Abstract
Background
Alcohol use is a risk factor for non-adherence to antiretroviral therapy (ART) among people living with HIV/AIDS (PLWHA); however, differences in ART adherence across levels of alcohol use are unclear. The current study examined whether “at-risk” alcohol use, defined by National Institute of Alcohol Abuse and Alcoholism guidelines, was associated with ART non-adherence among PLWHA.
Methods
Participants were 535 HIV-infected adults enrolled in studies at the HIV Neurobehavioral Research Program. ART non-adherence was identified by either self-reported missed dose or plasma viral load detectability (≥50 copies/mL). Potential covariates for multivariable logistic regression included demographics, depression, and substance use disorders.
Results
Using a stepwise model selection procedure, we found that at-risk alcohol use (OR = 0.64; p = 0.032) and low education (OR = 1.09 per one year increase in education; p = 0.009) significantly predict lower ART adherence.
Conclusion
A greater focus on the treatment of at-risk alcohol use may improve ART adherence among HIV-infected persons.
Keywords: HIV/AIDS, At-risk drinking, Adherence, Antiretroviral therapy
INTRODUCTION
Combination antiretroviral therapy (ART) has allowed people living with HIV/AIDS to maintain a high quality of life and achieve life expectancies near those without HIV (Palella Jr et al., 1998). The optimal benefits of ART, however, cannot be attained unless persons living with HIV/AIDS (PLWHA) are able to adhere to ART as prescribed (Bartlett, 2002). Non-adherence to ART is associated with poorer HIV suppression, decreased CD4 cell count, and an increased risk for antiretroviral drug resistance (Bangsberg et al., 2007, Bartlett, 2002). In addition to their impact on individual disease progression and health status, these consequences of ART non-adherence can also increase the likelihood of HIV transmission. For these reasons, accurate treatment monitoring with the goal of optimizing ART adherence is an issue of major relevance to the clinical care of PLWHA.
Alcohol use is common among PLWHA, with an estimated prevalence of current heavy drinking of almost twice the rate of the general population (Galvan et al., 2002). A recent national cohort study found that nearly half of PLWHA have a lifetime history of an alcohol use disorder (Byrd et al., 2011). Excessive alcohol exposure in the context of HIV disease has been linked to higher viral replication and accelerated disease progression (Hendershot et al., 2009, Samet et al., 2007, Bryant, 2006). Although the mechanisms underlying the impact of alcohol use on HIV disease remain under investigation, ART adherence is likely an important contributory factor (Hahn and Samet, 2010). For example, one study examining the relationship between alcohol use and HIV disease progression found no direct association between alcohol consumption and CD4 cell count in participants receiving ART when controlling for ART adherence (Samet et al., 2007). Another recent study also failed to show a direct relationship between alcohol use and viral load detectability when controlling for other factors (Kalichman et al., 2014).
The most robust evidence of the association between alcohol use and ART non-adherence in the literature finds that those who report any alcohol use are significantly more likely to be non-adherent compared to those who completely abstain from drinking (Chander et al., 2006, Golin et al., 2002, Samet et al., 2004), regardless of the amount of alcohol used (i.e., level of alcohol use). Although there is research examining the influence of level of alcohol use on medication adherence, results are inconsistent. Some studies have found significant associations between level of alcohol use and medication adherence (i.e., as level of alcohol use increases, medication adherence decreases; Braithwaite et al., 2005, Catz et al., 2001), while others have found no difference in medication adherence between those with different levels of alcohol use (Kleeberger et al., 2001, Samet et al., 2004). These studies have also measured level of alcohol use in many different ways. Some studies used continuous variables such as frequency of alcohol drinking (i.e., number of days used) and quantity of drinks (Catz et al., 2001, Heckman et al., 2004, Johnson et al., 2003), whereas others used categorical variables indicating the presence of an alcohol use disorder (AUD) (Hinkin et al., 2004, Peretti-Watel et al., 2006, Azar et al., 2010), binge-drinking (often defined as >5 drinks per occasion) (Berg et al., 2004, Moss et al., 2004), and risky drinking, which is often defined by a certain quantity of drinks per day or per week, though this quantity is also inconsistent across studies (Braithwaite et al., 2008, Braithwaite et al., 2005, Kleeberger et al., 2001, Samet et al., 2004).
A meta-analysis, attempting to shed light on these inconsistencies among PLWHA, found that individuals who either meet probable current AUD diagnosis, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) criteria for at-risk drinking were about half as likely to be adherent to medications compared to otherwise (Hendershot et al., 2009). A recent systematic review also consistently found that AUD diagnosis was associated with decreased ART adherence across studies; however, AUD diagnostic criteria (e.g., hazardous drinking, heavy drinking, binge drinking) differed across studies (Azar et al., 2010). These findings still do not clearly parse apart different levels of alcohol use in association with ART adherence in the context of those who use alcohol.
The current study examined the association between at-risk alcohol use (National Institue on Alcohol Abuse and Alcoholism, 2005) and ART adherence among PLWHA who reported drinking at least once in the last 30 days. By excluding non-drinkers, this study attempted to elucidate whether at-risk drinkers were more likely to be ART non-adherent compared to those who drank less (i.e., not at-risk drinkers). Importantly, we examined the association of alcohol use and ART adherence in the context of other important variables such as use of other substances (e.g., methamphetamine, cocaine), major depressive disorder (MDD), global cognitive function. We hypothesized that PLWHA who are at-risk drinkers will have lower ART adherence than PLWHA who are not at-risk drinkers, and that at-risk alcohol use will still be significantly predictive of ART adherence when controlling for substance use, MDD, and global cognitive function.
MATERIALS AND METHODS
Participants
Participants consisted of 535 HIV-infected adults enrolled in NIH-funded research studies at the University of California, San Diego (UCSD) HIV Neurobehavioral Research Program (HNRP) from 2003 to 2015. The current study is a secondary analysis of existing data from each participant’s baseline visit at the HNRP. All participants were receiving ART at the time of the visit and reported drinking alcohol in the previous 30 days.
Measures
Non-adherence to ART was determined based on 1) response to part of the self-report AIDS Clinical Trial Group (ACTG) Questionnaire indicating any missed ART doses in the previous four days, or 2) detectable plasma HIV RNA (≥50 copies/mL) (Mekuria et al., 2016, Nieuwkerk and Oort, 2005). The viral load assay was performed the same day as the alcohol assessment.
Alcohol use was measured using the HNRP Substance Use History form, which is a timeline follow-back measure of the amount of daily alcohol used in the previous 30 days. Participants were stratified based on the daily limit defined by the NIAAA guideline (National Institute on Alcohol Abuse and Alcoholism, 2005) for individuals at risk for developing an AUD: >3 drinks per day for women and >4 drinks per day for men, hereafter referred to as “at-risk drinking.” Individuals who consume more than these daily limits are at a near fourfold increase risk of developing alcohol abuse and a sevenfold increase risk of developing alcohol dependence compared to those who drink within those limits (Dawson et al., 2008). Current and lifetime substance use disorders and major depressive disorder were identified via the Composite International Diagnostic Interview (CIDI) (World Health Organization, 1997). Global cognitive function was measured via a standardized battery of well-validated neurocognitive tests [see Heaton et al. (2011) for descriptions of tests in this battery]. For all neurocognitive measures, raw scores were converted to demographically adjusted T scores used to construct one summary Global Mean T score.
Statistical Methods
Unadjusted association between at-risk drinking and adherence was assessed using simple logistic regression. A multivariable logistic model was used to regress the adherence measure on at-risk drinking adjusted for covariates. Potential covariates included demographic characteristics (age, education, sex, ethnicity); current AUD and current cannabis use; lifetime AUD; lifetime substance use disorder (SUD) for cannabis, cocaine, hallucinogens, inhalants, methamphetamine, opioids, phencyclidine (PCP), sedatives, and other substances combined; current and lifetime major depressive disorder (MDD); global cognitive function; estimated years of HIV infection; and ART regimen type. Current use disorders for substances other than alcohol and cannabis were not considered in this analysis because of low frequencies (i.e., <5) of participants with such diagnoses. Covariates were considered for inclusion into a multivariable model if they either showed an association with the adherence measure (via logistic regression) or differed between drinking level groups at a 0.10 significance level. Group comparisons were done with two-sample t-tests for numeric covariates and chi-square or Fisher’s exact tests for categorical covariates. Backward model selection was applied and the final model was chosen to include only the predictor of primary interest (i.e., at-risk drinking) and the covariates with p-values less than 0.05. Odds ratio (OR) was used as the effect size for the strength of these associations, such that OR>1 would indicate a predictor’s association with higher odds of adherence. All analyses were performed using R version 3.2.1 statistical software (R Core Team, 2015).
RESULTS
The cohort consisted of mostly men (86%) with ages between 20 and 74 years. Seventy six percent (N=405) of participants had a history of AIDS, and median current CD4 was 412 cells/mm3 (interquartile range (IQR) 240–608). The median duration of exposure to ART was 63 months (IQR 28–105). Seventy-nine participants (14.8%) endorsed missing an ART dose in the last four days, 46 of whom also had detectable plasma viral load. An additional 192 participants (35.9%) had detectable plasma viral load without reporting a missed ART dose in the last four days. Based on our criteria determining ART non-adherence (i.e., endorsement of missing ART dose in the last four days, or detectable plasma viral load), 271 participants (50.7%) were identified as ART non-adherent. See Table 1 for a full list of sample characteristics.
Table 1.
Sample characteristics (N=535).
| Variable | Mean (SD) or Median [Q1, Q3] or N (%) |
|---|---|
| Age (years) | 44.2 (8.6) |
| Education (years) | 13 (2.7) |
| Sex (% Male) | 85.6% |
| Ethnicity | 234 (43.7%) |
| White | 227 (42.4%) |
| Black | 74 (13.8%) |
| Other | |
| Detectable plasma viral load | 238 (45.5%) |
| Endorsed missing ART dose | 79 (14.8%) |
| AIDS status | 405 (75.7%) |
| Current CD4 | 412 [240, 608] |
| Nadir CD4 | 107 [23, 208] |
| Months ART exposure | 62.6 [4, 29] |
| Months current regimen | 11.3 [4, 29] |
| Current MDD | 78 (14.6%) |
| Lifetime MDD | 271 (50.7%) |
Approximately a quarter of the cohort met the criterion for at-risk drinking (N=133). Table 2 shows comparisons of demographic, psychiatric, and substance use characteristics between the two drinking level groups. Participants meeting the criterion for at-risk drinking were younger, less educated, and more likely to be diagnosed with lifetime alcohol use and lifetime cocaine use disorders.
Table 2.
Covariates by drinking level group (N=535).
| Covariate | Not At-Risk Drinking Group (N=402) |
At-Risk Drinking Group (N=133) |
P-value |
|---|---|---|---|
| Age (years)1 | 44.6 (8.8) | 42.9 (7.5) | 0.034 |
| Education (years)1 | 13.2 (2.8) | 12.3 (2.3) | <0.001 |
| Male | 86% | 85% | 0.81 |
| Ethnicity | 0.39 | ||
| White | 45.8% | 37.6% | |
| Black | 41% | 46.6% | |
| Hispanic | 10.7% | 12% | |
| Other | 2.5% | 3.8% | |
| Current MDD | 14% | 17% | 0.55 |
| Lifetime MDD | 50% | 53% | 0.53 |
| Estimated years of HIV infection | 11.4 (6.9) | 10.0 (5.9) | 0.032 |
| ART regimen type | 0.51 | ||
| NRTI/PI | 51.7% | 54.9% | |
| NRTI/NNRTI | 36.6% | 36.8% | |
| Other2 | 11.7% | 8.3% | |
| Global Mean T score1 | 46.4 (6.7) | 45.8 (6.7) | 0.40 |
| Substance Use Disorders | |||
| Current Alcohol | 2% | 3% | 0.75 |
| Lifetime Alcohol | 52% | 71% | <0.001 |
| Current Cannabis | 1% | 4% | 0.067 |
| Lifetime Cannabis | 27% | 32% | 0.27 |
| Lifetime Cocaine | 36% | 50% | 0.005 |
| Lifetime Hallucinogen | 5% | 8% | 0.52 |
| Lifetime Inhalant | 2% | 5% | 0.15 |
| Lifetime Methamphetamine | 13% | 19% | 0.17 |
| Lifetime Opioid | 10% | 12% | 0.55 |
| Lifetime PCP | 1% | 3% | 0.28 |
| Lifetime Sedatives | 5% | 10% | 0.10 |
| Lifetime Other | 2% | 0% | 0.21 |
Values are given as mean and standard deviation
Includes less common (<5% of sample) ART regimen types (i.e., other combinations of the six ART drug classes)
MDD = major depressive disorder; NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; PCP = phencyclidine.
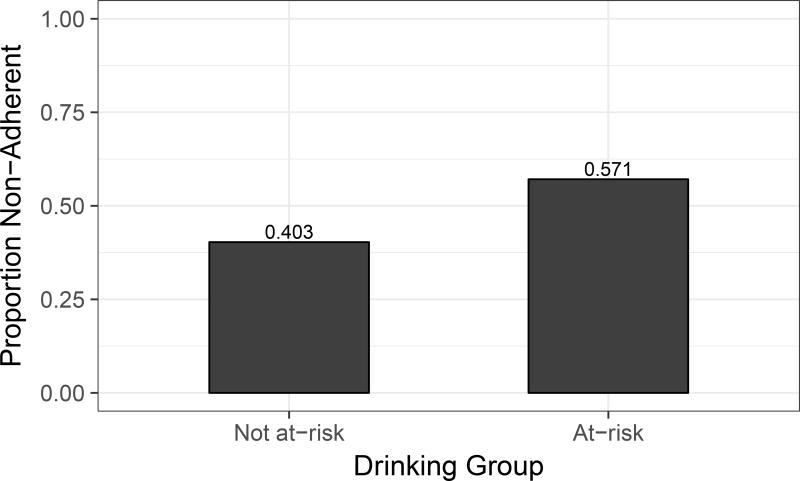
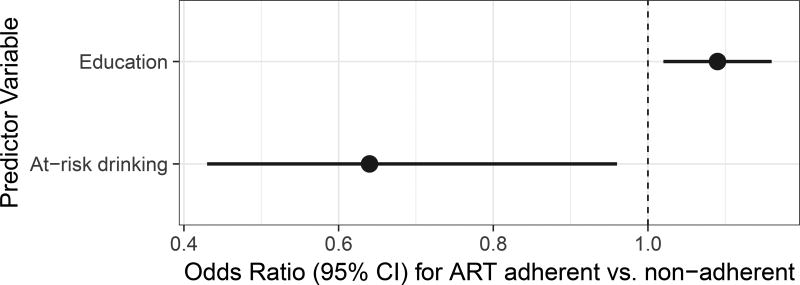
Table 3 lists results of univariable and multivariable analyses. In unadjusted analyses, the at-risk drinking group was associated with significantly lower odds of adherence (OR=0.60, p=0.012). 57.1% (N=76) of the participants in the at-risk drinking group were identified as non-adherent compared to 40.3% (N=162) of the participants in the not at-risk drinking group (see Figure 1). Among covariates, older age, greater education, absence of lifetime cocaine substance use disorder and absence of lifetime MDD were associated with greater adherence at a 0.10 significance level. These covariates were included into multivariable model selection along with three additional covariates that differed by group at a 0.10 level (estimated years of HIV infection, lifetime AUD, current cannabis SUD, and lifetime sedatives SUD). Following the stepwise procedure, the final model included only at-risk drinking and education. The effects for the remaining covariates did not achieve a 0.05 significance level in the multivariable analysis. The adjusted effect of at-risk drinking remained significant (OR=0.64, p=0.032). Education was the only other significant predictor, with OR=1.09, p=0.009 per one year increase. See Figure 2 for odds ratios and corresponding confidence intervals for at-risk drinking and education in the final model.
Table 3.
Results of univariable (unadjusted) and multivariable (adjusted) logistic regressions for predicting adherence.
| OR (95% CI) | P-value | |
|---|---|---|
| Unadjusted Analysis | ||
| At-risk Drinking Level1 | 0.60 (0.40, 0.89) | 0.012 |
| Adjusted Analysis | ||
| At-risk Drinking Level1 | 0.64 (0.43, 0.96) | 0.032 |
| Education (1 year increase) | 1.09 (1.02, 1.16) | 0.009 |
Compared to the not at-risk drinking level
OR = odds ratio; CI = confidence interval.
Figure 1.
Figure 2.
DISCUSSION
Understanding the factors associated with ART non-adherence is critical for developing strategies to improve patient outcomes for those living with HIV/AIDS. Although alcohol use is broadly associated with worse ART adherence among PLWHA, there is little existing literature on the level of alcohol use that portends ART non-adherence. Results of the current study showed that persons with lower education and persons who meet the NIAAA criteria for at-risk drinking, which indicate consumption of a high level of alcohol per day, are more likely to be ART non-adherent compared to not-at-risk drinkers.
This finding is consistent with the studies that have found a positive relationship between level of alcohol use and non-adherence to medications in samples of HIV-positive (HIV+) and HIV-negative (HIV−) participants (Braithwaite et al., 2005, Catz et al., 2001). The most notable of those found that regardless of HIV status, binge drinking veterans (≥5 drinks in one day) were more likely to be non-adherent to medications on drinking days, post-drinking days, and non-drinking days compared to non-binge drinking veterans (Braithwaite et al., 2005). They also found a temporal association within each drinking level group, meaning that participants were most likely to be non-adherent to medications on drinking days, followed by post-drinking days and non-drinking days. For non-binge drinkers, this trend was significantly stronger in HIV+ participants compared to matched HIV− participants. For binge drinkers, the strength of the trend was comparable for HIV+ and HIV− participants, suggesting that PLWHA may be more susceptible to medication non-adherence at lower levels of alcohol consumption compared to HIV− counterparts.
The current study supports and augments these previous findings by demonstrating a similar alcohol-adherence association using the widely accepted NIAAA guideline that defines a daily level of alcohol consumption that puts individuals at risk for developing an AUD. Research has shown that individuals who drink within the NIAAA limits are at a much lower risk of having an AUD (i.e., about 1%) compared to those who drink beyond those limits (i.e., between 20% and 50%; US Department of Health and Human Services, 2005). These standard NIAAA criteria are easy to assess and are a consistent method of stratifying alcohol use severity. The criteria for binge drinking, on the other hand, are defined differently by different governmental organizations (i.e., NIAAA criteria versus Substance Abuse and Mental Health Administration [SAMHSA] criteria), welcoming inconsistencies across studies that use binge drinking to stratify drinking level groups. The current study demonstrates that the daily limit defined by the NIAAA guideline for at-risk drinking provides a meaningful demarcation of drinking level in the context of ART adherence among PLWHA.
Notably, after consideration of all potential covariates, education was the only other significant predictor in the final logistic regression model besides drinking level. The results showed that at-risk drinking was a better predictor of ART adherence than lifetime cocaine use and methamphetamine use. Although cocaine and methamphetamine use have been shown to have associations with ART adherence (Malta et al., 2008, Moore et al., 2012a), there are several potential reasons why the results of this study found these relationships non-significant. First, alcohol is more widely used in our sample, as the studies conducted at the HNRP generally exclude participants who are current users of drugs other than alcohol and marijuana. Second, it is more socially and societally acceptable to report high levels of alcohol use than it is to report use of cocaine and methamphetamine. Last, we used a more informative variable for alcohol use (i.e., drinks per day) compared to our variables for cocaine use and methamphetamine use (i.e., lifetime SUD). That is, the results presented here do not necessarily point to alcohol use as more detrimental to ART adherence than use of other substances. In fact, polysubstance use was very high in our sample and level of alcohol use was found to be significantly associated with lifetime cocaine SUD. A potential future direction may be to more closely examine polysubstance use in the context of level of use for each substance.
Despite the lack of significant associations between ART adherence and use of other substances, education showed a strong association to ART adherence. Previous research suggests that low education may be correlated with decreased ART adherence because each is associated with health literacy (Kalichman et al., 1999, Kalichman and Rompa, 2000). Health literacy is defined as the ability to process and understand information about health and health services in order to make the best health-related decisions (Patient Protection and Affordable Care Act, 2010). Studies of health literacy among PLWHA have shown that higher education is associated with increased health literacy (Kalichman et al., 2000) and that greater health literacy is associated with health status and ART adherence (Kalichman et al., 1999, Kalichman and Rompa, 2000). The results of the current study may suggest that PLWHA with higher education may be more likely to understand and appreciate the importance of staying adherent to their ART regimen, even in the context of at-risk drinking. Education and health literacy among PLWHA who drink alcohol, however, has yet to be studied on its own. This is an important topic for future research and subsequent development of better interventions for improving health status among PLWHA, as level of education is immutable but health literacy can be easily enhanced.
There are several strengths of the current study including the large sample size and a relatively large group of at-risk drinkers; however, this study also has some limitations. First, alcohol use data are self-reported. The HNRP Substance Use History form is a retrospective self-report assessment that relies on participants’ memory of past drinking patterns. Although most studies rely on self-report to measure alcohol use, requiring participants to give day-by-day or real-time reports may be more reliable. Additionally, we were not able to calculate weekly alcohol quantity from this measure. This might have aided our classification of at-risk drinkers, as the NIAAA guideline also specifies a weekly drinking limit for at-risk drinking (i.e., >7 drinks per week for women and >14 drinks per week for men); however, previous research has shown that exceeding the daily limit is associated with a much greater risk of developing an AUD compared to that of exceeding the weekly limit (Dawson et al., 2008). We also did not administer other alcohol screening tools such as the Alcohol Use Disorders Identification Test (AUDIT), which may have aided our characterization of severity of participants’ drinking patterns.
Our adherence measure, using either self-reported non-adherence in the last four days or plasma HIV viral load detectability, also has limitations. We attempted to overcome the social desirability bias that is expected of self-reported adherence by using plasma viral load detectability as a proxy for adherence. Although viral load detectability does not equal non-adherence, non-adherence is likely the greatest indicator of viral detectability. Other reasons for viral load detectability, including virologic failure, drug resistance, and transient viral blips, are not very common and are often associated with ART non-adherence (Nettles et al., 2005, Parienti et al., 2004). Further, we did not collect data on other characteristics related to viral load and ART adherence, including drug-resistant genotype, homelessness, and criminal justice history. We did, however, assess for ART regimen type, which may represent a particularly important variable in the use of viral load detectability as a proxy for adherence, as some forms of ART (e.g., NNRTIs) have longer half-lives (Villani et al., 1999) and are thus more forgiving with missed doses. We found no differences in ART regimen type across drinking groups or adherence groups, providing further evidence in support of our use of viral load detectability as a proxy for ART adherence. Lastly, however, using viral load detectability as a proxy for ART non-adherence does not give as detailed information about medication adherence as would a real-time measure. Such real-time reports and ecological momentary assessments (EMA) are becoming increasingly feasible as mobile and web technologies grow and advance (Shiffman et al., 2008). Obtaining EMA data via cellular phones has become increasingly popular and has already been used to collect information about alcohol and drug use (Shiffman, 2009) as well as ART adherence (Dowshen et al., 2013) among PLWHA. EMA for both alcohol/drug use and medication adherence may be highly useful for clinicians and health care providers in order to ensure patients are receiving and optimally adhering to treatments.
Data from the current study additionally do not address other related factors that may be influencing or driving the association between at-risk drinking and ART non-adherence. For example, impulsivity measures (e.g., delay discounting) have been found to have associations with both alcohol use and medication adherence (Dick et al., 2010, Moore et al., 2012b), potentially suggesting impulsivity as a mechanism for the relationship between alcohol use and medication adherence. Alternatively, the relationship between alcohol use and ART adherence may be explained by beliefs about mixing alcohol and ART doses (Kalichman et al., 2012, Kalichman et al., 2013). Lastly, heavy drinking has been shown to be associated with cognitive deficits, especially among PLWHA (e.g., Rothlind et al., 2005). Such deficits (e.g., prospective memory impairments) are commonly associated with ART non-adherence (Woods et al., 2009). Although the current study measured and controlled for global cognitive function as a way to address these relationships, this measure of cognition was not associated with at-risk drinking or ART adherence, and does not capture any acute deficits that occur during and acutely after alcohol consumption. Such induced acute deficits may manifest as memory lapses or “blurring” of dose timing, contributing to ART non-adherence (Parsons et al., 2008, Braithwaite et al., 2005). These are factors that were not measured in the current study but certainly warrant further examination in future research.
Overall, our findings suggest that screening PLWHA for at-risk drinking, years of education, and plasma viral load may provide clinicians with a better indication of patients at the greatest risk for ART non-adherence compared to self-reported ART adherence alone. Our findings also support the use of the NIAAA guidelines for at-risk drinking as a marker of ART non-adherence risk among PLWHA. There is a clear recommendation for future research to continue clarifying the association between level of alcohol use and ART adherence. Future directions include examining the temporal relationship between alcohol use and ART adherence among PLWHA, similar to the Braithwaite et al. (2005) study. Such a future study may benefit from taking advantage of EMA via mobile technologies to ensure more accurate self-reporting of alcohol use and ART adherence. Additionally, while much research is being done on treatment for opioid dependence and injection drug use among PLWHA, the literature is scant for treating AUDs in this population. Future studies among PLWHA may explore the efficacy of evidenced-based interventions known to reduce alcohol use in HIV-uninfected populations, including pharmacotherapy and psychotherapy. For example, pharmacotherapy for problematic alcohol use can involve the prescription of FDA-approved medications such as acamprosate or naltrexone, which have shown to be equally effective in reducing alcohol consumption when paired with psychotherapy co-interventions (e.g., Jonas et al., 2014). Psychotherapy for problematic alcohol use often includes one or more of the following treatment modalities (McCrady, 2015): self-help groups (e.g., Alcoholics Anonymous; McCrady, Horvath, & Delaney, 2003), individual therapy (e.g., cognitive behavioral therapy, motivational interviewing; McCrady, 2015, Vasilaki, et al., 2006), group therapy (e.g., Monti et al., 2002, Sobell and Sobell, 2011), or couple therapy (e.g., McCrady et al., 2009, McCrady, Owens, & Brovko, 2013). The current study and supporting literature suggests that simply decreasing alcohol consumption may be a step in the right direction for many PLWHA. Considering the elevated prevalence of AUDs and heavy drinking patterns as well as the stronger association between alcohol use and mortality among PLWHA compared to uninfected adults (Justice et al., 2016, Braithwaite et al., 2007, Galvan et al., 2002), it may be beneficial to place a greater emphasis on educating health care providers on this topic as well as treating AUDs and decreasing alcohol consumption in this patient population.
Acknowledgments
Funding/Support: Emily W. Paolillo is supported by an Institutional Ruth L. Kirschstein National Research Service Award (NRSA) T32 grant funded by the NIAAA within the National Institutes of Health (Award T32 AA013525). Data for this study was collected as part of five larger ongoing studies: 1) The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is supported by awards N01 MH22005, HHSN271201000036C, and HHSN271201000030C from NIH; 2) the California NeuroAIDS Tissue Network (CNTN) is supported by awards U01MH083506, R24MH59745 from NIMH; 3) the HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH; 4) the Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA); and 5) a NIDA grant award P01DA1206507.
The San Diego HIV Neurobehavioral Research Program group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry Jernigan, Ph.D., Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S., Christi Kao, M.S.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112:178–93. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]
- Bartlett JA. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002;29(Suppl 1):S2–10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004;19:1111–7. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Mcginnis KA, Maisto SA, Bryant K, Justice AC. Adjusting alcohol quantity for mean consumption and intoxication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcohol Clin Exp Res. 2008;32:1645–51. doi: 10.1111/j.1530-0277.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, Mcginnis K, Rodriguez MC, Rabeneck L, Bryant K, Justice AC. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19:459–66. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Mcginnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JF, Justice AC. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–7. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Bryant KJ. Expanding research on the role of alcohol consumption and related risks in the prevention and treatment of HIV/AIDS. Subst Use Misuse. 2006;41:1465–507. doi: 10.1080/10826080600846250. [DOI] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, Mccutchan JA, Duarte NA, Simpson DM, Mcarthur J, Grant I, Group C. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58:154–62. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz SL, Heckman TG, Hkochman A, Dimarco M. Rates and correlates of HIV treatment adherence among late middle-aged and older adults living with HIV disease. Psychol Health Med. 2001;6:47–58. [Google Scholar]
- Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43:411–7. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug and alcohol dependence. 2008;95:62–72. doi: 10.1016/j.drugalcdep.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–26. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowshen N, Kuhns LM, Gray C, Lee S, Garofalo R. Feasibility of interactive text message response (ITR) as a novel, real-time measure of adherence to antiretroviral therapy for HIV+ youth. AIDS Behav. 2013;17:2237–43. doi: 10.1007/s10461-013-0464-6. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–86. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, Kaplan AH, Wenger NS. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7:226–33. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, Mccutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, Mcarthur JC, Simpson DM, Abramson I, Gamst A, Fennema-notestine C, Jernigan TL, Wong J, Grant I, Grp CH. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman BD, Catz SL, Heckman TG, Miller JG, Kalichman SC. Adherence to antiretroviral therapy in rural persons living with HIV disease in the United States. AIDS Care. 2004;16:219–30. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MO, Catz SL, Remien RH, Rotheram-borus MJ, Morin SF, Charlebois E, Gore-felton C, Goldsten RB, Wolfe H, Lightfoot M, Chesney MA, Team NHLP. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the Healthy Living Project. AIDS Patient Care STDS. 2003;17:645–56. doi: 10.1089/108729103771928708. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Justice AC, Mcginnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, Edelman EJ, Fiellin LE, Freiberg MS, Gordon AJ, Kraemer KL, Marshall BD, Williams EC, Fiellin DA. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, White D, Swetsze C, Kalichman MO, Cherry C, Eaton L. Alcohol and adherence to antiretroviral medications: interactive toxicity beliefs among people living with HIV. J Assoc Nurses AIDS Care. 2012;23:511–20. doi: 10.1016/j.jana.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Benotsch E, Suarez T, Catz S, Miller J, Rompa D. Health literacy and health-related knowledge among persons living with HIV/AIDS. Am J Prev Med. 2000;18:325–31. doi: 10.1016/s0749-3797(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Grebler T, Amaral CM, Mcnerey M, White D, Kalichman MO, Cherry C, Eaton L. Intentional non-adherence to medications among HIV positive alcohol drinkers: prospective study of interactive toxicity beliefs. J Gen Intern Med. 2013;28:399–405. doi: 10.1007/s11606-012-2231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Grebler T, Amaral CM, Mcnerney M, White D, Kalichman MO, Cherry C, Eaton L. Viral suppression and antiretroviral medication adherence among alcohol using HIV-positive adults. Int J Behav Med. 2014;21:811–20. doi: 10.1007/s12529-013-9353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. J Gen Intern Med. 1999;14:267–73. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Rompa D. Functional health literacy is associated with health status and health-related knowledge in people living with HIV-AIDS. J Acquir Immune Defic Syndr. 2000;25:337–44. doi: 10.1097/00042560-200012010-00007. [DOI] [PubMed] [Google Scholar]
- Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26:82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- Malta M, Strathdee SA, Magnanini MMF, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103:1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- Mccrady BS. Alcohol Use Disorders. In: Barlow DH, editor. Clinical handbook of psychological disorders: A step-by-step treatment manual. 5. New York: Guilford Press; 2015. pp. 533–587. [Google Scholar]
- Mccrady BS, Epstein EE, Cook S, Jenson NK, Hildebrandt T. A randomized trial of individual and couple behaivoral aclohol treatment for women. Jounral of Consulting and Clinical Psychology. 2009;77:243–256. doi: 10.1037/a0014686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccrady BS, Horvath AT, Delaney SI. Self-help groups. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. 3. Boston: Allyn & Bacon; 2003. pp. 165–187. [Google Scholar]
- Mccrady BS, Owens M, Brovko J. Couples and family treatment methods. In: McCrady BS, Epstein EE, editors. Addictions: A comprehensive guidebook. 2. New York: Oxford University Press; 2013. pp. 454–481. [Google Scholar]
- Mekuria LA, Prins JM, Yalew AW, Sprangers MAG, Nieuwkerk PT. Which adherence measure - self-report, clinician recorded or pharmacy refill - is best able to predict detectable viral load in a public ART programme without routine plasma viral load monitoring? Tropical Medicine & International Health. 2016;21:856–869. doi: 10.1111/tmi.12709. [DOI] [PubMed] [Google Scholar]
- Monti PM, Kadden RM, Rosenow DJ, Cooney NL, Abrams DB. Treating alcohol dependence: A coping skills training guide. 2. New York: Guilford Press; 2002. [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, Grant I, Grp H, Grp T. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral nonadherence. Aids Care-Psychological and Socio-Medical Aspects of Aids/Hiv. 2012a;24:1504–1513. doi: 10.1080/09540121.2012.672718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Posada C, Parikh M, Arce M, Vaida F, Riggs PK, Gouaux B, Ellis RJ, Letendre SL, Grant I, Atkinson JH Program, H. I. V. N. R. HIV-infected individuals with co-occurring bipolar disorder evidence poor antiretroviral and psychiatric medication adherence. AIDS Behav. 2012b;16:2257–66. doi: 10.1007/s10461-011-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss AR, Hahn JA, Perry S, Charlebois ED, Guzman D, Clark RA, Bangsberg DR. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin Infect Dis. 2004;39:1190–8. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- National Institue on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much. A Clinician's Guide. Washington, D.C.: National Institutes of Health, U.S. Department of Health and Human Services; 2005. [Google Scholar]
- Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, Cofrancesco J, Jr, Gallant JE, Quinn TC, Jackson B, Flexner C, Carson K, Ray S, Persaud D, Siliciano RF. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–29. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response - A meta-analysis. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2005;38:445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Parienti JJ, Massari V, Descamps D, Vabret A, Bouvet E, Larouze B, Verdon R. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–6. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, Mustanski B. The temporal relationship between alcohol consumption and HIV-medication adherence: A multilevel model of direct and moderating effects. Health Psychology. 2008;27:628–637. doi: 10.1037/a0012664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient Protection and Affordable Care Act, 5 § U.S.C. 5002. 2010 [Google Scholar]
- Peretti-watel P, Spire B, Lert F, Obadia Y, Group V. Drug use patterns and adherence to treatment among HIV-positive patients: evidence from a large sample of French outpatients (ANRS-EN12-VESPA 2003) Drug Alcohol Depend. 2006;82(Suppl 1):S71–9. doi: 10.1016/s0376-8716(06)80012-8. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing [Online] Vienna, Austria: 2015. Available: https://www.R-project.org/ [Accessed] [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–9. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–7. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–97. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. National Institutes of Health. National Institute on Alcohol Abuse and Alcoholism. NIH Publication, (07-3769) 2005. Helping patients who drink too much: a clinician’s guide. [Google Scholar]
- Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol and Alcoholism. 2006;41(3):328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- Villani P, Regazzi MB, Castelli F, Viale P, Torti C, Seminari E, Maserati R. Pharmacokinetics of efavirenz (EFV) alone and in combination therapy with nelfinavir (NFV) in HIV-1 infected patients. British journal of clinical pharmacology. 1999;48:712–715. doi: 10.1046/j.1365-2125.1999.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH HIV Neurobehavioral Research Center, G. Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. J Int Neuropsychol Soc. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview, Version 2.1. Geneva: World Health Organization; 1997. [Google Scholar]