Abstract
Esophageal squamous cell carcinoma (ESCC) is the most common esophageal cancer associated with poor prognosis and additional therapeutic strategies must be implemented to optimize ESCC treatment. Meanwhile, the important biologic role and potential prognostic and therapeutic implications of a tumors immunologic microenvironment (IM) have been recognized in various cancers.
In order to investigate the contexture and the prognostic relevance of the IM in ESCC, we immunohistochemically evaluated the extent of overall/intraepithelial TILs (CD3+/CD8+) and of PD-1 / PD-L1 expression in a cohort of 125 therapy-naive ESCCs, additionally assessing PD-L1 copy number status via fluorescence in-situ hybridization.
High intraepithelial CD3+ TILs (CD3ihigh) and high PD-L1 expression on tumor cells (PD-L1high) were each significantly associated with improved overall- (OS) (CD3+: p = 0.019; PD-L1: p = 0.028), disease specific- (DSS) (CD3+: p = 0.05; PD-L1: p = 0.006) and disease free survival (DFS) (CD3+: p = 0.009; PD-L1: p < 0.001). CD3ihigh- and PD-L1high cases were significantly associated with one another (p < 0.001). Subgrouping of ESCC revealed decreased OS (p = 0.031), DSS (p = 0.012) and DFS (p < 0.001) for CD3ilow/PD-L1low cancers.
Our data not only associate CD3ihigh- and PD-L1high ESCC with a beneficial outcome, but also demonstrate PD-L1high- and CD3ihigh status to be closely intertwined. Furthermore, our study demarcates a prognostically unfavorable, “non-immunoreactive” CD3ilow / PD-L1low ESCC-subgroup, potentially forming the basis for an immune-based stratification of ESCC.
Keywords: esophageal squamous cell carcinoma, immunologic microenvironment, PD-L1, tumor infiltrating lymphocytes, intraepithelial CD3, Pathology Section
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is a substantial cause of cancer-related death worldwide, accounting for approximately 80% of esophageal cancers [1–6]. ESCC is usually associated with poor patient prognosis, with reported 5-year overall survival rates varying from 15-40% [2, 4, 7]. Although diagnostic and therapeutic advances have led to slight improvements in the clinical management and outcome of ESCC [2, 5, 7], the implementation of additional treatment strategies and novel prognostic biomarkers must be expedited to optimize ESCC treatment.
The recognition of the immunologic microenvironment (IM) of a malignant tumor as a potentially powerful biomarker of prognostic and therapeutic relevance has led to a resurgence of immune based therapy strategies in solid cancers. Firstly, the immunologic tumor-host relationship appears to be of substantial importance for the carcinogenic process [8, 9] and especially the extent of CD3+ and CD8+ tumor infiltrating lymphocytes (TILs) seems to be of special interest for the evaluation of a potential immunogenic antitumor response [10–13]. Particularly, intraepithelial CD3+ T-cells have been identified as valid prognosticators in a variety of adenocarcinomas [14–17]. Secondly, immune-checkpoint blockade, especially via inhibition of the potent immunoevasive effects of the Programmed Death-1 (PD-1) / Programmed Death – Ligand 1 (PD-L1) axis, has become a powerful tool of tumor immunotherapy, with variable responses in diverse cancer types [18, 19, 20] and partially conflicting results regarding the predictive value of immunohistochemical PD-L1 expression [18]. Furthermore, a recent pan-cancer classification approach proposes a model of four different types of tumor microenvironments, based on a tumors T-cell infiltration and PD-L1 positivity [13].
However, in ESCC, data regarding its IM are limited and the prognostic value of PD-L1 expression is still a matter of debate, as some studies associate PD-L1 expression with a rather favorable [21, 22] prognosis, while others postulate a less favorable [23–25] disease course for PD-L1 positive cancers.
In order to investigate the contexture and possible clinicopathological implications of the IM including the PD-1 / PD-L1 axis in ESCC, we immunohistochemically analyzed the extent and distribution of overall and intraepithelial CD3+ / CD8+ TILs as well as PD-1 / PD-L1 expression in a tumor series of 125 primary resected, therapy-naive ESCCs. Additionally, the tumors PD-L1 copy number status was evaluated through Fluorescence in situ Hybridization (FISH).
The questions we addressed focused on (1) the specific composition of the IM in ESCC, (2) the identification of possible associations of overall and intraepithelial TILs with specific clinicopathological and survival parameters, (3) the predictive value of PD-L1 immunohistochemistry in ESCC and (4) the relationship of the PD-1 / PD-L1 axis to the IM, particularly focusing on potential immunologic ESCC subgroups based on their extent of T-cell infiltration and PD-L1 positivity.
RESULTS
Clinicopathological characteristics
95 of the 125 ESCC patients in our tumor series were male (76%), 30 were female (24%). Mean age at diagnosis was 60 years (range: 39-83). Rather locally advanced cancers (pT2-pT4: 70/125; 56%) and early stage carcinomas (pT1: 55/125; 44%) were roughly evenly distributed, synchronous lymph node metastases (pN+) were detectable in 57 cases (46%) and four cases showed synchronous distant metastases (pM1: 3%). According to the WHO classification of the digestive system [26], most ESCCs were moderately (G2; 49%) or poorly differentiated (G3; 46%) with only a minor subset of well differentiated tumors (G1; 5%). 53 (42%) patients suffered from local and/or distant relapse and 79 (63%) patients died during follow up, of which 82% (65/79) were tumor specific deaths. Mean follow-up time for patients alive at the endpoint of overall survival analysis was 65.09 months (Table 1).
Table 1. Association of immunological and clinicopathological factors with survival parameters (univariate).
| Overall | Events (OS) | Mean overall survival (SE) | p-value | Events (DSS) | Mean disease specific survival (SE) | p-value | Events (DFS) | Mean disease free survival (SE) | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 125 | 79 | 76.9 (7.1) | 65 | 89.0 (7.9) | 53 | 86.6 (7.4) | |||||
| Age | 0.447 | 0.401 | 0.710 | ||||||||
| median and below | 68 | 45 | 77.9 (8.9) | 36 | 89.6 (10.0) | 33 | 82.4 (8.7) | ||||
| above median | 57 | 35 | 57.6 (6.6) | 29 | 65.3 (7.0) | 20 | 77.4 (7.4) | ||||
| Sex | 0.140 | 0.067 | 0.192 | ||||||||
| male | 95 | 64 | 73.1 (8.0) | 54 | 82.3 (8.8) | 43 | 82.4 (8.2) | ||||
| female | 30 | 16 | 72.4 (8.3) | 11 | 83.7 (8.6) | 10 | 83.8 (8.8) | ||||
| pT | <0.001 | <0.001 | mean DFS not reached | 0.002 | |||||||
| 1 | 55 | 26 | 106.2 (11.5) | 31 | 119.0 (12.2) | 15 | |||||
| 2 | 40 | 36 | 38.9 (5.5) | 29 | 44.9 (6.5) | 25 | |||||
| 3 | 28 | 17 | 76.8 (13.2) | 3 | 86.8 (14.0) | 13 | |||||
| 4 | 2 | 1 | 36.9 (17.7) | 2 | 36.9 (17.7) | 0 | |||||
| pN | 0.012 | 0.014 | mean DFS not reached | 0.005 | |||||||
| 0 | 68 | 38 | 82.8 (8.6) | 42 | 92.9 (9.2) | 24 | |||||
| 1 | 47 | 37 | 60.7 (9.5) | 34 | 74.0 (11.6) | 23 | |||||
| 2 | 8 | 3 | 63.9 (14.0) | 3 | 63.9 (14.0) | 4 | |||||
| 3 | 2 | 2 | 13.9 (4.8) | 2 | 13.9 (4.8) | 2 | |||||
| pM | 0.001 | <0.001 | <0.001 | ||||||||
| 0 | 121 | 76 | 79.0 (7.2) | 61 | 91.6 (8.1) | 49 | 89.4 (7.5) | ||||
| 1 | 4 | 4 | 6.0 (2.8) | 4 | 14.5 (6.0) | 4 | 8.9 (5.6) | ||||
| UICC Stage | 0.008 | 0.002 | <0.001 | ||||||||
| 1 | 51 | 21 | 66.6 (6.8) | 19 | 74.7 (7.2) | 23 | 85.7 (7.2) | ||||
| 2 | 49 | 24 | 76.8 (10.1) | 24 | 85.6 (11.0) | 25 | 79.5 (9.7) | ||||
| 3 | 21 | 37 | 45.1 (7.9) | 34 | 55.6 (8.8) | 36 | 64.1 (9.6) | ||||
| 4 | 4 | 4 | 14.5 (16.0) | 4 | 14.5 (6.0) | 4 | 8.9 (5.6) | ||||
| Grade (WHO) | 0.039 | 0.071 | 0.402 | ||||||||
| 1 | 6 | 1 | 143.2 (19.0) | 1 | 143.2 (19.0) | 2 | 114.2 (24.7) | ||||
| 2 | 61 | 39 | 81.2 (9.9) | 31 | 94.3 (11.2) | 28 | 85.2 (9.6) | ||||
| 3 | 58 | 40 | 52.5 (6.0) | 33 | 60.1 (6.6) | 53 | 69.6 (7.5) | ||||
| CD3i | 0.019 | 0.050 | 0.009 | ||||||||
| low | 81 | 55 | 62.1 (7.4) | 44 | 73.9 (8.6) | 39 | 78.1 (8.9) | ||||
| high | 44 | 25 | 96.4 (11.9) | 21 | 106.1 (12.5) | 14 | 104.9 (11.1) | ||||
| Distribution of CD3i | 0.007 | 0.003 | 0.004 | ||||||||
| focal | 75 | 54 | 58.3 (7.1) | 46 | 65.5 (7.8) | 37 | 73.4 (8.9) | ||||
| diffuse | 50 | 26 | 100.1 (11.8) | 19 | 118.7 (12.6) | 16 | 106.5 (11.1) | ||||
| CD8i | 0.163 | 0.486 | 0.185 | ||||||||
| low | 87 | 58 | 73.3 (8.4) | 45 | 90.3 (9.6) | 39 | 81.5 (8.7) | ||||
| high | 38 | 22 | 83.5 (10.7) | 20 | 88.0 (11.0) | 14 | 100.3 (12.2) | ||||
| Distribution of CD8i | 0.015 | 0.080 | 0.030 | ||||||||
| focal | 85 | 60 | 61.6 (8.0) | 47 | 74.6 (10.3) | 40 | 70.8 (6.9) | ||||
| diffuse | 40 | 20 | 94.1 (11.0) | 18 | 98.7 (11.1) | 13 | 110.1 (11.7) | ||||
| PD1i | 0.035 | 0.117 | 0.169 | ||||||||
| low | 84 | 53 | 60.6 (7.4) | 42 | 72.2 (8.7) | 35 | 80.8 (9.3) | ||||
| high | 41 | 27 | 95.5 (12.5) | 21 | 103.2 (13.0) | 18 | 88.9 (9.0) | ||||
| PD-L1+ TILs | 0.870 | 0.815 | 0.844 | ||||||||
| low | 89 | 56 | 77.2 (8.6) | 47 | 87.2 (9.5) | 38 | 82.8 (8.4) | ||||
| high | 36 | 23 | 69.6 (10.7) | 18 | 84.1 (12.4) | 15 | 96.4 (12.8) | ||||
| PD-L1 TCs | 0.028 | 0.006 | <0.001 | ||||||||
| low/absent | 87 | 54 | 65.0 (8.8) | 47 | 71.7 (9.7) | 42 | 62.4 (6.8) | ||||
| high | 38 | 26 | 87.8 (9.1) | 18 | 103.8 (10.6) | 11 | 122.4 (10.6) | ||||
| Staining intensity of PD-L1 | 0.720 | 0.883 | 0.293 | ||||||||
| weak | 30 | 18 | 55.9 (8.1) | 13 | 68.2 (9.0) | 14 | 60.9 (9.2) | ||||
| intermediate | 31 | 15 | 73.9 (11.6) | 12 | 97.9 (14.3) | 12 | 117.6 (13.3) | ||||
| strong | 28 | 20 | 87.1 (13.3) | 17 | 96.1 (12.8) | 8 | 96.8 (14.2) | ||||
| PD-L1 copy number status | 0.781 | 0.533 | 0.951 | ||||||||
| Amplification | 3 | 2 | 25.4 (15.1) | 2 | 25.4 (15.1) | 1 | 37.2 (17.4) | ||||
| Polysomy | 19 | 6 | 66.2 (10.3) | 3 | 66.2 (10.3) | 4 | 69.2 (11.4) | ||||
| Disomy | 92 | 61 | 76.5 (8.1) | 49 | 89.2 (9.0) | 39 | 88.5 (8.3) | ||||
| Deletion | 10 | 11 | 52.5 (10.0) | 11 | 64.4 (10.0) | 9 | 56.5 (11.4) | ||||
| CD3i/PD-L1 subgroups | 0.031 | 0.012 | 0.001 | ||||||||
| low/low | 60 | 44 | 46.8 (5.3) | 38 | 51.6 (5.8) | 33 | 53.8 (6.3) | ||||
| low/high | 21 | 11 | 76.9 (7.1) | 6 | 117.4 (16.0) | 6 | 113.9 (16.8) | ||||
| high/high | 27 | 15 | 86.4 (16.0) | 12 | 100.6 (12.8) | 5 | 130.3 (12.7) | ||||
| high/low | 17 | 10 | 95.4 (21.4) | 9 | 101.4 (22.0) | 9 | 77.4 (14.7) |
Composition of intraepithelial tumor infiltrating lymphocytes in ESCC
Intraepithelial CD3+ TIL (CD3i) count was heterogeneous showing a wide range from 0-70/100 (median: 12) tumor cells (TC). 44/125 ESCCs exceeded the 66. percentile, harboring >20 CD3is (43%; CD3ihigh), while the remaining cancers had <20 CD3is and were therefore classified as CD3ilow. 50 tumors (40%) showed diffuse CD3i distribution. Intraepithelial CD8+ TIL (CD8i) count fluctuated from 0-70/100 TCs (median: 6). 38 tumors clustered within the upper third and had >13 CD8is (30%; CD8ihigh). Diffuse CD8is were detectable in 40 (32%) cases. Intraepithelial PD-1+ TIL (PD1i) count varied from 0-25/100 TCs (median: 3) and 41 cases had a high PD1i count of >6 PD1is, exceeding the 66. percentile (33%; PD1ihigh) (Table 1, Figure 1).
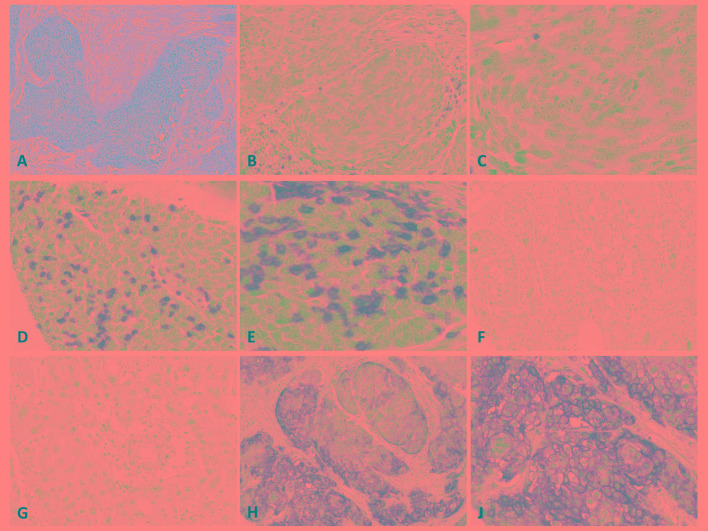
Figure 1.
A. Hematoxylin-Eosin stain of moderately differentiated ESCC; medium and high magnification of an ESCC with low B., C. and high D., E. CD3i count; medium and high magnification of an ESCC without F., G. and with high PD-L1 expression H., I..
Density of tumor infiltrating lymphocytes in the whole tumor area
Overall density of CD3+ TILs occupying the whole tumor area fluctuated from 1-60% (median: 10%; high overall CD3+ TILs: 15%, 83/125). Overall density of CD8+ TILs varied from 1-50% (median: 5%; high overall CD8+ TILs: 10%, 48/125), whereas overall PD1+ TILs ranged from 0-60% (median: 3%; high overall PD1+ TILs: 4%, 49/125) (Supplementary Figure 1).
Expression of PD-L1
Immunohistochemically, at least partial membranous PD-L1 staining was detectable in 71% of ESCCs (89/125, median: 3%), showing a range from 0-90% positive tumor cells. 38/125 (30%) cases were classified as PD-L1high as their amount of PD-L1 positive TCs exceeded the 66. percentile (>10% positive TCs). Weak (34%), intermediate (31%) and strong (35%) staining intensities were almost evenly distributed among the PD-L1 positive cases.
PD-L1+ TILs were detectable in 109/125 cases (87%) and their density within the whole tumor area varied from 0-60% (median: 3%; high overall PD-L1+ TILs: 5%, 36/125) (Table 1, Figure 1).
PD-L1 copy number status
Assessment of the PD-L1 copy number status via FISH revealed PD-L1 amplifications in 3/125 (2%) and PD-L1 deletions in 10/125 (8%) ESCCs. The remaining cases displayed PD-L1 disomy (92/125; 74%) or polysomy (19/125, 15%) (Table 1, Supplementary Figure 1).
Subgrouping of ESCCs
Slightly modifying the recently proposed immune-based cancer classification system by Teng et al. [13], ESCCs were stratified into 4 different subgroups based on their CD3i status and their amount of PD-L1 positive TCs. The resulting four subgroups were the following: subgroup 1: CD3ihigh / PD-L1high (27/125; 21.6%); subgroup 2: CD3ilow / PD-L1low (60/125; 48.0%); subgroup 3: CD3ilow / PD-L1high (21/125; 16.8%); subgroup 4: CD3ihigh / PD-L1low (17/125; 13.6%).
Correlation of immunological factors with each other
As highlighted in Table 2, CD3ihigh and CD8ihigh cases were each significantly associated with diffuse CD3i/CD8i distribution (p<0.001, respectively) and PD1ihigh - (p<0.001, respectively) as well as PD-L1high tumors (CD3: p<0.001; CD8: p=0.005). Additionally, diffusely distributed CD3i and CD8i tumors were significantly more frequent in cases with PD1ihigh - (CD3: p<0.001; CD8: p=0.014) and PD-L1high tumors (CD3: p<0.001; CD8: p=0.003). A high PD1i count was not associated with PD-L1high tumors. CD3i/CD8i/PD1i count correlated with the overall density of TILs of their respective subpopulation (CD3: p=0.002; CD8: p<0.001; PD1: p<0.001). Details of the correlations of overall TIL density are given in Supplementary Table 1.
Table 2. Rank-order correlations of intraepithelial lymphocytes with immunological factors in ESCC.
| High CD3i | High CD8i | Diffuse CD3i | Diffuse CD8i | High PD1i | High PD-L1+ TILs | PD-L1 high | |
|---|---|---|---|---|---|---|---|
| >20 CD3+ TILs/100 TCs | >13 CD8+ TILs/100 TCs | >6 PD-1+ TILs/100 TCs | >10% PD-L1+ TCs | ||||
| High CD3i | |||||||
| >20 CD3+ TILs/100 TCs | x | r = 0.642 | r = 0.595 | r = 0.392 | r = 0.456 | r = 0.091 | r = 0.348 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.409 | p < 0.001 | ||
| High CD8i | |||||||
| >13 CD8+ TILs/100 TCs | x | r = 0.419 | r = 0.591 | r = 0.442 | r = 0.093 | r = 0.265 | |
| p < 0.001 | p < 0.001 | p < 0.001 | p = 0.533 | p = 0.005 | |||
| Diffuse CD3i | |||||||
| x | r = 0.385 | r = 0.326 | r = 0.09 | r = 0.363 | |||
| p < 0.001 | p < 0.001 | p = 0.072 | p < 0.001 | ||||
| Diffuse CD8i | |||||||
| x | r = 0.265 | r = 0.056 | r = 0.269 | ||||
| p = 0.014 | p = 0.533 | p = 0.003 | |||||
| High PD1i | |||||||
| >6 PD-1+ TILs/100 TCs | x | r = 0.193 | r = 0.170 | ||||
| p = 0.04 | p = 0.081 | ||||||
| High PD-L1+ TILs | |||||||
| x | r = 0.152 | ||||||
| p = 0.1 |
Correlation of immunological factors with clinicopathologic variables
CD3ihigh tumors were significantly associated with lower pT stage (p=0.01), lower UICC stage (p=0.001) and tumor free local lymph nodes (p=0.001). Cases showing a diffuse distribution of CD3is were also more frequent in lower pT stage (p=0.02) and lacked nodal involvement more often (p=0.03). PD1ihigh tumors showed lower pT stage (p=0.015) and frequently lacked nodal involvement (p=0.02). CD8ihigh and PD-L1high status, PD-L1 expression in TILs and PD-L1 copy number status were not associated with clinicopathologic variables. Overall density of TILs across the tumor area (including TCs and tumor stroma) showed no significant associations with clinicopathologic features.
Correlation of clinicopathological factors with survival parameters
UICC stage (OS: p=0.008; DSS: p=0.002; DFS: p<0.001), pT (OS: : p<0.001; DSS: : p<0.001; DFS: p=0.002), pN (OS: p=0.012; DSS: p=0.014; DFS: p=0.005) and pM (OS: p=0.001; DSS: p<0.001; DFS: p<0.001) stadium were all significantly associated with survival parameters, while conventional histopathologic grade only displayed a comparatively weak association with OS (p=0.039), but not with DSS or DFS. Age and sex played no role in survival prediction (Table 1).
Correlation of immunological factors with survival parameters and clinical outcome
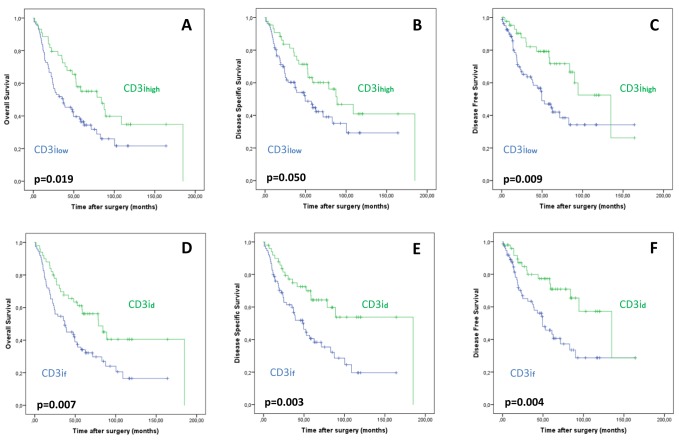
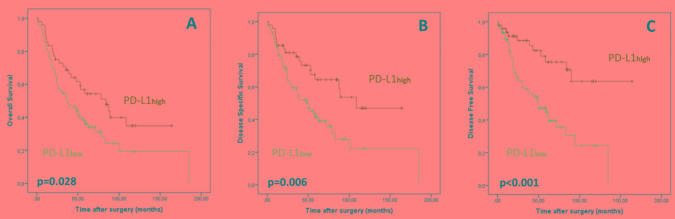
As demonstrated in Table 1 and Figure 2, univariate analysis revealed that patients with CD3ihigh tumors showed favorable OS (p=0.019), DSS (p=0.05) and DFS (p=0.009) compared to patients with low CD3is. While patients with CD3ihigh tumors displayed a mean OS of 96.4 months (DSS: 106.1 months; DFS: 104.9 months), mean OS (62.1 months) in cases harboring low CD3is was significantly shorter (DSS: 73.9 months; DFS: 78.1 months). Additionally, ESCC-patients with diffusely distributed CD3is displayed favorable OS (p=0.007), DSS (p=0.003) and DFS (p=0.004) (Figure 2). PD1ihigh cancers were associated with favorable OS (p=0.035). Pure CD8i count did not show prognostic impact. Overall density of TILs across the tumor area (including tumor cells and tumor stroma) had no impact on prognosis. As highlighted in Figure 3, PD-L1high ESCCs displayed improved OS (p=0.028), DSS (p=0.006) and DFS (p<0.001), while the extent of PD-L1+ TILs, PD-L1 staining intensity in TCs and PD-L1 copy number status were not associated with survival parameters. Multivariate survival analyses (including gender, age, pT, pN) revealed improved survival parameters for CD3ihigh tumors (OS: p=0.011; DSS: p=0.045; DFS: p=0.004), diffusely distributed CD3is (OS: p=0.01; DSS: p=0.005; DFS: p=0.009), diffusely distributed CD8is (OS: p=0.005; DSS: p=0.03; DFS: p=0.01) and PD-L1high ESCCs (OS: p=0.026; DSS: p=0.009; DFS: p=0.001). Details on multivariate survival analyses are given in Table 3 and Supplementary Table 2.
Figure 2.
Association of CD3ihigh status with improved overall A., disease-specific B. and disease-free C. survival. Correlation of diffuse (CD3id) and focal (CD3if) distribution of CD3i on overall D., disease-specific E. and disease-free F. survival.
Figure 3.
Association of PD-L1high status with improved overall A., disease-specific B. and disease-free C. survival.
Table 3. Multivariate analysis of the impact of CD3i – and PD-L1 status on overall survival.
| HR (OS) | lower CI (95%) | upper CI (95%) | p-value | ||
|---|---|---|---|---|---|
| Gender | male | 1.000 | 0.026 | ||
| female | 0.518 | 0.290 | 0.925 | ||
| Age | per year | 1.022 | 0.994 | 1.051 | 0.121 |
| pT | 1 | 1.000 | <0.001 | ||
| 2 | 3.345 | 1.918 | 5.834 | ||
| 3 | 1.693 | 0.875 | 3.276 | ||
| 4 | 2.068 | 0.223 | 19.211 | ||
| pN | 0 | 1.000 | 0.038 | ||
| 1 | 1.441 | 0.879 | 2.361 | ||
| 2 | 0.554 | 0.149 | 2.057 | ||
| 3 | 6.048 | 1.288 | 28.387 | ||
| CD3i | high | 1.000 | 0.011 | ||
| low | 1.925 | 1.159 | 3.200 | ||
| HR (OS) | lower CI (95%) | upper CI (95%) | p-value | ||
| Gender | male | 1.000 | 0.044 | ||
| female | 0.551 | 0.308 | 0.985 | ||
| Age | per year | 1.026 | 0.997 | 1.056 | 0.077 |
| pT | 1 | 1.000 | 0.001 | ||
| 2 | 3.025 | 1.736 | 5.272 | ||
| 3 | 1.723 | 0.893 | 3.325 | ||
| 4 | 2.962 | 0.343 | 25.556 | ||
| pN | 0 | 1.000 | 0.040 | ||
| 1 | 1.471 | 0.892 | 2.426 | ||
| 2 | 0.602 | 0.168 | 2.163 | ||
| 3 | 5.952 | 1.269 | 27.904 | ||
| CD3i | diffuse | 1.000 | 0.010 | ||
| Distribution | focal | 1.941 | 1.173 | 3.210 | |
| HR (OS) | lower CI (95%) | upper CI (95%) | p-value | ||
| Gender | male | 1.000 | 0.088 | ||
| female | 0.597 | 0.329 | 1.080 | ||
| Age | per year | 1.022 | 0.995 | 1.049 | 0.112 |
| pT | 1 | 1.000 | <0.001 | ||
| 2 | 3.415 | 1.978 | 5.895 | ||
| 3 | 1.956 | 1.013 | 3.777 | ||
| 4 | 5.081 | 0.529 | 48.760 | ||
| pN | 0 | 1.000 | 0.048 | ||
| 1 | 1.248 | 0.773 | 2.014 | ||
| 2 | 0.388 | 0.105 | 1.432 | ||
| 3 | 5.210 | 1.110 | 24.457 | ||
| PD-L1 TCs | high | 1.000 | 0.026 | ||
| low | 1.801 | 1.074 | 3.021 |
Additional ESCC subgrouping based on T-cell infiltration (CD3i status) and PD-L1 status revealed the CD3ilow / PD-L1low subgroup to be significantly associated with reduced OS (p=0.031), DSS (p=0.012) and DFS (p=0.001) compared to the other subgroups. Mean OS (DSS; DFS) of patients with CD3ilow/PD-L1low ESCC was 46.8 months (51.6 months; 53.8 months) compared to 76.9 months (117.4 months; 113.9 months) for patients with CD3ilow / PD-L1high tumors, 86.4 months (100.6 months; 130.3 months) for patients with CD3ihigh / PD-L1high cancers and 95.4 months (101.4 months; 77.4 months) for patients with CD3ihigh / PD-L1low ESCCs (Table 1; Figure 4). The observed impact on survival was confirmed by a subsequent multivariate analysis including age, gender and stage (OS p=0.031; DSS p=0.017; DFS p=0.002; Table 3; Supplementary Table 2).
Figure 4.
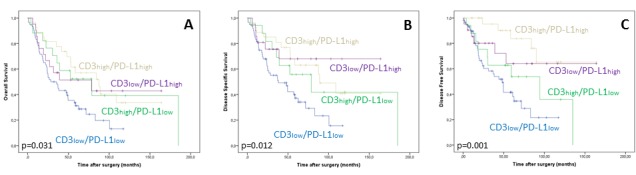
Decreased overall A., disease specific B. and disease free survival C. of the CD3ilow / PD-L1low ESCC subgroup.
DISCUSSION
In this study, we analyzed the composition of the IM in a comparatively large cohort of 125 primary resected, therapy-naive ESCCs and demonstrate CD3ihigh - as well as PD-L1high tumors not only to be significantly associated with one another, but also to be independent prognosticators of a beneficial ESCC disease course in uni- and multivariate statistical analyses.
Furthermore, an additional, immune-based ESCC stratification demarcated a prognostically unfavorable subgroup of CD3ilow / PD-L1low cancers.
High densities of CD3+ / CD8+ TILs have been associated with improved survival and a favorable disease course in various cancers [8–11, 14, 15, 27, 28] and high CD3i levels have been identified as strong and independent prognosticators for example in human colorectal [14] and ovarian cancer [16, 17]. CD3, a transmembranous protein virtually exclusive to the T-cell lineage, stains all T-cell subgroups, represents the gold standard for the assessment of overall T-cell infiltration in daily clinicopathological practice [29] and can therefore, in our opinion, be used to determine whether a given cancer is in the state of a so called “T - cell inflamed microenvironment” or not, a condition which has been postulated to be of crucial importance for the efficacy of immune checkpoint inhibitors [12, 13, 30]. In line with these findings, our data show a high count and a diffuse distribution of CD3is, but not of overall CD3+ TILs, to be prognostic of favorable OS, DSS and DFS in ESCC, suggesting that the assessment of CD3is is a comparatively specific and standardized approach to evaluate the T-cell mediated tumor host relationship, hypothesizing that intraepithelial lymphocytes are more likely to truly interact with cancer cells than their stromal counterparts. Surprisingly, to our knowledge, the prognostic and clinicopathologic influence of overall CD3+ TILs and especially of CD3is has not yet been studied in ESCC, as the few, and rather loosely connected studies regarding TILs in ESCC rather focused their efforts on studying potential effects of the PD-1 / PD-L1 axis [21, 24] or overall CD8+ TILs [21]. Interestingly, in contrast to previous work in ESCC [21], neither pure CD8i- or overall CD8+ TIL count displayed predictive relevance in our cohort, emphasizing that the complex interplay between distinctive T-cell subgroups and cancer cells is not sufficiently recognized through detection of CD8+ TILs alone [31].
Data on expression rates and prognostic value of PD-L1 immunohistochemistry in ESCC is rather scarce and partially conflicting, as some studies associate PD-L1 positive ESCC with poor patient outcome [24, 25, 32], while two recent studies [21, 22] demonstrated PD-L1 positivity to be predictive of a rather favorable disease course. Pointing in the same direction, our data, generated with a robust and highly specific PD-L1 (SP263) antibody [33] [34–36] in a comparatively large and extensively investigated, therapy naive tumor series, associate PD-L1high ESCC with favorable OS, DSS and DFS survival in uni- and multivariate analyses. However, comparability between ESCC studies is either hampered by small cohort sizes [25], varying PD-L1 antibodies and cutoffs, or differences in the amount of investigated cancerous tissue [21, 24, 25, 32]. Noteworthy, PD-L1 copy number analysis revealed PD-L1 amplifications to be a far less frequent event in ESCC (2%) than in squamous cell carcinoma of the oral cavity (19%) [37], indicating an intertumoral spread width regarding the biologic mechanisms underlying PD-L1 expression between primary squamous cell carcinomas of different localizations [38]. Furthermore, PD-L1 staining intensity showed no predictive value in our ESCC tumor series.
Besides in ESCC, the association of immunohistochemical PD-L1 expression to a beneficial outcome has been observed in several entities like colorectal carcinoma [39], non-small cell lung cancer [40], melanoma [41], Merkel cell carcinoma [42] or breast cancer [43], although the mechanisms underlying the observed positive effects remain poorly understood. Nevertheless, as PD-L1high ESCC was significantly associated with a concurrent CD3ihigh, CD8ihigh and PD1ihigh status in our cohort, one might hypothesize that high PD-L1 expression in ESCC might rather be interpreted as an adaptive mechanism of a given cancer in response to an immunoactive tumor-host relationship [41], that could therefore contribute to an improved disease course and speculatively, to a potential response towards immune checkpoint therapy [44].
Taking our analyses one step further, we stratified our ESCC cohort into four immunogenic subgroups based on their PD-L1 / CD3i status. Interestingly, our subgrouping approach, which represents a slightly adjusted version of the TIL/PD-L1 based cancer classification proposed by Teng et al. [13], unmasked a comparatively large subgroup of “non-immunogenic”, CD3ilow / PD-L1low ESCCs (48% of all tumors), which were significantly associated with reduced survival parameters in uni- and multivariate analyses, while certain tendencies, but no distinct differences in patient survival were observed between the other subgroups. Although no clear prognostic separation of the CD3ihigh / PD-L1high subgroup was visible, we believe that these stratification data are concordant with our general findings and with those from certain previous studies [21, 22], as they prognostically segregate those tumors that show at least partial immunoreactivity in a certain manner from those who find themselves in a completely “non-immunogenic” state.
Our work has some limitations: (1) our investigations were performed on TMA-basis, although we examined a substantial number of tumor cores deriving from the diverse tumor compartments (apical and central tumor region, invasive margin). Furthermore (2), only resection specimens (3) without postoperative checkpoint blockade were investigated. As our work is retrospective in nature, our data need to be validated in larger, prospective ESCC cohorts as well as on biopsy material. Furthermore, the suitability of PD-L1 IHC, intraepithelial CD3+ TILs and the subsequent immunologic ESCC subgroups as a potential rationale for immune checkpoint therapy regimens clearly needs to be investigated by subsequent clinical studies in appropriate patient cohorts.
Summarizing, we investigated the contexture of ESCCs IM and demonstrate increased intraepithelial CD3+ TILs and high PD-L1 expression on tumor cells to be independent predictors of a beneficial clinical outcome in ESCC. This study not only highlights the comparatively frequent expression of PD-L1 in this tumor entity and underlines the close association of PD-L1 positive ESCCs with increased numbers of intraepithelial T-lymphocytes, but also identifies a subset of non-immunogenic ESCCs with a distinct clinical course, potentially forming the basis for an immune-based stratification in this tumor entity.
MATERIALS AND METHODS
Cohort recruitment
Our tumor series comprised 125 ESCC patients, who underwent surgical resection between 1994 and 2007 at Klinikum Rechts der Isar of the Technical University of Munich, Germany and at the University Hospital Heidelberg, Germany. All tumors were chemo-/radiation-naive at the time of resection and none of the patients received immune checkpoint inhibitors or underwent other immune therapy regimens. Only tissue from primary tumors was investigated. Hematoxylin-Eosin (H&E) stained sections were initially reviewed by two pathologists (MJ, MB), who confirmed the diagnosis of ESCC. Grading and staging of ESCC at the time of diagnosis was performed according to the current World Health Organization Classification of Tumors of the Digestive System [26] and the UICC tumor, node, metastasis (TNM) classification [45]. Additional clinicopathologic characteristics (sex, age, tumor localization, local, nodal / distant relapse) and established survival parameters such as overall survival (OS), disease-specific survival (DSS) and disease-free survival (DFS) were collected for all patients. This study has been approved by the Ethics Review Committee of the Technical University of Munich (503/16 S).
Tissue microarray construction
Formalin-fixed paraffin-embedded (FFPE) tumor samples from the central and apical tumor region as well as from the invasive margin were assembled into a tissue microarray (TMA) using a Tissue Microarrayer (Beecher Instruments, Sun Praierie, USA) with a core size of 0.6 mm. All samples of a respective tumor region (central/apical tumor region, invasive margin) were extracted from areas harboring a high tumor/stroma ratio. Mean tumor cell content per core was 79.3%, ranging from 40% - 99 %. Obvious inflammatory hotspots (such as lymph follicles or areas of ulceration) were avoided. Considering tumor heterogeneity, a minimum of 3 and (where feasible) up to 6 tumor cores were taken from the primary tumors in areas previously marked by five pathologists (MJ, MB, JS, ED, RL).
Immunohistochemistry
IHC was performed on 2 μm sections from each TMA using a PD-L1 primary antibody (VENTANA, clone (c): SP-263; dilution (d): 1:100), a PD-1 primary antibody (Cell Marque, c: 11RQ-22, d: 1:50) and primary antibodies against CD3 (Cell Marque, c: MRQ39, d: 1:500) and CD8 (DAKO, c: C8/144B, d: 1:50), using an automated immunostainer with an iVIEW DAB detection kit (Ventana Medical Systems, Roche, Mannheim, Germany).
Scoring of PD-1+, CD3+ and CD8+ tumor infiltrating lymphocytes
Scoring of TILs (PD-1+, CD3+, CD8+) was performed jointly by two pathologists (MJ, MB) blinded to clinicopathological outcome. In general, TILs were separately evaluated in every core of the TMA and an average score resulting from all cores (and all tumor regions) was assigned as the final TIL count for the respective case. The analysis of TIL- subpopulations was performed in two ways: (1) Scoring of TILs was performed in the tumor region of the respective core showing the highest density of the particular TIL subpopulation on low power magnification (4x). Within this region, the amount of intraepithelial CD3+ TILs (CD3i), intraepithelial CD8+ TILs (CD8i) and intraepithelial PD-1+ TILs (PD1i) was scored manually by counting the quantity of the respective lymphocyte subpopulation within tumor cell clusters of 100 tumor cells using high power magnification (40x). (2) In analogy to previous TIL-scoring approaches [46, 47] overall density of TILs was evaluated via determination of the percent proportion of the tumor area occupied by the respective TIL subpopulations. The tumor area was defined as tumor cells (TCs) and tumor stroma, while areas of tumor necrosis or inflamed peritumoral areas were not taken into account. All corresponding tumor cores of each case were analyzed and the average density across all cores was calculated. The infiltration pattern of (CD3+/CD8+) TILs was classified as diffuse, if the respective neoplasms showed a continuous intraepithelial infiltration, whereas a patchy, discontinuous infiltrate was reported as focal.
Immunohistochemical scoring of PD-L1 in tumor cells and tumor infiltrating lymphocytes
PD-L1 expression in TCs and in TILs was jointly determined by two pathologists (MJ, MB) and was evaluated separately in every TMA core before a final score resulting from all investigated cores (and all tumor regions) was assigned. In TCs, the absolute percentage of positive cells was determined. Only membranous staining patterns were scored as positive. The intensity of PD-L1 staining was scored using a 4-tiered grading system established in a recent study regarding the efficacy of the used PD-L1 SP263 antibody [33], including “no staining” (0), “weak staining” (1+), “intermediate staining” (2+) and “strong staining” (3+). Immunohistochemical expression of PD-L1 in TILs was assessed via a determination of the percent proportion of the tumor area occupied by PD-L1+ TILs, as described previously [47]. Since no obvious differences in staining intensity were noted, staining intensity of PD-L1 in TILs was not taken into account.
Determination of immunohistochemical scoring groups / cut offs
Following the general scoring process, the respective data were quantified regarding their respective percentile value and patients were stratified into three subgroups: lower (below 33. percentile), intermediate (33.-66. percentile) and upper third (exceeding 66. percentile). For further analyses, cases within the upper third were classified as “high”, whereas all cases ranging within the intermediate or the lower third were classified as “low”.
PD-L1 Fluorescence in situ Hybridization
FFPE tissue sections (thickness 2μm) of TMAs were used for dual-color FISH analysis using a SPEC CD274, PDCD1LG2/CEP9 dual color probe and PD-L1 copy number status (CNS) was evaluated as described previously counting at least 20 tumor cell nuclei per tumor core [37]. PD-L1 amplification was defined as PD-L1/CEP9 ratio ≥2.0, with high level amplification defined as ≥4.0 and low level amplification defined as ≥2.0 and ≤4.0. Polysomy 9 was defined as average PD-L1 copy number >3 signals/cell. PD-L1 deletion was defined as PD-L1/CEP9 ratio <0.8.
Statistical analyses
Statistical analyses were performed using SPSS 23 (SPSS Inc, Chicago, IL, USA). Correlations between immunologic characteristics and clinicopathological parameters were calculated using Χ2 test and Fisher's exact test. Associations of immunologic factors with each other were calculated with Spearman's rank order correlation. Survival probabilities were plotted using the Kaplan-Meier method and a log-rank test was used to probe the significance of differences in survival probabilities. Multivariate survival analysis was performed utilizing the Cox proportional hazard model. All significances were two-sided, p-values ≤0.05 were considered significant.
SUPPLEMENTARY MATERIALS FIGURE AND TABLE
Acknowledgments
The authors thank Klara Fizi for excellent technical assistance.
Abbreviations
- CD3ihigh
High intraepithelial CD3+ tumor infiltrating lymphocytes
- CD8ihigh
High intraepithelial CD8+ tumor infiltrating lymphocytes
- DSS
Disease specific survival
- DFS
Disease free survival
- ESCC
Esophageal squamous cell carcinoma
- FISH
Fluorescence in situ Hybridization
- FFPE
Formalin-fixed paraffin-embedded
- IM
immunogenic microenvironment
- OS
Overall survival
- PD1ihigh
High intraepithelial PD1+ tumor infiltrating lymphocytes
- PD1
Programmed Death-1
- PD-L1
Programmed Death – Ligand 1
- TC
Tumor cells
- TIL
Tumor infiltrating lymphocyte
- TMA
Tissue micro array
Author contributions
Moritz Jesinghaus and Melanie Boxberg designed this study and wrote this paper with assistance from Katja Specht, Björn Konukiewitz and Wilko Weichert. Marcus Feith, Kathrin Wieczorek, Katja Ott, Julia Slotta-Huspenina and Enken Drecoll helped gain clinical data. Moritz Jesinghaus and Melanie Boxberg performed tissue analyses with assistance from Petra Mayer, Nicole Pfarr, Marcus Bettstetter, Rupert Langer and Katja Steiger. Statistical analyses were performed by Moritz Jesinghaus and Melanie Boxberg with assistance from Wilko Weichert.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
FUNDING
This study has been supported by the Else-Kröner Fresenius Stiftung (to MJ and MB).
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149:1700–15. doi: 10.1053/j.gastro.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 3.Chen YB, Jia WH. A comprehensive genomic characterization of esophageal squamous cell carcinoma: from prognostic analysis to in vivo assay. Chin J Cancer. 2016;35:76. doi: 10.1186/s40880-016-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennathur A, Farkas A, Krasinskas AM, Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ, Luketich JD. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048–1054. doi: 10.1016/j.athoracsur.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 6.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–87. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 7.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Fridman WH, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–86. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–64. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383–91. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–79. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Čermáková P, Melichar B, Tomšová M, Zoul Z, Kalábová H, Spaček J, Doležel M. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in patients with endometrial carcinoma. Anticancer Res. 2014;34:5555–61. [PubMed] [Google Scholar]
- 16.Tomsová M, Melichar B, Sedláková I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–20. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Bosmuller HC, Wagner P, Peper JK, Schuster H, Pham DL, Greif K, Beschorner C, Rammensee HG, Stevanovic S, Fend F, Staebler A. Combined Immunoscore of CD103 and CD3 Identifies Long-Term Survivors in High-Grade Serous Ovarian Cancer. Int J Gynecol Cancer. 2016;26:671–679. doi: 10.1097/IGC.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–39. doi: 10.2147/OTT.S105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunturi A, McDermott DF. Nivolumab for the treatment of cancer. Expert Opin Investig Drugs. 2015;24:253–60. doi: 10.1517/13543784.2015.991819. [DOI] [PubMed] [Google Scholar]
- 20.Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J Immunother Cancer. 2015;3:36. doi: 10.1186/s40425-015-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatogai K, Kitano S, Fujii S, Kojima T, Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T, Ochiai A. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget. 2016;7:47252–64. doi: 10.18632/oncotarget.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang D, Song Q, Wang H, Huang J, Wang H, Hou J, Li X, Xu Y, Sujie A, Zeng H, Tan L, Hou Y. Independent prognostic role of PD-L1expression in patients with esophageal squamous cell carcinoma. Oncotarget. 2017;8:8315–29. doi: 10.18632/oncotarget.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang J, Wu C. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol. 2014;7:6015–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen MF, Chen PT, Chen WC, Lu MS, Lin PY, Lee KD. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. 2016;7:7913–24. doi: 10.18632/oncotarget.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 26.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. World Health Organization; 2010 [Google Scholar]
- 27.Krpina K, Babarovic E, Jonjic N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Arch. 2015;467:443–448. doi: 10.1007/s00428-015-1808-6. [DOI] [PubMed] [Google Scholar]
- 28.Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, Perisanidis C, Kontos CK, Giotakis AI, Scorilas A, Rimm D, Sasaki C, Fountzilas G, Psyrri A. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22:704–713. doi: 10.1158/1078-0432.CCR-15-1543. [DOI] [PubMed] [Google Scholar]
- 29.Chetty R, Gatter K. CD3: structure, function, and role of immunostaining in clinical practice. J Pathol. 1994;173:303–07. doi: 10.1002/path.1711730404. [DOI] [PubMed] [Google Scholar]
- 30.Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu YX. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;29:285–96. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 32.Leng C, Li Y, Qin J, Ma J, Liu X, Cui Y, Sun H, Wang Z, Hua X, Yu Y, Li H, Zhang J, Zheng Y, et al. Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8+ T cells. Oncol Rep. 2016;35:699–708. doi: 10.3892/or.2015.4435. [DOI] [PubMed] [Google Scholar]
- 33.Smith J, Robida MD, Acosta K, Vennapusa B, Mistry A, Martin G, Yates A, Hnatyszyn HJ. Quantitative and qualitative characterization of two PD-L1 clones: SP263 and E1L3N. Diagn Pathol. 2016;11:44. doi: 10.1186/s13000-016-0494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebelatto MC, Midha A, Mistry A, Sabalos C, Schechter N, Li X, Jin X, Steele KE, Robbins PB, Blake-Haskins JA, Walker J. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016;11:95. doi: 10.1186/s13000-016-0545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, Al-Masri H, Rebelatto M, Walker J. Agreement between Programmed Cell Death Ligand-1 diagnostic assays across multiple protein expression cut-offs in non-small cell lung cancer. Clin Cancer Res. 2017 Jan 10; doi: 10.1158/1078-0432.CCR-16-2375. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, Shi K, Soria JC. A Phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17:232–236.e1. doi: 10.1016/j.cllc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Straub M, Drecoll E, Pfarr N, Weichert W, Langer R, Hapfelmeier A, Götz C, Wolff KD, Kolk A, Specht K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget. 2016;7:12024–34. doi: 10.18632/oncotarget.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel SP, Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–56. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 39.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–42. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt LH, Kümmel A, Görlich D, Mohr M, Bröckling S, Mikesch JH, Grünewald I, Marra A, Schultheis AM, Wardelmann E, Müller-Tidow C, Spieker T, Schliemann C, et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One. 2015;10:e0136023. doi: 10.1371/journal.pone.0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, Anders RA, Topalian SL, Taube JM. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, Holliday A, Cooper CL, Robbins E, Gillett D, Kennedy CW, Gluch L, Carmalt H, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69:25–34. doi: 10.1111/his.12904. [DOI] [PubMed] [Google Scholar]
- 44.Dong ZY, Wu SP, Liao RQ, Huang SM, Wu YL. Potential biomarker for checkpoint blockade immunotherapy, treatment strategy. Tumour Biol. 2016;37:4251–4261. doi: 10.1007/s13277-016-4812-9. [DOI] [PubMed] [Google Scholar]
- 45.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7th edition. Hoboken, New Jersey: John Wiley & Sons; 2011. p. 336. [Google Scholar]
- 46.Buisseret L, Garaud S, de Wind A, Van den Eynden G, Boisson A, Solinas C, Gu-Trantien C, Naveaux C, Lodewyckx JN, Duvillier H, Craciun L, Veys I, Larsimont D, et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. OncoImmunology. 2016;6:e1257452. doi: 10.1080/2162402X.2016.1257452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.