Abstract
Rationale: Fibrosis after lung injury is related to poor outcome, and idiopathic pulmonary fibrosis (IPF) can be regarded as an exemplar. Vascular endothelial growth factor (VEGF)-A has been implicated in this context, but there are conflicting reports as to whether it is a contributory or protective factor. Differential splicing of the VEGF-A gene produces multiple functional isoforms including VEGF-A165a and VEGF-A165b, a member of the inhibitory family. To date there is no clear information on the role of VEGF-A in IPF.
Objectives: To establish VEGF-A isoform expression and functional effects in IPF.
Methods: We used tissue sections, plasma, and lung fibroblasts from patients with IPF and control subjects. In a bleomycin-induced lung fibrosis model we used wild-type MMTV mice and a triple transgenic mouse SPC-rtTA+/−TetoCre+/−LoxP-VEGF-A+/+ to conditionally induce VEGF-A isoform deletion specifically in the alveolar type II (ATII) cells of adult mice.
Measurements and Main Results: IPF and normal lung fibroblasts differentially expressed and responded to VEGF-A165a and VEGF-A165b in terms of proliferation and matrix expression. Increased VEGF-A165b was detected in plasma of progressing patients with IPF. In a mouse model of pulmonary fibrosis, ATII-specific deficiency of VEGF-A or constitutive overexpression of VEGF-A165b inhibited the development of pulmonary fibrosis, as did treatment with intraperitoneal delivery of VEGF-A165b to wild-type mice.
Conclusions: These results indicate that changes in the bioavailability of VEGF-A sourced from ATII cells, namely the ratio of VEGF-Axxxa to VEGF-Axxxb, are critical in development of pulmonary fibrosis and may be a paradigm for the regulation of tissue repair.
Keywords: idiopathic pulmonary fibrosis, vascular endothelial growth factor, animal models of pulmonary fibrosis
At a Glance Commentary
Scientific Knowledge on the subject
Failure of tissue repair leads to fibrosis and organ dysfunction and is an increasing cause of disease. Idiopathic pulmonary fibrosis (IPF) is an exemplar of this process in which vascular endothelial growth factor (VEGF)-A has been pathogenically implicated.
What This Study Adds to the Field
We show that IPF is associated with an increased expression of an inhibitory VEGF-A splice variant (VEGF-A165b) in IPF lung tissue, isolated IPF lung fibroblasts, and plasma of patients with IPF with progressive disease. In a mouse model of pulmonary fibrosis, alveolar type II cell–specific deficiency of VEGF-A or constitutive overexpression of VEGF-A165b inhibited the development of pulmonary fibrosis, as did treatment with intraperitoneal delivery of VEGF-A165b to wild-type mice. These results indicate that changes in the bioavailability of VEGF-A sourced from alveolar type II cells, namely the ratio of VEGF-Axxxa to VEGF-Axxxb, are critical in the development of pulmonary fibrosis and may be a paradigm for the regulation of tissue repair.
Fibrosis in the lung or elsewhere is part of the normal repair mechanism after injury. Idiopathic pulmonary fibrosis (IPF) can be considered as a paradigm of an aberrant repair process in response to repeated and unidentified injury. IPF is a devastating progressive fibrosing lung disease of unknown cause that is increasing in incidence (1). Median survival is 3–5 years and treatment options are limited (2, 3), leading to an estimated mortality rate of more than 50 deaths per 1,000,000 persons in the United States (4) and United Kingdom (1).
Vascular endothelial growth factor (VEFG)-A, originally described as an angiogenic factor, belongs to a super-family of glycoproteins, and signals through tyrosine kinase receptors VEGF receptor 1 (VEGFR1) and VEGF receptor 2 (VEGFR2) and coreceptors, neuropilin (NP) 1 and 2 (5, 6). However, VEGF biology has undergone radical revision since its discovery. It has been identified in primitive organisms that have no vasculature (7), is not specific to endothelial cells, and is increasingly recognized as vital in the function and maintenance of nonendothelial cells (8, 9). In vitro studies have confirmed that VEGF-A is abundant in lung tissue, especially in alveolar epithelium (10–14), and has a role in lung development (15) and maturation (16). In the healthy lung processes classically associated with VEGF-A are extremely restricted (14, 17, 18). This apparent paradox has led to controversy about the role of VEGF-A in the normal and diseased lung (19).
Several pathogenic mechanisms for the development of IPF have been proposed but currently a form of recurrent alveolar type II (ATII) epithelial injury leading to aberrant collagen deposition from fibroblasts is most widely accepted (20). Evidence on the role of VEGF-A in IPF conflicts (21–23). Evidence that VEGF-A may facilitate fibrogenesis comes from the extensive trials of Food and Drug Administration–approved nintedanib, a triple tyrosine kinase inhibitor, including the VEGFR2 receptor (24, 25). In contrast, several clinical studies have indicated a reparative role for VEGF-A in the lung (17, 18), with preclinical studies suggesting a protective role for VEGF-A against the development of excessive pulmonary fibrosis as a result of lung injury (26–28).
Differential splicing of the VEGF-A gene produces multiple functional isoforms (29): proximal splice site selection with exon 8 produces the conventional family of VEGF-Axxxa isoforms. Distal splice site selection within exon 8 produces an alternative family of isoforms (VEGF-Axxxb family), which have the same number of amino acids as the conventional family in humans, but six different amino acids at the C terminus (30). The most widely studied of these isoforms, VEGF-A165b, is functionally different from VEGF-A165a (30, 31). VEGF-A165b inhibits canonical VEGF-A signaling by competitive interference with conventional isoforms at the VEGFR2–NP1 receptor complex, preventing full downstream signaling by VEGFR2 (32). Knowledge of inhibitory VEGF-A variants in clinical tissue repair syndromes is limited (33). We have shown that they inhibit VEGF-A165a-induced ATII and pulmonary microvascular proliferation in vitro (10). Functional murine VEGF-A165b has been described (33). Thus a number of regulatory events at the 3′ end of the VEGF-A gene are required for precise physiologic control of VEGF-A bioavailability (34).
Until now, studies have examined VEGF as a single molecule. Differential expression of the numerous VEGF-A isoforms offers one explanation for the apparent discrepancies observed in the literature. In this study we have focused on the role of VEGF-A in relation to fibroblast function. We show that ATII cell–specific production of VEGF-A is a key checkpoint for controlling lung fibrogenesis. VEGF-A165b acts as a natural regulator of this process and has potential therapeutic applications for restoring homeostasis to the lung and treating fibrosis. Some of the results have been previously reported in abstract form (35–37).
Methods
Human Tissue Collection
Anonymized human lung biopsies and bronchoalveolar lavage (BAL) were donated from patients undergoing clinically indicated procedures. The Bristol institutional review boards granted ethical approval. Additional IPF tissue and plasma samples came from the Lung Tissue Research Consortium and Toby Maher (Royal Brompton Hospital, London UK) (38).
Primary Lung Fibroblast Culture
Primary lung fibroblasts were explanted from lung biopsies as previously described or obtained from the American Type Culture Collection.
Reverse Transcription–Polymerase Chain Reaction and Quantitative Reverse Transcription–Polymerase Chain Reaction
Total RNA was extracted using the Quick RNA kit (Zymo Research Irvine, CA) and reverse transcribed with Taqman High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). Individual quantitative reverse transcription–polymerase chain reaction (qRT-PCR) reaction mixes were made using manufacturer recommendations (2× SensiFAST SYBR Hi-ROX; Bioline, London, UK). Human and murine primer sets are in Tables E1 and E2 in the online supplement. Expression of human VEGF-Axxxa and VEGF-Axxxb isoforms were also quantified (39).
Cell Immunofluorescence
Cells were fixed, permeabilized, and blocked before incubation with primary antibody overnight and then secondary antibody before image capture.
Western Blot Analysis
Cell lysates were prepared using radioimmunoprecipitation buffer (Sigma, St. Louis, MO), containing protease and phosphatase inhibitor cocktails (Sigma). Lysates were fractionated, immunoblotted, blocked, then incubated with primary antibodies and loading controls.
HPLC
HPLC was used to quantify fibroblast collagen production (40).
Immunohistochemistry
Slides were dewaxed before rehydration and antigen retrieval. Endogenous peroxidases were blocked and sections incubated with primary antibody or IgG control.
ELISA of Pan-VEGF-A and VEGF-A165b
Pan-VEGF-A and VEGF-A165b levels were quantified using Pan-VEGF-A and VEGF-A165b ELISA sets (R&D Systems, Oxford, UK).
Cell Proliferation Assay and Wound Healing–Migration Assay
Fibroblasts were seeded into 24-well plates or IBIDI culture chambers (Ibidi, Martinsried, Germany); quiesced; and after stimulation, counted or imaged.
Animal Housing and Breeding
All experiments were performed in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and University of Bristol ethical review panel approval. Transgenic (TG) mice constitutively overexpressing VEGF-A165b (MMTV-VEGF165b+/−) were generated as previously described (41). SPC-rtTA+/−Tet-O-Cre+/−LoxP VEGF+/+ mice were generated on C57Bl6 background by crossing three transgenic mouse lines: LoxP-VEGF (Genentech, San Francisco, CA) (42), Cre-recombinase, under control of a tetracycline-responsive promoter element (Tet-O) and TG mice expressing the reverse tetracycline-controlled transactivator (rtTA) protein under control of surfactant, pulmonary-associated protein C, promoter (SPC) (Jackson) (43).
Animal Studies
The bleomycin (BLM)-induced model of pulmonary fibrosis was used (44). Lung fibrosis was assessed by fibronectin and procollagen-1α mRNA expression and histologically by Masson trichrome staining and lung fibrosis score. Further experimental details are available in the online supplement.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA). Unpaired Student’s t test was used, with or without Welch correction dependent on the variance of data. Analysis of variance with post hoc Holm-Šidák multiple comparisons analysis was used for multiple comparisons. For all tests a P less than 0.05 was considered statistically significant.
Results
Pan-VEGF-A and VEGF-Axxxb Expression in Patients with IPF
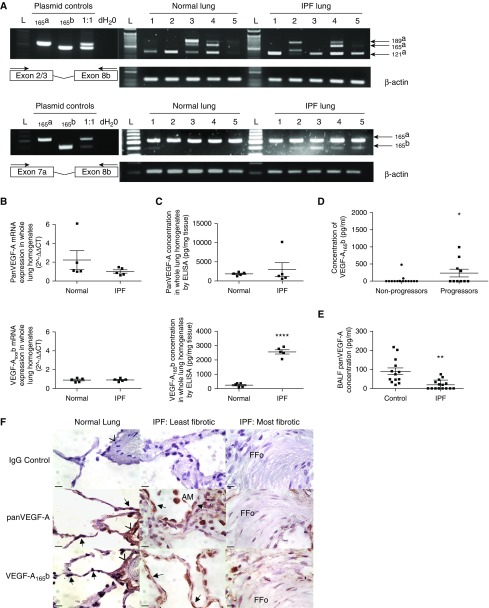
Whole-lung tissue expression of pan-VEGF-A (collective VEGF-A isoforms), VEGF-Axxxb, VEGF-A121a, VEGF-A165a, and VEGF-A189a was detected at mRNA level (Figure 1A), confirmed by sequencing (see Figure E1) and quantified (Figure 1B). Pan-VEGF-A protein levels in whole lung were comparable between normal and IPF, but intriguingly VEGF-A165b protein expression was dramatically increased in IPF lung tissue (Figure 1C). VEGF-A165b, but not pan-VEGF-A, in baseline plasma samples of subsequent progressors (death or >10% decline in FVC at 12 mo) were significantly greater than nonprogressors (Figure 1D).
Figure 1.
Pan–vascular endothelial growth factor (VEGF)-A and VEGF-Axxxb expression in the lungs of patients with idiopathic pulmonary fibrosis (IPF). (A) Pan-VEGF-A reverse transcription–polymerase chain reaction (RT-PCR) of whole-lung RNA extract using VEGF-A Exon2/3 For and 8b Rev primers (top) and Exon 7a For and 8b Rev primers (bottom) (n = 5 normal lung; n = 5 IPF lung). VEGF-A121a, VEGF-A165a, VEGF-A165b, and VEGF-A189a isoforms were identified by RT-PCR and verified by direct sequencing (see Figure E1). L = 50-bp marker. (B) Quantitative RT-PCR of pan-VEGF-A and VEGF-Axxxb mRNA expression in whole-lung tissue homogenates of normal (n = 5) and IPF lung (n = 5). No significant difference was detected in the expression of pan-VEGF-A or VEGF-A165b (unpaired Student’s t test) isoforms between normal and IPF lung samples. (C) Pan-VEGF-A and VEGF-A165b ELISA data from lung whole-tissue lysates in normal (n = 5) and IPF (n = 5) subjects. There was no significant difference in pan-VEGF-A expression (unpaired Student’s t test with Welch correction), but a significant increase in VEGF-A165b expression in the IPF lung was observed (****P < 0.0001; unpaired Student’s t test). Data are presented as means with SEM. (D) VEGF-A165b ELISA data from plasma samples of progressor (death or >10% decline in FVC at 12 mo follow-up) and nonprogressor patients with IPF (38). There was a significant difference in plasma levels of VEGF-A165b in progressors (n = 10) compared with nonprogressors (n = 15) (*P < 0.05; unpaired Student’s t test). (E) Pan-VEGF-A levels in bronchoalveolar lavage fluid (BALF) of patients with IPF (n = 15) compared with control subjects (n = 13) using an antibody that does not discriminate between isoforms. Patient demographics were statistically comparable by unpaired Student’s t test (see Figure E2A). Pan-VEGF-A BALF levels were significantly lower in the IPF group compared with control subjects, **P < 0.01, unpaired Student’s t test with Welch correction. Data are presented as means with SEM. Mean pan-VEGF-A expression in control group 85.7 pg/ml ± 17.1, versus IPF 18.0 pg/ml ± 6.1. VEGF-A165b expression was below the limit of detection in both patients with IPF and control subjects (10 pg/ml). (F) Pan-VEGF-A and VEGF-A165b immunohistochemical staining. Intense staining of the alveolar epithelium was observed for both pan-VEGF-A and VEGF-A165b in normal and IPF lungs (least and most fibrotic designated as in Ashcroft and colleagues [59] and Ebina and colleagues [60]) (arrows). Additional sites of localization included vessel walls (arrowheads), fibroblasts, lymphocytes, and alveolar macrophages. Isotype IgG shown as negative control. Scale bars = 10 μm; original magnification, ×100. Lower-magnification images are shown in Figure E2B. AM = alveolar macrophages; FFo = fibrotic focus.
Levels of VEGF-A (representing only the soluble isoforms, VEGF-A121 and VEGF-A165), investigated by pan-VEGF-A ELISA, were significantly lower in BAL fluid (BALF) of patients with IPF compared with control subjects (Figure 1E), as previously reported (13, 45–47). Patient demographics were comparable (see Figure E2A). VEGF-A165b was undetectable in BALF, using an ELISA specific for an epitope encoded by exon 8b suggesting it was not secreted into an area accessible by this procedure or was below the detection limit (10 pg/ml). Using immunohistochemistry, we found that in both normal and IPF lung pan-VEGF-A and VEGF-A165b were prominent within alveolar epithelium (Figure 1F; see Figure E2B) but also localized to macrophages, lymphocytes, and fibroblasts, particularly outside the fibrotic focus. These data suggest that there is an increased expression of VEGF-A165b in IPF, one source being ATII cells as previously shown in normal lung (10). To understand the biologic potential of these findings in IPF we investigated the expression of the receptors and coreceptors necessary for functional activity.
VEGF Receptor and Coreceptors in Patients with IPF
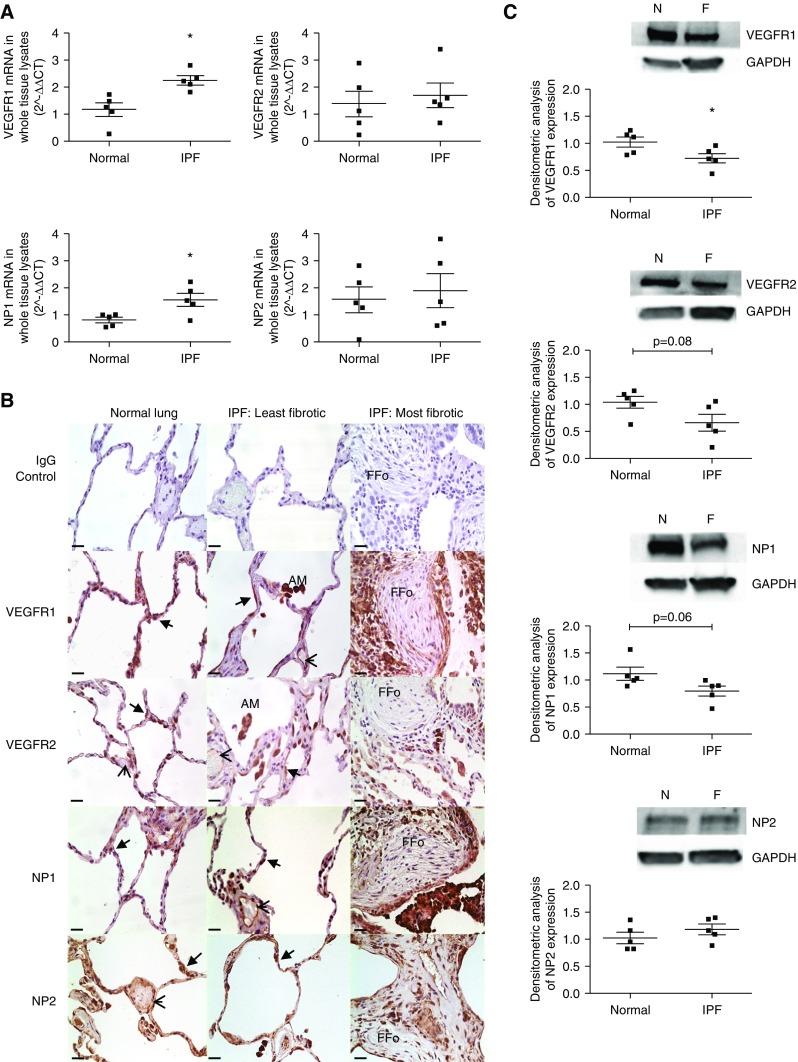
Expression of VEGFR1, VEGFR2, NP1 and NP2 was detected at the mRNA level in normal and IPF lung whole-lung tissue (Figure 2A). Expression of VEGFR1 and NP1 was significantly up-regulated in IPF lung.
Figure 2.
Vascular endothelial growth factor (VEGF)-A receptor and coreceptor expression in the lungs of patients with idiopathic pulmonary fibrosis (IPF). (A) Quantitative reverse transcription–polymerase chain reaction of VEGFR1, VEGFR2, neuropilin (NP) 1, and NP2 mRNA expression in whole-lung RNA extracts of normal (n = 5) and IPF (n = 5) lung. VEGFR1 (*P < 0.05; unpaired Student’s t test) and NP1 (*P < 0.05; unpaired Student’s t test) mRNA expression was significantly up-regulated in the IPF lung. Data are presented as mean fold change in expression (2−ΔΔCT) with SEM, data analysis performed on ΔΔCT values. (B) VEGF-A receptor and coreceptor immunohistochemical staining. Intense staining of the alveolar epithelium was observed for VEGFR1, VEGFR2, NP1, and NP2 (arrows) in both the normal and IPF lung. Additional sites of localization included the vascular endothelium (arrowheads), fibroblasts, lymphocytes, and alveolar macrophages. Isotype IgG shown as negative control subject. Images were taken at ×40 magnification; scale bars = 25 μm. Additional higher-magnification images are shown in Figure E3B. (C) Expression of VEGFR1, VEGFR2, NP1, and NP2 in whole-tissue lysates of normal (n = 5) and IPF (n = 5) lung by Western blotting (top) with semiquantitative densitometric analysis (bottom). VEGFR1, VEGFR2, NP1, and NP2 were expressed in both the normal and IPF lung. VEGFR1 (*P < 0.05) was significantly down-regulated in the IPF lung (unpaired Student’s t test, data presented as mean densitometry score with SEM). AM = alveolar macrophages; F = IPF lung; FFo = fibrotic focus; N = normal lung.
Using immunohistochemistry, we found that in normal and IPF lung VEGFR1, VEGFR2, NP1, and NP2 were expressed on alveolar epithelium, macrophages, lymphocytes, and fibroblasts surrounding fibrotic foci with reduced expression within the foci (Figure 2B; see Figure E3A).
Lung whole-tissue lysates expressed VEGFR1, VEGFR2, NP1, and NP2 protein (Figure 2C). There was a significant reduction in protein expression of VEGFR1 in the whole IPF lung samples with a trend to reduced VEGFR2 and NP1. Costaining of VEGFR1 and VEGFR2 with epithelial-specific E-cadherin suggests that nonendothelial cells are VEGF-A targets (see Figure E3B).
These data demonstrated the potential for VEGF-A to have functional effects in fibrotic lung. In this context, we then looked at isolated fibroblast cultures, the potential effector cells, from IPF and normal subjects.
VEGF-A Receptors and Coreceptors Are Expressed by Primary Lung Fibroblasts, and VEGF-A Stimulation Stimulates Downstream Signaling Pathways
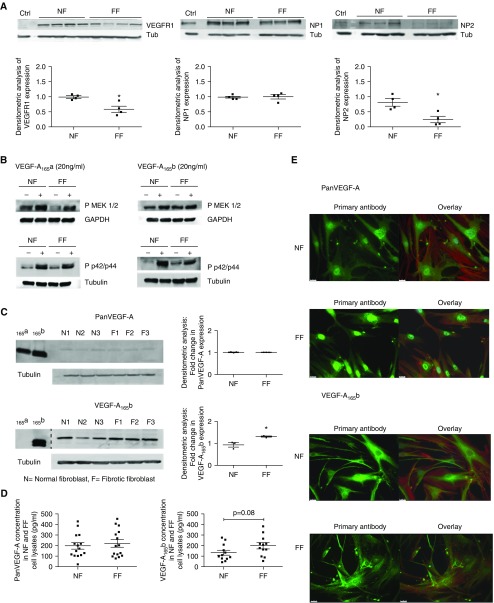
VEGFR1, VEGFR2, NP1, and NP2 were endogenously expressed by fibroblasts from normal subjects (NF) and patients with IPF (FF), at the mRNA level (see Figure E3C). FF expressed less VEGFR1 and NP2 protein (Figure 3A). Surprisingly, these cells did not express mature VEGFR2 protein by Western blotting. These results were corroborated by ELISA; the expression of VEGFR2 was below the detection limit (≤30 pg/ml). VEGFR2 is conventionally considered the main signaling receptor so we investigated whether signaling could occur. Treatment of NF and FF cultures with rhVEGF-A165a or rhVEGF-A165b proteins led to increased phosphorylation of MEK1/2 (Mitogen/Extracellular signal related Kinase) and p42/44 MAPK (Mitogen Activated Protein Kinase) proteins (Figure 3B; see Figure E4).
Figure 3.
Vascular endothelial growth factor receptor (VEGFR) and vascular endothelial growth factor (VEGF)-A isoform expression in NF and FF. (A) Expression of VEGFR1, VEGFR2, neuropilin (NP) 1, and NP2 in NF and FF by Western blotting with semiquantitative densitometric analysis. Using glomerular endothelial cells as a positive control (Ctrl), bands were observed by Western blotting that were consistent with the expression of VEGFR1, NP1, and NP2 in both NF and FF. Mature VEGFR2 protein was not expressed by NF or FF fibroblasts (data not shown). Semiquantification of expression by densitometry demonstrated a significant reduction in the expression of VEGFR1 (*P < 0.05) and NP2 (*P < 0.05) in unstimulated FF cultures compared with NF (data presented as means with SEM; NF, n = 4; FF, n = 4, n = 3 or 4 shown; unpaired Student’s t test). (B) Western blot of phosphorylated MEK1/2 and phosphorylated p42/p44 MAPK (mitogen-activated protein kinase) expression in response to 24 hours of stimulation with VEGF-A165a (20 ng/ml) or VEGF-A165b (20 ng/ml). In the absence of mature VEGFR2 expression in NF and FF, the activation of known VEGF-A signaling pathways was explored. Stimulation of NF and FF led to the increased phosphorylation of MEK1/2 and p42/p44 in response to treatment of cells with VEGF-A165a or VEGF-A165b. For densitometric analysis see Figure E4. (C) Western blot of pan-VEGF-A and VEGF-A165b expression in NF and FF cell lysates with semiquantitative densitometric analysis (data presented as means with SEM). Recombinant proteins were used as positive control subjects to highlight the specificity of the VEGF-A165b antibody in detecting VEGF-A165b proteins. The dashed line in the lower blot indicates where the blot has been manually cut and components imaged separately. VEGF-A165b proteins were significantly up-regulated in FF cell lysates compared with NF (n = 3; *P < 0.05; unpaired Student’s t test). No significant difference in pan-VEGF-A isoform expression was shown (n = 3; unpaired Student’s t test). (D) Quantification of protein expression of pan-VEGF-A and VEGF-A165b expression in NF and FF cell lysates. By ELISA there was no significant difference in the expression of pan-VEGF-A or VEGF-A165b in NF or FF cell lysates (n = 6 performed in duplicate cell lysates of different passage, unpaired Student’s t test). In the conditioned medium extracted from these cultures pan-VEGF-A expression was significantly up-regulated in the FF supernatants (n = 6; *P < 0.05; unpaired Student’s t test) (see Figure E7). VEGF-A165b expression in these same cell supernatant samples was below the limit of detection of the ELISA (10 pg/ml). (E) Cell immunofluorescence of pan-VEGF-A and VEGF-A165b expression in NF and FF. Comparable patterns were observed for NF and FF, with cytoplasmic and perinuclear expression of pan-VEGF-A and cytoplasmic expression of VEGF-A165b. Images were taken at ×40 magnification with scale bar = 25 μm. Primary antibody shown in green with an overlay image of the primary antibody, phalloidin (red) for F-actin and DAPI (blue) for nuclear staining. Isotype IgG control subjects and separate phalloidin and DAPI images shown in Figure E8. FF = patients with idiopathic pulmonary fibrosis; NF = normal subjects; Tub = tubulin.
VEGF-A Isoforms Are Expressed in Primary Lung Fibroblasts
NF and FF were characterized as expressing three major VEGF-A isoforms detectable at the RNA level by RT-PCR and confirmed by sequencing (see Figures E5 and E6). Comparable levels of pan-VEGF-A and VEGF-Axxxb mRNA were demonstrated in NF and FF by qRT-PCR (see Figure E7).
In contrast, comparable protein expression of pan-VEGF-A, but increased expression of VEGF-Axxxb isoforms in FF cell lysates, was identified by Western blotting (Figure 3C). Quantification of pan-VEGF-A and VEGF-Axxxb protein expression in cell lysates of NF and FF cultures by ELISA supported these findings (Figure 3D).
Localization of pan-VEGF-A and VEGF-A165b expression by immunofluorescence demonstrated cytoplasmic localization in all NF and FF, with additional pan-VEGF-A perinuclear staining (Figure 3E; see Figure E8). These data suggest an additional potential for autocrine effects of pan-VEGF-A and VEGF-A165b on fibroblasts.
Having shown the potential for functional effects of VEGF-A165a and VEGF-A165b on fibroblasts we then explored how they might relate to IPF pathology.
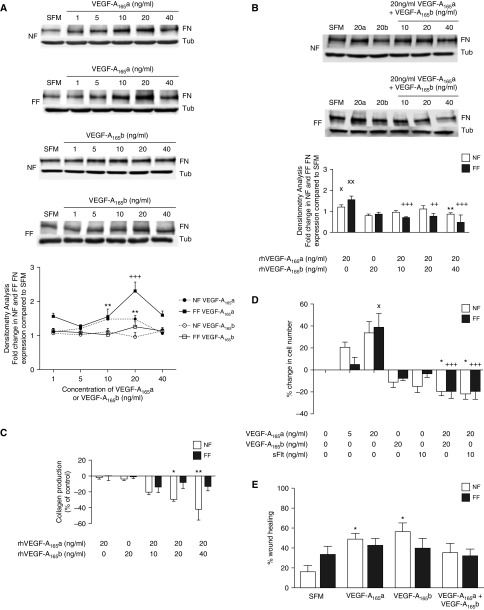
VEGF-A165a and VEGF-A165b Recombinant Proteins Have Differential Effects on Fibroblast Proliferation, Migration, and ECM Expression
We examined fibroblastic fibronectin and collagen expression (Figures 4A–4C). Administration of rhVEGF-A165a significantly increased protein expression of fibronectin but not collagen by NF and FF. No significant effect was observed for either protein after rhVEGF-A165b administration (Figures 4A and 4C). However, with increasing levels of VEGF-A165b, the VEGF-A165a-induced increase in fibronectin was inhibited, an effect more apparent for FF (Figure 4B). In the presence of both isoforms, VEGF-A165b inhibited collagen production in NF but not FF (Figure 4C).
Figure 4.
Functional response of normal subjects (NF) and patients with idiopathic pulmonary fibrosis (FF) to recombinant vascular endothelial growth factor (VEGF)-A proteins. (A) The effect of VEGF-A on the expression of fibronectin (FN) by NF and FF in response to 24 hours of stimulation with VEGF-A165a (20 ng/ml) or VEGF-A165b (20 ng/ml) as measured by Western blotting (top) with densitometry analysis (bottom). A significant increase in the expression of FN in response to VEGF-A165a stimulation in both NF (**P < 0.01 with 10 ng/ml and 20 ng/ml) and FF (+++P < 0.001 at 20 ng/ml; n = 3) was observed. There was no significant effect of VEGF-A165b on the expression of FN in either NF or FF. (B) The combined effect of VEGF-A165a and VEGF-A165b on the expression of FN in NF and FF as measured by Western blotting (top) and densitometry (bottom). Used in isolation, VEGF-A165a (20 ng/ml) was shown to increase FN expression in NF (XP < 0.05) and FF (XXP < 0.01), whereas VEGF-A165b had no significant effect, as shown in the previous experiments. VEGF-A165b inhibited the VEGF-A165a–induced increase in FN expression in both NF (*P < 0.05 at 40 ng/ml of VEGF-A165b) and FF (+++P < 0.001 at 10 and 40 ng/ml of VEGF-A165b, ++P < 0.01 at 20 ng/ml of VEGF-A165b), with FF appearing to be more susceptible to this effect (n = 3). Data are presented as means with SEM. (C) The effect of VEGF-A on the expression of collagen by NF and FF in response to 24 hours of stimulation with VEGF-A165a or VEGF-A165b as measured by HPLC. In the presence of both isoforms, VEGF-A165b inhibited collagen production in NF but not FF (*P < 0.05; **P < 0.01; NF and FF n = 3). Data are presented as means with SEM. (D) The effect of VEGF-A recombinant proteins on the proliferation of NF and FF. A significant increase in FF cell number was observed in response to 20 ng/ml VEGF-A165a compared with SFM (XP < 0.05). VEGF-A165a-induced proliferation was inhibited by the concomitant addition of 20 ng/ml VEGF-A165b (+++P < 0.001) or 10 ng/ml sFlt (+++P < 0.001). VEGF-A165a had no statistically significant effect on NF cell proliferation compared with SFM, but concomitant treatment of NF with 20 ng/ml VEGF-A165a and 20 ng/ml VEGF-A165b (*P < 0.05) or sFlt (*P < 0.05) inhibited cell proliferation compared with 20 ng/ml VEGF-A165a alone (*P < 0.05; NF and FF n = 5). Data are presented as means with SEM. (E) The effect of VEGF-A recombinant proteins on NF and FF wound healing. Image analysis performed using ImageJ. VEGF-A165a and VEGF-A165b significantly increased the migration of NF at 48 hours (*P < 0.05). This effect was blocked by the concomitant treatment of VEGF-A165a and VEGF-A165b. Recombinant VEGF-A proteins had no significant effect on the migration of FF. Statistical analysis: analysis of variance with post hoc Holm-Šidák multiple comparisons analysis used throughout (n = 4). Representative images shown in Figure E9. SFM = serum-free media; Tub = tubulin.
Recombinant VEGF-A165a also significantly increased the proliferation of FF, inhibited by the concomitant addition of VEGF-A165b (Figure 4D). Concomitant treatment of NF with VEGF-A165a and VEGF-A165b inhibited cell proliferation compared with VEGF-A165a alone.
Furthermore, VEGF-A165a and VEGF-A165b significantly increased NF migration. This effect was blocked by the concomitant treatment of VEGF-A165a and VEGF-A165b. In contrast, recombinant VEGF-A proteins had no significant effect on FF migration (Figure 4E; see Figure E9).
Collectively these data supported an in vitro mechanism by which VEGF-A165a could induce a fibrotic response ameliorated by VEGF-A165b. In the context of IPF, VEGF-A165b could ameliorate the development of IPF with ATII cells as the predominant source and fibroblasts as the likely effector cells, so to consider this in more detail we turned to an animal model.
Alveolar Epithelial Expression of VEGF-Axxxa Is Essential for Development of Pulmonary Fibrosis
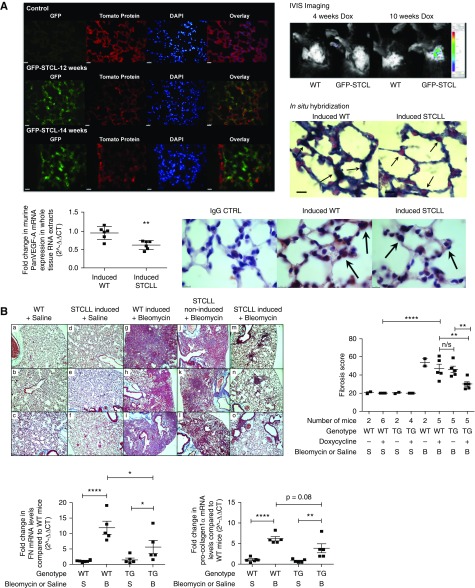
To determine the effect of VEGF-A on the development of BLM-induced pulmonary fibrosis (44), we generated a triple transgenic mouse SPC-rtTA+/−TetoCre+/−LoxP-VEGF-A+/+ (termed STCLL) on a C57Bl/6 background to conditionally induce the deletion of VEGF-A isoforms specifically in the ATII cells of adult mice. Tissue specificity of Cre-recombinase activity after doxycycline induction was confirmed by in vivo imaging of doxycycline-induced GFP-SPC-rtTA+/−TetoCre+/−LoxP-VEGF-A+/− mice (termed GFP-STCL) (Figure 5A; see Figure E10). Quantitative RT-PCR of whole-lung tissue RNA extracts demonstrated reduced pan-VEGF-A in doxycycline-induced TG mice. In situ hybridization indicated a lack of VEGF-A mRNA in the murine ATII cells and pan-VEGF-A immunohistochemistry corroborated findings of reduced ATII-derived VEGF-A (Figure 5A).
Figure 5.
The effect of postnatal deletion of vascular endothelial growth factor (VEGF)-A from alveolar type II (ATII) cells on the development of pulmonary fibrosis. (A) GFP-reporter mice (ROSAmT/mG) were crossed with SPC+/−TC+/−LoxP+/− transgenic (TG) mice (STCL) to determine the period of doxycycline induction required to activate the Cre-recombinase and to identify the tissue specificity of Cre-recombinase activity after doxycycline induction. The reporter mice possess LoxP sites on either side of a membrane-targeted tdTomato (mT) cassette and express red fluorescence in all tissues. In the presence of Cre recombinase, the mT cassette is deleted, allowing expression of the membrane-targeted EGFP (mG) cassette located just downstream. The in vivo imaging system (IVIS) demonstrated evidence of GFP fluorescence in the GFP-STCL mouse after 10 weeks of doxycycline induction, absent at 4 weeks of induction and in wild-type (WT) mice. Direct visualization of the lungs of induced mice (at 12 and 14 wk of doxycycline treatment) demonstrated GFP fluorescence in ATII cells with an absence of GFP fluorescence in the bronchial epithelium (14-wk induction image; arrows). Images were taken at ×40 magnification; scale bar = 25 μm, n = 3. GFP fluorescence was absent in the kidneys and liver (see Figure E10). Using a murine VEGF-A RNA probe, in situ hybridization demonstrated a reduction in ATII cell staining (reduction in blue/black signal) in induced-TG mice compared with induced-WT mice (arrows). Pink staining represents nuclear fast red counterstain (n = 3; images taken at ×100 magnification; scale bar = 10 μm). Pan-VEGF-A mRNA expression was significantly reduced in whole-tissue RNA extracts from TG mice compared with WT mice (n = 6; **P < 0.01; unpaired Student’s t test; data presented as means with SEM). Consistent with these findings, immunohistochemical staining for pan-VEGF-A also showed a reduction in ATII cell staining in induced-TG mice compared with induced-WT mice (n = 3; images taken at ×100 magnification; scale bar = 10 μm). (B) Postnatal deletion of VEGF-A from ATII cells ameliorates the development of bleomycin (BLM)-induced pulmonary fibrosis. Masson trichrome staining of mouse lung sections, with lung fibrosis score (above) and quantitative reverse transcription–polymerase chain reaction of FN and procollagen-1α mRNA levels (below). As demonstrated by Masson trichrome staining, administration of BLM to both WT (g–i) and noninduced STCLL (j–l) mice resulted in extensive pulmonary fibrosis. This effect was ameliorated in BLM-treated doxycycline-induced STCLL mice (m–o). Images were originally ×10 magnification; scale bar = 100 μm; n = 5 or 6; n = 3 shown. The lung fibrosis score of BLM-treated, doxycycline-induced STCLL mice was significantly reduced compared with BLM-treated control animals (induced-WT mice **P < 0.01 and noninduced STCLL mice **P < 0.01; n = 5). The expression of FN and pro-collagen-1α mRNA was significantly up-regulated in the lungs of BLM-treated, doxycycline-induced WT mice compared with saline-treated control animals (****P < 0.001; n = 5 or 6 analyzed). FN and pro-collagen-1α expression was significantly up-regulated in BLM-treated, doxycycline-induced TG mice compared with TG saline controls (*P < 0.05; **P < 0.01; n = 5). Furthermore, FN mRNA levels were significantly reduced in BLM-treated TG mice compared with BLM-treated WT mice (*P < 0.05; n = 5), with a trend toward a reduction in pro-collagen-1α levels in these same mice (P = 0.08, n = 5). Statistical analysis: analysis of variance with Holm-Šidák multiple comparisons test used throughout. n/s = not significant; STCLL = SPC-rtTA+/−TetoCre+/−LoxP-VEGF-A+/+.
Deletion of ATII VEGF-A significantly reduced the development of BLM-induced pulmonary fibrosis by both a blindly assessed lung fibrosis score and reduced collagen deposition and architectural distortion (Figure 5B). Furthermore, lung fibronectin mRNA expression was significantly reduced in BLM-treated STCLL mice compared with BLM-treated wild-type (WT) control animals, with a trend toward a reduction in pro-collagen-1α mRNA levels in these TG mice (Figure 5B). This demonstrates that ATII expression of VEGF-A is required for fibrosis to occur.
VEGF-A165b May Be Protective against the Formation of Pulmonary Fibrosis
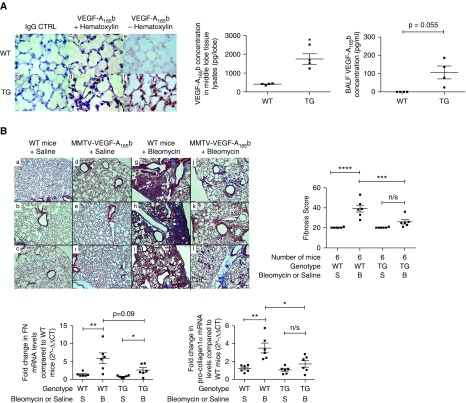
A TG mouse that overexpresses VEGF-A165b using a MMTV promoter as a driver of expression has been described (41). This mouse has increased levels of the MMTV-VEGF-A165b transgene and human VEGF-A165b in the lung (see Figure E11A). Hematoxylin-eosin-stained lung sections of TG mice were histologically normal, in particular with no vascular abnormalities, as might be expected in view of the established compartmentalization of lung VEGF (see Figure E11B) (14). Immunohistochemical staining of TG mouse lung sections demonstrated increased VEGF-A165b expression localized to alveolar epithelial cells, compared with both mouse IgG and WT control animals (Figure 6A). Homogenized lung tissue extracts from MMTV-VEGF-A165b mice expressed significantly more VEGF-A165b protein than littermate WT control animals by ELISA, with detectable VEGF-A165b levels in BALF of TG mice, but below the detection limit of the ELISA in WT control animals (Figure 6A).
Figure 6.
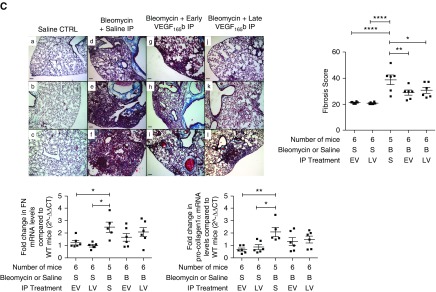
The effect of vascular endothelial growth factor (VEGF)-A165b on the development of pulmonary fibrosis. (A) Lung phenotype of the MMTV-VEGF-A165b transgenic (TG) mouse. Immunohistochemical staining for VEGF-A165b in the lung of TG mice with quantification of VEGF-A165b expression by ELISA, in whole-tissue lysates and bronchoalveolar lavage fluid (BALF). VEGF-A165b expression was increased in the alveolar type II (ATII) cells of the TG mouse lung (d and f, indicated by arrows) compared with the wild-type (WT) lung (c and e). Isotype IgG staining was used as a negative control (a and b). Images were taken at ×100 magnification; scale bars = 10 μm, (n = 3, n = 1 shown). VEGF-A165b expression was significantly up-regulated in whole-tissue lysates of TG mice compared with WT mice (*P < 0.05; unpaired Student’s t test with Welch correction; n = 4). The expression of VEGF-A165b was below the limit of detection of the ELISA in all BALF samples of WT mice but present in detectable levels in all BALF samples of TG mice. There was no statistical difference between these groups (P = 0.055; unpaired Student’s t test with Welch correction; n = 4). (B) Overexpression of VEGF-A165b in ATII cells ameliorates the development of bleomycin (BLM)-induced pulmonary fibrosis. Representative images of Masson trichrome staining with lung fibrosis score and quantitative reverse transcription–polymerase chain reaction (RT-PCR) of fibronectin (FN) and pro-collagen-1α mRNA levels (bottom). Masson trichrome staining of mouse lung sections, 21 days after oropharyngeal (OP) instillation of BLM to MMTV-VEGF-A165b TG mice (j–l) or littermate control animals (g–i). The development of BLM-induced pulmonary fibrosis was ameliorated in TG mice. Saline control subjects are shown in a–f (n = 6 per group; n = 3 shown). Scale bar = 100 μm; original magnification, ×10. The lung fibrosis score of BLM-treated WT mice was significantly greater than saline-treated WT mice (****P < 0.0001; n = 6 per group). The lung fibrosis score of BLM-treated TG mice was significantly lower than BLM-treated WT mice (***P < 0.001; n = 6 per group). FN mRNA was significantly up-regulated in the lungs of WT BLM-treated mice compared with WT saline-treated control animals (**P < 0.01; n = 6 analyzed) and in TG BLM-treated mice compared with TG saline-treated control animals (*P < 0.05; n = 6). There was no statistical difference between BLM-treated WT and TG mice (P = 0.09; n = 6). In contrast, pro-collagen-1α mRNA levels were significantly reduced in BLM-treated TG mice compared with BLM-treated WT littermates (*P < 0.05; n = 6). Data are presented as means with SEM. Statistical analysis: analysis of variance with Holm-Šidák multiple comparisons test used throughout. (C) IP instillation of rhVEGF-A165b ameliorates the development of pulmonary fibrosis. Masson trichrome staining of lung sections with lung fibrosis score and quantitative RT-PCR of FN and pro-collagen-1α mRNA levels (bottom). Representative images of Masson trichrome staining of mouse lung sections, 21 days after OP instillation of BLM with or without additional IP rhVEGF-A165b instillation. (a–c) OP saline control (no differences between early IP VEGF-A165b [EV] or late IP VEGF-A165b [LV] plus saline). (d–f) OP BLM with saline IP. (g–i) Early IP VEGF-A165b treatment with subsequent BLM OP instillation. (j–l) BLM OP with subsequent late IP VEGF-A165b instillation. Early and late rhVEGF-A165b instillation ameliorated the development of lung fibrosis (n = 6 per group; n = 3 shown). Scale bar = 100 μm; original magnification, ×10. By qRT-PCR, FN and pro-collagen-1α mRNA was significantly up-regulated in the lungs of BLM-treated mice compared with saline-treated control animals (*P < 0.05; **P < 0.01; n = 5). In contrast, FN and pro-collagen-1α expression in mice treated with either early (EV) or late (LV) rhVEGF-A165b and BLM was not significantly different from saline control (n = 6). The lung fibrosis score in both early (EV, **P < 0.01) and late (LV, *P < 0.05) IP rhVEGF-A165b treatment groups was significantly reduced compared with BLM OP–saline IP–treated mice; n = 5 or 6 per group; data are presented as means with SEM. Statistical analysis: analysis of variance with Holm-Šidák multiple comparisons test used throughout. CTRL = control; n/s = not significant.
In this preclinical model the development of pulmonary fibrosis was significantly reduced in TG mice compared with WT mice (Figure 6B). Pro-collagen-1α mRNA levels were significantly reduced in BLM-treated TG mice compared with BLM-treated WT littermates, with a trend to reduction in fibronectin mRNA in these same TG mice (Figure 6B). These data show a protective effect of VEGF-A165b within the lung when expressed particularly by the alveolar epithelium, supporting a potential for therapeutic application.
To determine the effect of VEGF-A165b on the development of BLM-induced pulmonary fibrosis, WT C57/Bl/6 mice received oropharyngeal BLM with or without the additional intraperitoneal (IP) delivery of rhVEGF-A165b. Importantly, in one arm of the study, to specifically address the potential therapeutic benefit of VEGF-A165b, the compound was delivered during the fibrotic rather than the inflammatory phase of the model (late VEGF-A165b group).
IP injection of VEGF-A165b significantly reduced the development of pulmonary fibrosis in WT mice. This protective effect of VEGF-A165b was evident in the early VEGF-A165b and late VEGF-A165b groups suggesting a protective effect of VEGF-A165b (Figure 6C). Although the expression of fibronectin and pro-collagen-1α mRNA in whole-lung tissue RNA extracts was significantly up-regulated in mice treated with BLM plus saline IP, the expression in both VEGF-A165b IP groups was not significantly different from oropharyngeal saline-treated control subjects, supporting the notion that VEGF-Axxxa is profibrotic and VEGF-Axxxb is protective.
Discussion
Although VEGF-A has been implicated in the response of the lung to injury there are conflicting reports as to whether it functions as a contributory or protective factor (21, 22, 27, 28). Processes classically associated with VEGF-A are extremely restricted within the normal lung (14, 17, 18), which reflects the increasing recognition of VEGF as vital in the function and maintenance of nonendothelial cells (8, 9. Cumulative evidence from several studies has suggested that the alternatively spliced VEGF-Axxxb isoforms may have opposing/inhibitory functions compared with the conventional VEGF-Axxxa family (31) and appreciation of the existence of numerous VEGF-A isoforms and their interaction with VEGF-A receptors and cofactors determining VEGF-A bioavailability, offers one potential explanation for these discrepant findings.
We show that differential expression of VEGF-Axxxa and VEGF-Axxxb isoforms in the IPF lung can result in functional changes to the fibrotic process, and that ATII cell VEGF-A is critical for the development of fibrosis in a preclinical murine model. The finding that in this model fibrosis can be prevented or resolved by expression of the inhibitory isoform indicates that it is the VEGF-Axxxa family that is profibrotic and the VEGF-Axxxb family is inhibitory/regulatory. This leads to two potential new therapeutic approaches for IPF: selective inhibition of VEGF-Axxxa, or stimulation (or administration) of VEGF-A165b. The development of a specific VEGF-A165a antibody (48, 49) and small molecule splicing agents that alter the relative balance of VEGF-A isoforms (50, 51) could facilitate this.
We have shown significant up-regulation of VEGF-A165b within IPF lung tissue compared with normal lung. This was particularly seen in both ATII cells and fibroblasts (outside the fibrotic foci) in the most severely fibrotic areas of the IPF. These data were obtained from patients with established disease suggesting that dysregulated fibrosis has occurred despite these changes. We speculate that the increase in VEGF-A165b is a compensatory mechanism that was insufficient to prevent inappropriate fibrosis in these patients; however, in some patients at risk, fibrosis will be prevented. Sequential lung tissue data to support this is not available because these patients present with established disease. However, increased VEGF-A165b in baseline plasma samples from patients with subsequent progression supports this hypothesis (38). In contrast, we found a significant reduction in pan-VEGF-A expression in the BALF of patients with IPF by ELISA as previously observed (45–47) and VEGF-A165b levels were undetectable in identical BALF samples. The nature of BALF collection from IPF lung would favor least affected areas and the greatest intensity of immunohistochemical staining for VEGF-A165b was in the most fibrotic areas.
The isoform production switch, altering the ratio of VEGF-Axxxa to VEGF-Axxxb, could be viewed as a contributor to disease, or as a protective response of the ATII and other cells, a regulatory mechanism to maintain homeostasis as we have previously postulated in the lung (10) and others in systemic sclerosis (52, 53). Interestingly, it has become clear that IPF is associated with multiple splicing changes, such as that required for VEGF-A165b (54, 55).
VEGFR1 was down-regulated in IPF and expression was low in fibrotic fibroblasts, with additional trends toward a reduction in both VEGFR2 and NP1 expression in IPF. This may be caused by the presence of increased VEGF-A165b that has been shown to cause down-regulation of VEGFR2, because it results in internalization and degradation through the endosomal pathway (32). Although fibroblasts lack VEGFR2, historically considered the most biologically active of all the VEGF receptors (56), we demonstrated that recombinant VEGF-A proteins have differential effects on the proliferation, migration, and ECM protein expression by these cells. Given that VEGF-A165b levels are raised in IPF, and VEGF-A165a stimulates profibrotic effects in fibrotic fibroblasts, which were inhibited by VEGF-A165b, this could be a protective mechanism in IPF that is being overwhelmed by the other inflammatory processes being driven forward (57). VEGF-A has been shown to have both paracrine and autocrine effects on several cell types within the lung (6, 10, 15, 16). It is our opinion that although the statistically significant differences observed in fibroblast responses to VEGF-A isoforms might seem biologically modest, they could have important implications in a slowly progressive fibrosing disease, such as IPF, in which tissue remodeling and disease progression is best observed over months and years. Although in vitro studies using cocultures of IPF-derived alveolar epithelium and fibroblasts would be desirable to further study this mechanism, there are several recognized practical limitations to culturing IPF epithelial cells (58).
In our initial studies of a preclinical pulmonary fibrosis model, we sought to determine the critical source of VEGF-A. We demonstrated a significant amelioration of the fibrotic response in a specific doxycycline-induced ATII cell VEGF-A–deficient mouse showing that alveolar production of VEGF-A is required for fibrosis to occur. VEGF-A was not completely absent in this model, which is likely to reflect the situation in the human lung. We did not find any evidence of vascular abnormalities in our model, although functional vascular studies were not undertaken. This supports the widely accepted notion in the pathogenesis of IPF that is of initiation by recurrent epithelial injury (20).
Using this same preclinical model, we explored the potential therapeutic benefit of IP rhVEGF-A165b administration. The BLM-model of lung fibrosis has been characterized as having an early partially inflammatory phase followed by a fibrotic phase (44). In our study, both early IP VEGF-A165b and critically late IP VEGF-A165b, delivered specifically in the “fibrotic phase” of the fibrosis model, resulted in significant amelioration of pulmonary fibrosis. In conjunction with the previous data this demonstrates that it is VEGF-Axxxa isoforms that are required for fibrosis.
These data support our hypothesis that the coordinated expression of VEGF-Axxxa and VEGF-Axxxb isoforms is important in the development and progression of pulmonary fibrosis. Differential expression of these families of VEGF-A isoforms may explain, in part, some apparently contradictory studies describing VEGF-A as both a protective and contributory factor in fibrogenesis. We describe for the first time the apparent protective effects of VEGF-Axxxb expression in preclinical models of pulmonary fibrosis, an effect that may occur because of differential effects on fibroblastic synthetic function. Our findings support a “nonangiogenic/nonvascular” role for VEGF-A in the fibrotic lung. Future studies are required to elucidate further mechanisms by which VEGF-Axxxb may inhibit fibrogenesis and to address factors that may regulate alternative splicing of the VEGF-A gene.
Acknowledgments
Acknowledgment
The authors thank Andrea Cupp (University of Nebraska) for the MMTV-VEGF165b mice, S. Quaggin (University of Chicago) for reagents and mice, R. Coward (University of Bristol) for GFP reporter mouse, Genentech Inc. for loxp-VEGF mice, and the Lung Tissue Research Consortium for lung tissue samples.
Footnotes
Supported by the Wellcome Trust (95114), the Richard Bright Vascular Endothelial Growth Factor Research Trust, and the Medical Research Council (G10002073 and MR/K020366/1).
Author Contributions: S.L.B., S.O., C.J.S., G.I.W., D.O.B., and A.B.M. designed and performed experiments. T.B., T.J.H., C.J., K.O., G.S.-F., M.J.D., and S.H. performed experiments. T.M.M. contributed samples and Y.Q. developed the MMTV mouse line. S.L.B., G.I.W., C.J.S., D.O.B., and A.B.M. analyzed the data and wrote the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201603-0568OC on June 29, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, Hubbard RB. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66:462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 2.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 4.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 6.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 7.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 8.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87:262–271. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varet J, Douglas SK, Gilmartin L, Medford AR, Bates DO, Harper SJ, Millar AB. VEGF in the lung: a role for novel isoforms. Am J Physiol Lung Cell Mol Physiol. 2010;298:L768–L774. doi: 10.1152/ajplung.00353.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boussat S, Eddahibi S, Coste A, Fataccioli V, Gouge M, Housset B, Adnot S, Maitre B. Expression and regulation of vascular endothelial growth factor in human pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L371–L378. doi: 10.1152/ajplung.2000.279.2.L371. [DOI] [PubMed] [Google Scholar]
- 13.Koyama S, Sato E, Tsukadaira A, Haniuda M, Numanami H, Kurai M, Nagai S, Izumi T. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J. 2002;20:1449–1456. doi: 10.1183/09031936.02.00089802. [DOI] [PubMed] [Google Scholar]
- 14.Kaner RJ, Crystal RG. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol Med. 2001;7:240–246. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1001–L1010. doi: 10.1152/ajplung.2001.281.4.L1001. [DOI] [PubMed] [Google Scholar]
- 16.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 17.Thickett DR, Armstrong L, Christie SJ, Millar AB. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1601–1605. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- 18.Thickett DR, Armstrong L, Millar AB. A role for vascular endothelial growth factor in acute and resolving lung injury. Am J Respir Crit Care Med. 2002;166:1332–1337. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 19.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–626. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013;1832:911–921. doi: 10.1016/j.bbadis.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, Nakashima N, Maeyama T, Yoshimi M, Nakanishi Y. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol. 2005;175:1224–1231. doi: 10.4049/jimmunol.175.2.1224. [DOI] [PubMed] [Google Scholar]
- 22.Ou XM, Li WC, Liu DS, Li YP, Wen FQ, Feng YL, Zhang SF, Huang XY, Wang T, Wang K, et al. VEGFR-2 antagonist SU5416 attenuates bleomycin-induced pulmonary fibrosis in mice. Int Immunopharmacol. 2009;9:70–79. doi: 10.1016/j.intimp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Farkas L, Farkas D, Ask K, Möller A, Gauldie J, Margetts P, Inman M, Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest. 2009;119:1298–1311. doi: 10.1172/JCI36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhary NI, Roth GJ, Hilberg F, Müller-Quernheim J, Prasse A, Zissel G, Schnapp A, Park JE. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29:976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- 25.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 26.Kearns MT, Dalal S, Horstmann SA, Richens TR, Tanaka T, Doe JM, Boe DM, Voelkel NF, Taraseviciene-Stewart L, Janssen WJ, et al. Vascular endothelial growth factor enhances macrophage clearance of apoptotic cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L711–L718. doi: 10.1152/ajplung.00116.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockmann C, Kerdiles Y, Nomaksteinsky M, Weidemann A, Takeda N, Doedens A, Torres-Collado AX, Iruela-Arispe L, Nizet V, Johnson RS. Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc Natl Acad Sci USA. 2010;107:4329–4334. doi: 10.1073/pnas.0912766107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 30.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 31.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 32.Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P. Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood. 2011;118:816–826. doi: 10.1182/blood-2011-01-328773. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464–1471. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eswarappa SM, Potdar AA, Koch WJ, Fan Y, Vasu K, Lindner D, Willard B, Graham LM, DiCorleto PE, Fox PL. Programmed translational readthrough generates antiangiogenic VEGF-Ax. Cell. 2014;157:1605–1618. doi: 10.1016/j.cell.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barratt SL, Jarrett C, Blythe T, Welsh GI, Maher T, Bates DO, Millar AB.Bioavailability of VEGF in idiopathic pulmonary fibrosis Thorax 201267Suppl 2A34 [Google Scholar]
- 36.Barratt SL, Blythe T, Jarrett C, Welsh GI, Ourradi K, Scotton CS, Bates DO, Millar AB.Vascular endothelial growth factor (VEGF) expression in the IPF lung: a role for anti-angiogenic isoforms? Thorax 201469Suppl 2A37 [Google Scholar]
- 37.Barratt SL, Blythe T, Jarrett C, Ourradi K, Maher T, Welsh GI, Bates DO, Millar AB.Differential expression of conventional and inhibitory VEGFA isoforms in normal and fibrotic fibroblasts–a potential role in IPF pathogenesis? Thorax201368Suppl 3A139–A140. [Google Scholar]
- 38.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, Molyneaux PL, McKeever TM, Wells AU, Flynn A, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3:462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 39.Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scotton CJ, Krupiczojc MA, Königshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119:2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu Y, Bevan H, Weeraperuma S, Wratting D, Murphy D, Neal CR, Bates DO, Harper SJ. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- 42.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 43.Perl AK, Zhang L, Whitsett JA. Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol. 2009;40:1–3. doi: 10.1165/rcmb.2008-0011ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scotton CJ, Hayes B, Alexander R, Datta A, Forty EJ, Mercer PF, Blanchard A, Chambers RC. Ex vivo micro-computed tomography analysis of bleomycin-induced lung fibrosis for preclinical drug evaluation. Eur Respir J. 2013;42:1633–1645. doi: 10.1183/09031936.00182412. [DOI] [PubMed] [Google Scholar]
- 45.Meyer KC, Cardoni A, Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med. 2000;135:332–338. doi: 10.1067/mlc.2000.105618. [DOI] [PubMed] [Google Scholar]
- 46.Ando M, Miyazaki E, Ito T, Hiroshige S, Nureki SI, Ueno T, Takenaka R, Fukami T, Kumamoto T. Significance of serum vascular endothelial growth factor level in patients with idiopathic pulmonary fibrosis. Lung. 2010;188:247–252. doi: 10.1007/s00408-009-9223-x. [DOI] [PubMed] [Google Scholar]
- 47.Cosgrove GP, Brown KK, Schiemann WP, Serls AE, Parr JE, Geraci MW, Schwarz MI, Cool CD, Worthen GS. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242–251. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 48.Ye X, Abou-Rayyah Y, Bischoff J, Ritchie A, Sebire NJ, Watts P, Churchill AJ, Bates DO. Altered ratios of pro- and anti-angiogenic VEGF-A variants and pericyte expression of DLL4 disrupt vascular maturation in infantile haemangioma. J Pathol. 2016;239:139–151. doi: 10.1002/path.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter JG, Gammons MV, Damodaran G, Churchill AJ, Harper SJ, Bates DO. The carboxyl terminus of VEGF-A is a potential target for anti-angiogenic therapy. Angiogenesis. 2015;18:23–30. doi: 10.1007/s10456-014-9444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gammons MV, Fedorov O, Ivison D, Du C, Clark T, Hopkins C, Hagiwara M, Dick AD, Cox R, Harper SJ, et al. Topical antiangiogenic SRPK1 inhibitors reduce choroidal neovascularization in rodent models of exudative AMD. Invest Ophthalmol Vis Sci. 2013;54:6052–6062. doi: 10.1167/iovs.13-12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, et al. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurer B, Distler A, Suliman YA, Gay RE, Michel BA, Gay S, Distler JH, Distler O. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis. 2014;73:1880–1887. doi: 10.1136/annrheumdis-2013-203535. [DOI] [PubMed] [Google Scholar]
- 53.Hirigoyen D, Burgos PI, Mezzano V, Duran J, Barrientos M, Saez CG, Panes O, Mezzano D, Iruretagoyena M. Inhibition of angiogenesis by platelets in systemic sclerosis patients. Arthritis Res Ther. 2015;17:332. doi: 10.1186/s13075-015-0848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng N, Sanchez CG, Lasky JA, Zhu D. Detecting splicing variants in idiopathic pulmonary fibrosis from non-differentially expressed genes. PLoS One. 2013;8:e68352. doi: 10.1371/journal.pone.0068352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nance T, Smith KS, Anaya V, Richardson R, Ho L, Pala M, Mostafavi S, Battle A, Feghali-Bostwick C, Rosen G, et al. Transcriptome analysis reveals differential splicing events in IPF lung tissue. PLoS One. 2014;9:e92111. doi: 10.1371/journal.pone.0092111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 57.Kamio K, Sato T, Liu X, Sugiura H, Togo S, Kobayashi T, Kawasaki S, Wang X, Mao L, Ahn Y, et al. Prostacyclin analogs stimulate VEGF production from human lung fibroblasts in culture. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1226–L1232. doi: 10.1152/ajplung.00129.2007. [DOI] [PubMed] [Google Scholar]
- 58.Jenkins G, Blanchard A, Borok Z, Bradding P, Ehrhardt C, Fisher A, Hirani N, Johnson S, Königshoff M, Maher TM, et al. ECIPF workshop. In search of the fibrotic epithelial cell: opportunities for a collaborative network. Thorax. 2012;67:179–182. doi: 10.1136/thoraxjnl-2011-200195. [DOI] [PubMed] [Google Scholar]
- 59.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, Sasano H, Kondo T, Nukiwa T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;169:1203–1208. doi: 10.1164/rccm.200308-1111OC. [DOI] [PubMed] [Google Scholar]