, 3.3–59.7 ng/mL), respectively. Mean arterial umbilical cord to venous cord ratio of metoprolol was 1.1 ± 0.2 (range, 1.0–1.5) and for α-hydroxymetoprolol was 1.0 ± 0.2 (range, 0.6–1.1).
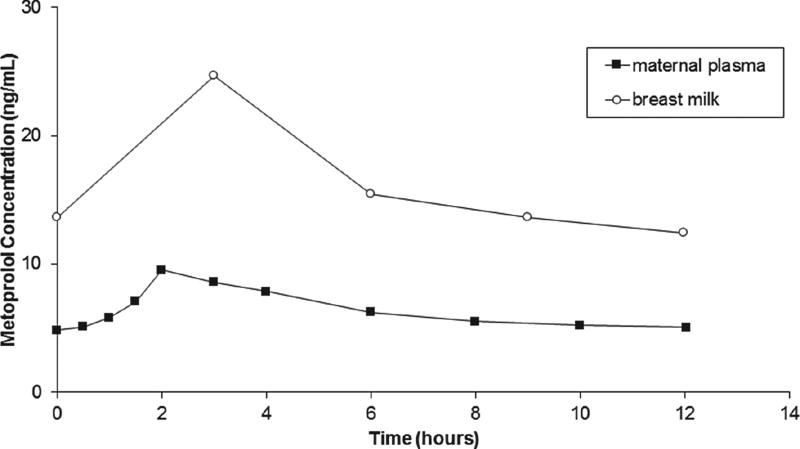
A representative breast milk and plasma metoprolol concentration–time profile over 1 dosing interval in an EM (CYP2D6*1/*1) is shown in Figure 3. In 3 subjects, the mean breast milk:plasma AUCτSS ratio of metoprolol was 2.4 ± 0.3. The average amount of metoprolol excreted in breast milk was 71.5 ± 76.3 µg/day (range, 17.0–158.7 µg/day). The mean relative infant dose was 0.5% ± 0.1% of the mother’s weight-adjusted dose. Subject 10 (CYP2D6*1/*41) did not produce enough milk for analytical determinations of metoprolol concentration after the sixth hour of collection. In this subject, using 6 hours for both milk and plasma AUC, the milk: plasma AUCτSS ratio of metoprolol was 1.9.
Figure 3.
Plasma and breast milk metoprolol concentration-versus-time curves in a representative subject receiving metoprolol extended release 25 mg twice daily.
Discussion
Metoprolol is considered a model CYP2D6 substrate, as this enzyme mediates 70%–80% of its metabolism and almost all its α-hydroxylation. In the present study, we report changes in metoprolol pharmacokinetics during pregnancy. Pregnancy enhanced the metabolism of metoprolol, as determined by significant increases in metoprolol CL/F, corresponding decreases in plasma metoprolol/α-hydroxymetoprolol metabolic ratio, and urinary recovery of unchanged parent drug. This study is the first to report metoprolol CL/F in extensive, intermediate, and poor metabolizers and metoprolol CLrenal and metoprolol/α-hydroxymetoprolol metabolic ratio in plasma and urine during pregnancy compared with postpartum.
Our results are in agreement with previous reports of enhanced CYP2D6 activity during pregnancy.2,3 Our study not only evaluated late pregnancy as reported by Högstedt et al, but also in early and mid-pregnancy. As expected, EMs in our study showed significant increases in metoprolol CL/F during mid-pregnancy (P < .05) and late pregnancy (P < .05) compared with postpartum. We saw a similar trend in early pregnancy; however, the small number of subjects limited the power for this comparison. In the nonpregnant population, marked differences in metoprolol pharmacokinetics have been reported when comparing CYP2D6 extensive and poor metabolizers.16A recent meta-analysis of nonpregnant healthy adults reported a 5.9-fold difference (P < .001) in metoprolol CL/F between EMs and PMs.16 The PM in our study had weight-adjusted CL/F in mid- and late pregnancy that was 14-fold and 19-fold lower, respectively, than the average values of EMs during mid- and late pregnancy. The apparently greater difference in metoprolol CL/F seen between EM and PM in pregnancy reflects the inability of pregnancy to modulate CYP2D6 in the PM, whereas pregnancy upregulated CYP2D6 in the IMs and EMs. The reason for the large variability within the EMs is unclear but may be suggestive of unidentified variant alleles, the presence of known alleles that were not tested in our study, or the presence of some other unknown contributing variable.
Historically, CYP2D6 was thought to be a non-inducible enzyme by classic pathways for enzyme induction (such as those involving the pregnane × receptor and the constitutive androstane receptor).17,18 However, it has been suggested that decreased expression of small heterodimer partner (SHP) may be partly responsible for CYP2D6 induction in pregnancy.19 In CYP2D6-humanized mice, increasing hepatic levels of retinoic acid, an endogenous compound that induces SHP during pregnancy, led to a significant decrease in CYP2D6 expression.19 The enhanced metabolism of other CYP2D6 substrates during pregnancy, including clonidine,20 dextromethorphan,17 fluoxetine,21 and nortriptyline,22 further supports CYP2D6 induction. The mean norfluoxetine/fluoxetine MR was found to be 58% higher at term (~36–37 weeks’ gestation) compared with 2 months postpartum.21 Other possible explanations for changes in metoprolol concentrations would include changes in blood flow, gastrointestinal absorption, and/or plasma protein binding. Changes in hepatic blood flow should only have a modest effect on intravenous metoprolol AUC, as metoprolol is an intermediate extraction ratio drug (ER = 0.5)23 and should have no effect on oral metoprolol AUC. With regard to absorption, Högstedt et al found a significant decrease in oral bioavailability of metoprolol during pregnancy and a trend toward increased urinary recovery of both metoprolol and its major metabolites (α-hydroxymetoprolol, O-demethylmetoprolol, and an acid metabolite).2 These data suggest that metoprolol oral absorption is not impaired but rather increased during pregnancy,2 and the decreased bioavailability reflects increased first-pass metabolism. Likewise, intestinal blood flow may increase during pregnancy, but this would be more likely to result in enhanced and not reduced absorption of metoprolol. As for protein binding, Högstedt et al found metoprolol plasma protein binding to be low and comparable during pregnancy (9%) compared with postpartum (11%).2 Therefore, increased CYP2D6 metabolism is the most likely explanation for increased metoprolol CL/F during pregnancy. We recognize that metoprolol bioavailability is dose dependent in nonpregnant subjects for both ER and IR formulations.24,25 To our knowledge, there are no published studies on nonlinear pharmacokinetics with low doses of metoprolol at steady state. Internal evaluation of those subjects receiving the same dose during pregnancy and postpartum (n = 4) demonstrated that metoprolol CL/F was significantly higher in pregnancy than postpartum (551 ± 195 vs 194 ± 59 L/h, respectively; P < .05). The median dose for each phase ranged from 75 to 100 mg, which would limit the impact of nonlinearity in dosing. Nevertheless, the limitation of our study is that subjects received varying metoprolol doses during each study phase because doses were based on clinical need and not adjusted for research purposes.
At delivery, umbilical cord plasma concentrations (both venous and arterial) were similar to maternal plasma concentrations 1–5 hours postdose for both metoprolol and α-hydroxymetoprolol, suggesting rapid equilibrium between the 2 circulations. There was a significant correlation between venous cord plasma and maternal plasma concentrations for both metoprolol and α-hydroxymetoprolol. In a previous study, Lindeburg et al reported the ratio of mixed cord plasma to maternal plasma concentrations of metoprolol to be approximately 1, with significant correlations between metoprolol mixed cord plasma and maternal plasma (r = 0.99, P < .001).26
Five subjects were treated with drugs known to interact with CYP2D6. Fluoxetine is a potent CYP2D6 inhibitor that has anecdotally been shown to lead to symptomatic bradycardia when given with metoprolol, possibly through inhibition of metoprolol metabolism.13 At the lowest recommended dose of fluoxetine, 20 mg/day, desipramine (a CYP2D6 substrate) AUC increased by 380%.27 Sertraline, on the other hand, is a weak to moderate dose-dependent CYP2D6 inhibitor.14,28 In 2 separate studies, healthy nonpregnant EM subjects given a single dose of metoprolol 100 mg with sertraline 100 mg/day showed significant increases in AUC, by 48% (n = 16) and 67% (n = 7).14 These results were consistent with earlier studies and demonstrated increased AUC of CYP2D6 substrates by an average of 18% with sertraline 50 mg/day, 30% with sertraline 100 mg/day, and 64% with sertraline 150 mg/day.28 Our subjects received 25–50 mg/day during pregnancy and 75 mg/day during postpartum, where an average increase in metoprolol AUC of 0%–30% may be expected. There was no significant change in the PK parameters or conclusions in this study when sertraline subjects were removed. Concomitant administration with hydroxychloroquine (400 mg daily) has been reported to significantly increase metoprolol AUC by 65% in 6 homozygous EM men by inhibition of CYP2D6.15 Currently there are no studies on low-dose interaction with metoprolol. Our subject, on hydroxychloroquine 200 mg daily, did not participate in the postpartum study to allow for comparison. When this subject was removed from the analysis, it did not alter the overall conclusions or the pharmacokinetic analysis of our study.
Changes observed in renal drug clearance during pregnancy can be attributed to altered glomerular filtration rate (GFR), active tubular secretion, and/or reabsorption.29 Metoprolol CLrenal was significantly higher in late pregnancy (139 ± 51 mL/min) compared with postpartum (92 ± 31 mL/min). Nevertheless, renal changes should only have a minor influence on the elimination half-life or the total clearance of metoprolol in pregnancy because of the low urinary excretion of unchanged drug (<3%).2 We have also found a poor correlation between renal clearance and creatinine clearance. A possible explanation for the poor correlation is that metoprolol undergoes a net renal reabsorption, which occurs via an unknown mechanism.
Observed elevated CLrenal –4 months postpartum) compared with postlactation (6–13 months postpartum) may be associated with renal function not having returned to baseline by 4 months postpartum. The decrease in prolactin concentrations over the first 6 months postpartum in lactating women may have affected the slow return to baseline. Prolactin concentrations initially increase with breastfeeding until 60 days postpartum but then decrease rapidly between 12 and 18 months postpartum.30 In humans and in rats, decreased renal function has been associated with increase in prolactin, possibly by direct action on renal prolactin receptors, alteration in renal plasma flow and GFR, enhancement of aldosterone secretion, and/or direct renal interaction with vasopressin.31,32 Further research is needed to investigate the mechanism behind higher metoprolol CLrenal during lactation.
Our findings indicate that metoprolol is concentrated in breast milk, as demonstrated by a mean milk:plasma AUC ratio of 2.4 ± 0.3. Because metoprolol is a weak base (pKa 9.7) with low plasma protein binding and moderate lipid solubility, it can be expected that the drug will readily transfer into milk and ionize because of milk’s slightly lower pH. However, the process of active transport of metoprolol cannot be ruled out. Future research is needed to characterize metoprolol transporters in the mammary epithelia. Our milk:plasma AUC ratio is within the range of those previously reported (2.0–3.1) in 3 women.33 Metoprolol concentrations in breast milk were on average 2.6 times higher (n = 3) than in plasma. Previous work reported similar findings, in which a single time point concentration of metoprolol in breast milk was on average 3–3.5 times higher than maternal plasma.2 However, the relative infant dose (<1.0% of the mother’s weight-adjusted dose) of metoprolol via breast milk is sufficiently low, so that exposure is unlikely to be clinically significant for infants.
It is expected that the enhanced metabolism of metoprolol in EM and IM subjects during pregnancy would result in decreased pharmacologic activity because α-hydroxymetoprolol is only about one-tenth as potent as metoprolol in β-adrenergic receptors blocking activity.34 Indeed, clinical observations indicate that without dosage adjustment, the efficacy of metoprolol in pregnant women may be reduced.35 Some of our subjects required higher doses during pregnancy compared with postpartum. However, varying indications for metoprolol use in our subjects made it difficult to rigorously evaluate the effect of PK changes in pregnancy on clinical response. The high intra- and intersubject variability as well as the gestational age-dependent changes in metoprolol pharmacokinetics and pharmacodynamics throughout pregnancy makes the therapeutic use of metoprolol particularly challenging. CYP2D6 genotyping may be considered to distinguish poor and intermediate metabolizers from extensive or ultra metabolizers prior to treatment. The effects of CYP2D6 genotype are as large as the effects of pregnancy on metoprolol PK. However, an evaluation of the relationship between clinical response and genotype will require further investigation. In contrast to metoprolol, atenolol, a renally eliminated beta-blocker, is a potential alternative during pregnancy. Atenolol CL/F in late pregnancy is not significantly different when compared with postpartum.9 The interindividual variability of atenolol’s CL/F is less than 27% during mid-pregnancy, 29% in late pregnancy, and 45% postpartum.9 Compared with metoprolol, atenolol has more predictable PK during pregnancy and postpartum, making it an easier agent to use.9
In conclusion, our study describes the changes in metoprolol pharmacokinetics during pregnancy. Because of the large genotype and pregnancy-induced changes in metoprolol pharmacokinetics in pregnant women, when a beta-blocker is required, challenges in dosing metoprolol should be anticipated. This is particularly true for EMs. If inadequate clinical responses are encountered, more aggressive dosing or treatment with an alternate beta-blocker should be considered.
Supplementary Material
Acknowledgments
We gratefully acknowledge the expert technical assistance of Tot Bui Nguyen from the UW Department of Pharmaceutics and the DNA Sequencing and Gene Analysis Center at UW School of Pharmacy, and Sengkeo Srinouanprachanh, Jesse M. Tsai, Zahra Afsharinejad, and Theo Bammler from the Functional Genomics Laboratory in the Department of Environmental and Occupational Health Science at University of Washington.
Funding
This research was supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health & Human Development through support of the Obstetric-Fetal Pharmacology Research Unit Network U10HD047892, U10HD047905, 5U10HD047891-10, National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR000423, UL1RR024153, and NIEHS Center for Ecogenetics & Environmental Health P30ES007033. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
None of the authors have a conflict of interest.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 8. Philadelphia: Lippincott, Williams & Wilkins; 2008. Metoprolol; pp. 947–950. [Google Scholar]
- 2.Högstedt S, Lindberg B, Peng DR, Regårdh CG, Rane A. Pregnancy-induced increase in metoprolol metabolism. Clin Pharmacol Ther. 1985;37(6):688–692. doi: 10.1038/clpt.1985.114. [DOI] [PubMed] [Google Scholar]
- 3.Högstedt S, Lindberg B, Rane A. Increased oral clearance of metoprolol in pregnancy. Eur J Clin Pharmacol. 1983;24(2):217–220. doi: 10.1007/BF00613820. [DOI] [PubMed] [Google Scholar]
- 4.Regårdh CG, Landahl S, Larsson M, et al. Pharmacokinetics of metoprolol and its metabolite alpha-OH-metoprolol in healthy, non-smoking, elderly individuals. Eur J Clin Pharmacol. 1983;24(2):221–226. doi: 10.1007/BF00613821. [DOI] [PubMed] [Google Scholar]
- 5.Johnson J, Burlew B. Metoprolol metabolism via cytochrome P4502D6 in ethnic populations. Drug Metab Dispos. 1996;24(3):350–355. [PubMed] [Google Scholar]
- 6.Bijl M, Visser L, van Schaik R, et al. Genetic variation in the CYP2D6 gene is associated with a lower heart rate and blood pressure in beta-blocker users. Clin Pharmacol Ther. 2009;85(1):45–50. doi: 10.1038/clpt.2008.172. [DOI] [PubMed] [Google Scholar]
- 7.Högstedt S, Rane A. Plasma concentration—effect relationship of metoprolol during and after pregnancy. Eur J Clin Pharmacol. 1993;44(3):243–246. doi: 10.1007/BF00271365. [DOI] [PubMed] [Google Scholar]
- 8.Zhu B, Ou-Yang DS, Chen XP, et al. Assessment of cytochrome P450 activity by a five-drug cocktail approach. Clin Pharmacol Ther. 2001;70(5):455–461. doi: 10.1067/mcp.2001.119813. [DOI] [PubMed] [Google Scholar]
- 9.Hebert MF, Carr DB, Anderson GD, et al. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005;45(1):25–33. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, National Center for Health Statistics. The WHO growth charts: data table for girls length-for-age and weight-for-age charts, birth to 24 months. [Accessed December 3, 2014]; http://www.cdc.gov/growthcharts/who/girls_length_weight.htm.
- 11.Hebert M, Easterling T, Kirby B, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington Specialized Center of Research study. Clin Pharmacol Ther. 2008;84(2):248–253. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 12.Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet. 2009;48(11):689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Walley T, Pirmohamed M, Proudlove C, Maxwell D. Interaction of metoprolol and fluoxetine. Lancet. 1993;341(8850):967–968. doi: 10.1016/0140-6736(93)91265-n. [DOI] [PubMed] [Google Scholar]
- 14.Preskorn S, Greenblatt D, Flockhart D, et al. Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharmacol. 2007;27(1):28–34. doi: 10.1097/00004714-200702000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Somer M, Kallio J, Pesonen U, Pyykkö K, Huupponen R, Scheinin M. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br J Clin Pharmacol. 2000;49(6):549–554. doi: 10.1046/j.1365-2125.2000.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake CM, Kharasch ED, Schwab M, Nagele P. A meta-Analysis of CYP2D6 metabolizer phenotype and metoprolol pharmacokinetics. Clin Pharmacol Ther. 2013;94(3):394–399. doi: 10.1038/clpt.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tracy T, Venkataramanan R, Glover D, Caritis S for the National Institute for Child Health and Human Development Network of Maternal-Fetal-Medicine Units. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5(1):6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- 19.Koh KH, Pan X, Shen HW, et al. Altered expression of small heterodimer partner governs cytochrome P450 (CYP) 2D6 induction during pregnancy in CYP2D6-humanized mice. J Biol Chem. 2014;289(6):3105–3113. doi: 10.1074/jbc.M113.526798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claessens AJ, Risler LJ, Eyal S, Shen DD, Easterling TR, Hebert MF. CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos. 2010;38(9):1393–1396. doi: 10.1124/dmd.110.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heikkinen T, Ekblad U, Palo P, Laine K. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003;73(4):330–337. doi: 10.1016/s0009-9236(02)17634-x. [DOI] [PubMed] [Google Scholar]
- 22.Wisner KL, Perel JM, Wheeler SB. Tricyclic dose requirements across pregnancy. Am J Psychiatry. 1993;150(10):1541–1542. doi: 10.1176/ajp.150.10.1541. [DOI] [PubMed] [Google Scholar]
- 23.Lennard MS, Tucker GT, Silas JH, Freestone S, Ramsay LE, Woods HF. Differential stereoselective metabolism of metoprolol in extensive and poor debrisoquin metabolizers. Clin Pharmacol Ther. 1983;34(6):732–737. doi: 10.1038/clpt.1983.242. [DOI] [PubMed] [Google Scholar]
- 24.Sandberg A, Abrahamsson B, Regårdh CG, Wieselgren I, Bergstrand R. Pharmacokinetic and biopharmaceutic aspects of once daily treatment with metoprolol CR/ZOK: a review article. J Clin Pharmacol. 1990;30(2 Suppl):S2–S16. doi: 10.1002/j.1552-4604.1990.tb03490.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnsson G, Regårdh CG, Sölvell L. Combined pharmacokinetic and pharmacodynamic studies in man of the adrenergic beta1-receptor antagonist metoprolol. Acta Pharmacol Toxicol (Copenh) 1975;36(Suppl 5):31–44. doi: 10.1111/j.1600-0773.1975.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 26.Lindeberg S, Sandstrom B, Lundborg P, Regardh CG. Disposition of the adrenergic blocker metoprolol in the late-pregnant woman, the amniotic fluid, the cord blood and the neonate. Acta Obstet Gynecol Scand Suppl. 1984;118:61–64. doi: 10.3109/00016348409157125. [DOI] [PubMed] [Google Scholar]
- 27.Preskorn SH, Alderman J, Chung M, Harrison W, Messig M, Harris S. Pharmacokinetics of desipramine coadministered with sertraline or fluoxetine. J Clin Psychopharmacol. 1994;14(2):90–98. [PubMed] [Google Scholar]
- 28.Preskorn SH. Reproducibility of the in vivo effect of the selective serotonin reuptake inhibitors on the in vivo function of cytochrome P450 2D6: An update (part I) J Psychiatr Pract. 2003;2:150–158. doi: 10.1097/00131746-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GD, Carr DB. Effect of pregnancy on the pharmacokinetics of antihypertensive drugs. Clin Pharmacokinet. 2009;48(3):159–168. doi: 10.2165/00003088-200948030-00002. [DOI] [PubMed] [Google Scholar]
- 30.Hennart P, Delogne-Desnoeck J, Vis H, Robyn C. Serum levels of prolactin and milk production in women during a lactation period of thirty months. Clin Endocrinol. 1981;14(4):349–353. doi: 10.1111/j.1365-2265.1981.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 31.Morrissey SE, Newth T, Rees R, Barr A, Shora F, Laycock JF. Renal effects of recombinant prolactin in anaesthetized rats. Eur J Endocrinol. 2001;145(1):65–71. doi: 10.1530/eje.0.1450065. [DOI] [PubMed] [Google Scholar]
- 32.Buckman MT, Peake GT, Robertson G. Hyperprolactinemia influences renal function in man. Metabolism. 1976;25(5):509–516. doi: 10.1016/0026-0495(76)90004-4. [DOI] [PubMed] [Google Scholar]
- 33.Kulas J, Lunell NO, Rosing U, Stéen B, Rane A. Atenolol and metoprolol. A comparison of their excretion into human breast milk. Acta Obstet Gynecol Scand Suppl. 1984;118:65–69. doi: 10.3109/00016348409157126. [DOI] [PubMed] [Google Scholar]
- 34.Regårdh C, Ek L, Hoffmann K. Plasma levels and beta-blocking effect of alpha-hydroxymetoprolol–metabolite of metoprolol–in the dog. J Pharmacokinet Biopharm. 1979;7(5):471–479. doi: 10.1007/BF01062389. [DOI] [PubMed] [Google Scholar]
- 35.Wichman K, Karlberg BE, Rydéen G. Metoprolol in the treatment of mild to moderate hypertension in pregnancy-effects on the mother. Clin Exper Hypertens Preg. 1985;B4:141–156. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.