Abstract
Hotspot mutations in isocitrate dehydrogenase 1 (IDH1) initiate low-grade glioma and secondary glioblastoma and induce a neomorphic activity that converts α-ketoglutarate (α-KG) to the oncometabolite D-2-hydroxyglutarate (D-2-HG). It causes metabolic rewiring that is not fully understood. We investigated the effects of IDH1 mutations (IDH1MUT) on expression of genes that encode for metabolic enzymes by data mining The Cancer Genome Atlas. We analyzed 112 IDH1 wild-type (IDH1WT) versus 399 IDH1MUT low-grade glioma and 157 IDH1WT versus 9 IDH1MUT glioblastoma samples. In both glioma types, IDH1WT was associated with high expression levels of genes encoding enzymes that are involved in glycolysis and acetate anaplerosis, whereas IDH1MUT glioma overexpress genes encoding enzymes that are involved in the oxidative tricarboxylic acid (TCA) cycle. In vitro, we observed that IDH1MUT cancer cells have a higher basal respiration compared to IDH1WT cancer cells and inhibition of the IDH1MUT shifts the metabolism by decreasing oxygen consumption and increasing glycolysis. Our findings indicate that IDH1WT glioma have a typical Warburg phenotype whereas in IDH1MUT glioma the TCA cycle, rather than glycolytic lactate production, is the predominant metabolic pathway. Our data further suggest that the TCA in IDH1MUT glioma is driven by lactate and glutamate anaplerosis to facilitate production of α-KG, and ultimately D-2-HG. This metabolic rewiring may be a basis for novel therapies for IDH1MUT and IDH1WT glioma.
Keywords: glioma, metabolism, isocitrate dehydrogenase 1, glycolysis, glutamate
INTRODUCTION
Diffuse gliomas are infiltrative neoplasms that arise in the cerebral hemispheres of adults and are graded as WHO II-IV based on histopathological characteristics. Grade II and III are low-grade gliomas (LGG) of which a subset may progress to secondary glioblastoma (WHO grade IV), whereas others may remain relatively stable for longer periods of time. In contrast to this gradual progression of malignancy, the majority of glioblastomas arise de novo, and are then called primary glioblastoma. Complete surgical resection of diffuse glioma is impossible due to their highly invasive nature and without exception residual tumor is a source of recurrence and malignant progression. Median overall survival of LGG ranges widely from 3 to 15 years and patients with de novo primary glioblastoma, the most common and aggressive primary brain tumor, have a median overall survival of 16 months. Despite all efforts to therapeutically target various pathways that are involved in glioma progression, significant improvement in survival has not been achieved since 2005, when temozolomide was added to irradiation as standard therapy [1, 2].
A subset of gliomas displays increased sensitivity towards chemo- and radiotherapy with prolonged median overall patient survival. Recently, it was demonstrated that these characteristics are strongly associated with mutations in the metabolic enzyme isocitrate dehydrogenase (IDH) [3]. Hotspot mutations in IDH1 and, less frequently, IDH2 occur in 80% of WHO grade II-III and secondary WHO grade IV gliomas and are ancestral events in the formation of these neoplasms [4, 5]. In addition to glioma, IDH1/2 mutations (IDH1/2MUT) occur in substantial percentages in various other tumor types, such as acute myeloid leukemia (20–40%), chondrosarcoma (60%), intrahepatic cholangiocarcinoma (20%) and melanoma (5–10%) [6].
IDH1 and IDH2 are homodimeric enzymes that reversibly convert isocitrate to α-ketoglutarate (α-KG) with concomitant reduction of NADP+ to NADPH in the cytoplasm and mitochondria, respectively. The mutations lead to a neomorphic activity of the enzyme that converts α-KG into the oncometabolite D-2-hydroxyglutarate (D-2-HG). D-2-HG competitively inhibits α-KG-dependent enzymes [7, 8], including histone demethylases and 5-methylcytosine hydroxylases that are essential for epigenetic regulation of gene expression, including that of metabolic genes [9–13]. IDH1/2MUT changes cellular metabolism via 4 mechanisms: 1) loss of wild-type IDH1/2 (IDH1/2WT) function that affects carbohydrate and NADP+/NADPH metabolism; 2) accumulation of D-2-HG that further restricts the activity of various enzymes such as α-ketoglutarate dehydrogenase (α-KGDH), succinate dehydrogenase (SDH) and complex IV of the mitochondrial electron transport chain, 3) epigenetic effects of D-2-HG on expression of genes involved in metabolism and 4) increased degradation of the hypoxia-response transcription factor HIF-1α, a major inducer of expression of genes involved in glycolysis [3, 6, 14, 15].
Reprogramming of cellular metabolism is one of the hallmarks of cancer [16]. It is generally assumed that malignant cells use pyruvate for lactate production instead of fluxing it into acetyl-CoA in the TCA cycle, even in the presence of oxygen [17]. This so-called Warburg effect is an accepted metabolic phenotype in cancer that allows rapid generation of biosynthetic intermediates at the expense of less efficient ATP production. As a result, cancer cells overexpress glucose transporters to compensate for their ATP demand via an abnormally high rate of glucose uptake. Presumably, the composition and availability of metabolites in the microenvironment, for example in the adult brain glucose, glutamate, glutamine and acetate, dictates which metabolites are the predominant carbon sources for cancer cells. These metabolites can all serve as precursors for macromolecules such as proteins, nucleotides and lipids [18, 19].
It has been reported that glioma cells have a higher rate of aerobic glycolysis than healthy brain tissue, and this is more pronounced in glioblastoma than in LGG [20, 21]. However, these observations may be biased because most analyses of glioblastoma tissue use resection material that likely contains angiogenic, hypoxic and necrotic regions. Metabolism in these areas may well differ from that in peripheral invasive regions that presumably do not experience hypoxia. Talasila et al. [22] reported recently that the centre the tumor centre of glioblastoma is particularly glycolytic, in line with the presence of perinecrotic hypoxia, and we previously observed similar metabolic phenotypes in preclinical orthopic xenograft models [23]. However, how metabolic pathways in glioma are affected by IDH1MUT is not completely clear.
In order to evaluate the metabolic rewiring associated with IDH1MUT, we performed an in silico analysis of the transcripts that encode for metabolic enzymes and substrate transporters. These gene expression data were correlated with IDH1 mutational status in 677 LGG and glioblastoma samples derived from The Cancer Genome Atlas (TCGA). This study provides a comprehensive overview of the expression of key glycolytic, TCA cycle and glucose/lactate/glutamate/acetate anaplerosis enzymes in IDH1MUT glioma versus IDH1WT glioma.
RESULTS
We determined differences in expression levels of metabolic enzymes in IDH1WT versus IDH1MUT glioma to increase our understanding of the metabolism of these two clinically very distinct types of gliomas. We included 399 IDH1MUT and 112 IDH1WT LGG (WHO stage II: 49% and WHO stage III: 51%) and 157 IDH1WT and 9 IDH1MUT WHO stage IV glioblastoma samples. The ratios of IDH1-mutated to IDH1 wild-type tumors are in agreement with mutation frequencies from earlier reports of TCGA glioma data and other datasets. In line with previous reports [4, 24, 25], our dataset shows an approximately 3-fold prolonged survival of IDH1MUT LGG and glioblastoma patients compared to IDH1WT LGG and glioblastoma patients (Supplementary Figure 1).
Expression of GLUT3 and glucose-metabolizing enzymes is low in IDH1MUT compared to IDH1WT glioma
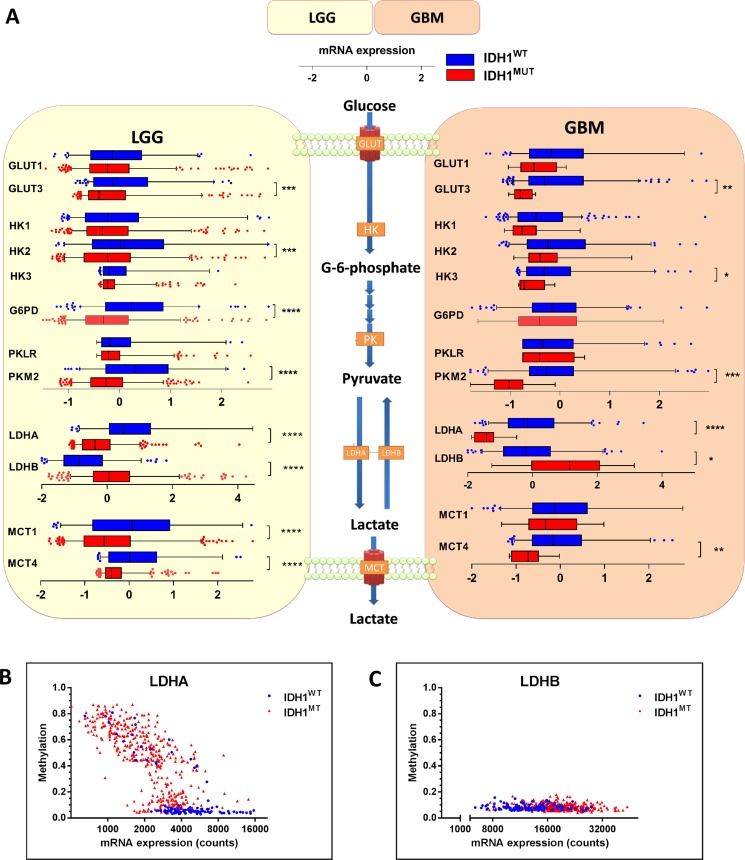
To investigate the first rate-limiting step of cellular glucose metabolism, i.e. transport of glucose across the plasma membrane, we examined expression levels of SLC2A1 and SLC2A3, the genes encoding for glucose transporters (GLUT1 and GLUT3). GLUT3 expression levels were low in IDH1MUT glioma when compared with IDH1WT glioma, whereas GLUT1 expression did not differ between IDH1MUT and IDH1WT glioma (Figure 1A). The rate-limiting, irreversible and glycolysis-related enzymes hexokinase (HK2 and HK3) and pyruvate kinase (PKM2), but not HK1 or the pyruvate kinase isoform PKLR, were also expressed at lower levels in IDH1MUT glioma compared with IDH1WT glioma (Figure 1). High expression of HK2 was significant in IDH1WT LGG, whereas HK3 expression was significant higher in IDH1WT glioblastoma. The rate-limiting enzyme of the pentose-phosphate pathway, glucose-6-phosphate dehydrogenase (G6PD) was expressed at higher levels in IDH1WT glioma, but only in LGG and not glioblastoma. These results suggest that IDH1WT glioma depend more on glycolysis than IDH1MUT glioma.
Figure 1. mRNA expression levels of enzymes involved in glucose metabolism in IDH1WT versus IDH1MUT glioma.
IDH1WT glioma show higher expression levels of genes that are typically upregulated in glycolytic cancer cells. (A) Analysis of gene expression levels of metabolic enzymes involved in transport and metabolism of glucose in IDH1WT (n = 399) and IDH1MUT (n = 112) LGG and IDH1WT (n = 157) and IDH1MUT (n = 9) glioblastoma samples obtained from the TCGA database. Relative mRNA expression levels are shown for IDH1WT (blue) and IDH1MUT (red). (B) LDHA and (C) LDHB expression and methylation according to IDH1MUT status (blue: IDH1WT, red: IDH1MUT). Abbreviations: GLUT, glucose transporter; HK, hexokinase; G6PD, glucose-6-phosphate dehydrogenase; PK, pyruvate kinase; LDH, lactate dehydrogenase.
IDH1WT glioma express Warburg-effect genes, whereas IDH1MUT glioma express TCA cycle genes
The relative activities of lactate fermentation in the glycolysis and the TCA cycle are controlled by two sets of enymes that determine the fate of pyruvate: pyruvate dehydrogenase (PDH) and the lactate dehydrogenases (LDHA and LDHB, where LDHA catalyzes conversion of pyruvate into lactate and LDHB catalyzes the reverse reaction). In IDH1WT glioma, we observed a striking overexpression of LDHA relative to IDH1MUT glioma (Figure 1A). Lower expression levels of LDHA in IDH1MUT glioma were associated with hypermethylation of its promoter (Figure 1B). Conversely, IDH1MUT glioma showed higher LDHB expression levels than IDH1WT glioma. To maintain pH homeostasis, cancer cells up-regulate transporters such as the monocarboxylate transporters 1 and 4 (MCT1 and MCT4) for secretion of lactate [26, 27]. In IDH1WT glioma, we observed higher expression of MCT1 and MCT4 as compared to IDH1MUT glioma, suggesting increased transport of lactate in IDH1WT glioma. These results indicate that IDH1WT gliomas catabolize glucose in the glycolytic pathway with lactate as end product whereas in IDH1MUT glioma lactate is converted into pyruvate.
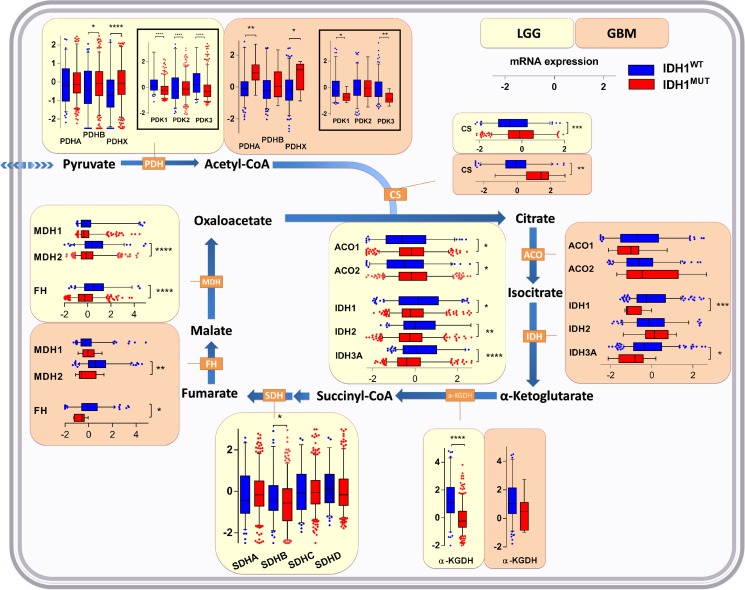
Conversion of pyruvate into acetyl-CoA for its entry into the TCA is accomplished by the pyruvate dehydrogenase (PDH) complex, the activity of which is controlled by pyruvate dehydrogenase kinase (PDK; isoforms PDK1, PDK2 and PDK3). PDK inactivates PDH via phosphorylation. PDH is a heterotrimeric enzyme complex composed of subunits PDHA and PDHB, forming the catalytic PDH component PDH E1, and PDHX, a non-catalytic regulatory subunit. In IDH1WT glioma, we observed higher expression of regulator genes PDK1 and PDK3 as compared to IDH1MUT glioma. On the other hand, IDH1MUT glioma showed higher expression levels of PDHA (in glioblastoma), PDHB (in LGG) and PDHX as compared to IDH1WTglioma (Figure 2). Combined with the aforementioned glycolysis data these results suggest that in contrast to IDH1WT glioma, IDH1MUT glioma actively prevents the generation of lactate by silencing LDHA and maintaining high expression of LDHB, PDHA/B and PDHX. These conditions ensure that pyruvate is maximally used for TCA entry.
Figure 2. Expression levels of genes of enzymes involved in the TCA cycle in IDH1WT versus IDH1MUT glioma.
Increased TCA upstream of isocitrate, but decreased metabolism downstream of isocitrate in IDH1MUT glioma as indicated by gene expression levels of enzymes involved in the TCA cycle in IDH1WT (n = 399) and IDH1MUT (n = 112) LGG and IDH1WT (n = 157) and IDH1MUT (n = 9) glioblastoma. Relative mRNA expression levels are shown for IDH1WT (blue) and IDH1MUT (red). Abbreviations: PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; CS, citrate synthase; ACO, aconitase; IDH, isocitrate dehydrogenase; a-KGDH, α-ketoglutarate dehydrogenase; SDH, succinate dehydrogenase; FH, fumarate hydratase; MDH, malate dehydrogenase.
IDH1MUT glioma have increased expression of TCA genes upstream of isocitrate and decreased expression of TCA genes downstream of isocitrate
Intermediate metabolites of glycolysis upstream of pyruvate can be shunted into macromolecular biosynthesis pathways without stalling glycolysis itself. In contrast, the TCA cycle must be continuously running and be completed to function optimally: generation of citrate by citrate synthase (CS) requires pyruvate-derived acetyl-CoA, the principal fuel of mitochondria, and the last TCA cycle metabolite (oxaloacetate). Mitochondrial products of a full TCA cycle are ATP and CO2. The lack of glycolysis in IDH1MUT gliomas suggests that these cancers apply alternative metabolic pathways that ensure sufficient energy and building blocks for macromolecular biosynthesis. To investigate this in detail, we examined expression levels of TCA-related enzymes in IDH1MUT and IDH1WT gliomas.
CS and aconitase (ACO1/2), enzymes that function upstream of citrate oxidation, were expressed at higher levels in IDH1MUT glioma than in IDH1WT glioma (Figure 2). In contrast, expression levels of enzymes involved in downstream metabolism of isocitrate, including IDH1, IDH2 and IDH3A, α-KGDH, succinate dehydrogenase (SDHB), fumarate hydratase (FH) and malate dehydrogenase (MDH2), were remarkably lower in IDH1MUT glioma versus IDH1WT glioma. These results suggest that IDH1MUT glioma have an imbalanced TCA, with increased enzyme activity upstream of isocitrate, and decreased TCA cycle metabolism downstream of isocitrate. The imbalance resulting from low oxaloacetate production and high pyruvate influx causes disbalance that may be problematic for efficient citrate production. As a compensatory mechanism, oxaloacetate can also be produced directly from pyruvate by the activity of pyruvate carboxylase (PC). This reaction is counteracted by phosphoenolpyruvate carboxykinase 2 (PCK2) which catalyzes the reaction of oxaloacetate to phosphoenolpyruvate. In IDH1MUT glioma, PC levels were markedly higher than in IDH1WT glioma, whereas PCK2 expression levels were significantly lower in IDH1MUT glioma although differences in PCK2 levels between IDH1WT and IDH1MUT in glioblastoma were less apparent (Figure 3). Collectively, these results indicate increased flux of pyruvate into the TCA cycle in IDH1MUT glioma compared to IDH1WT counterparts.
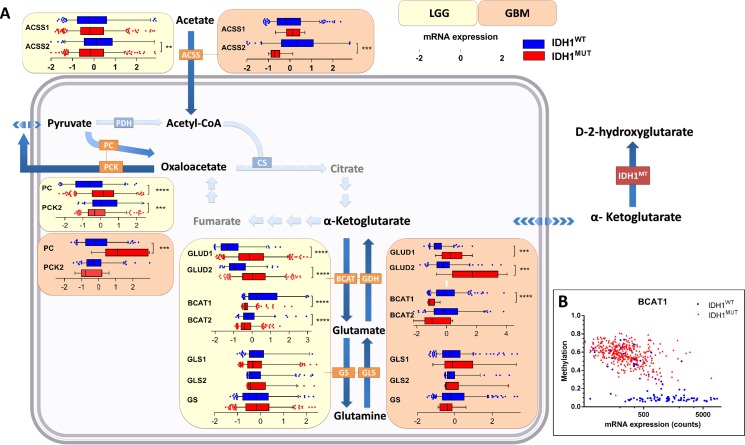
Figure 3. Contribution of glucose/glutamine/glutamate anaplerosis and acetate influx to TCA cycle metabolism in IDH1WT versus IDH1MUT glioma.
Increased glucose/glutamate anaplerosis in IDH1MUT glioma and high contribution of acetate in IDHWT glioma metabolism. (A) Gene expression levels of metabolic enzymes involved in glucose anaplerosis, glutaminolysis and acetate metabolism in IDH1WT (n = 399) and IDH1MUT (n = 112) LGG and IDH1WT (n = 157) and IDH1MUT (n = 9) glioblastoma. Relative mRNA expression levels are shown for IDH1WT (blue) and IDH1MUT (red). (B) BCAT1 expression and methylation according to IDH1MUT status (blue: IDH1WT, red: IDH1MUT). Abbreviations: PC, pyruvate carboxylase; GLUD, glutamate dehydrogenase; GLS, glutaminase; BCAT, branched-chain amino acid transferase; GS, glutamine synthetase; ACSS, acetyl-CoA synthetase.
Higher lactate/glutamate anaplerosis in IDH1MUT glioma and higher acetate anaplerosis in IDHWT glioma
We have previously postulated the hypothesis that glutamate could act as anaplerotic precursor for the TCA cycle in IDH1MUT glioma [28]. To test this assumption, we analyzed the expression levels of mRNA of enzymes that are involved in the transport and conversion of these metabolites.
Glutamine is a major source of TCA cycle metabolites via glutaminolyis, a process that converts glutamine to α-KG via two subsequent deamination steps. To investigate its contribution to α-KG replenishment in the TCA cycle, we determined mRNA expression levels of glutaminase (GLS and GLS2, converting glutamine to glutamate) and glutamate dehydrogenase (GLUD1 and GLUD2, converting glutamate to α-KG). We also investigated mRNA expression levels of branched-chain amino acid transferase (BCAT1 and BCAT2) and glutamine synthetase (GS), which catalyze the converse reaction of α-KG into glutamate and of glutamate into glutamine, respectively. In IDH1MUT glioma, we observed strikingly higher expression levels of GLUD1/2 than in IDH1WT glioma, whereas BCAT1/2 expression levels were much lower in IDH1MUT gliomas than in IDH1WT glioma (Figure 3), as has been reported previously [29]. Low BCAT1 mRNA expression levels in IDH1MUT glioma were associated with BCAT1 promoter hypermethylation (Figure 3B).
We observed no significant differences between mRNA expression levels of GLS, GLS2 or GS between IDH1WT glioma and IDH1MUT glioma. These results suggest that IDH1MUT gliomas predominantly utilize glutamate, rather than glutamine, for TCA cycle anaplerosis.
Acetate can be utilized as an alternative substrate to generate acetyl-CoA and is transferred through the plasma membrane by MCT1 and MCT4 [30]. Acetyl-CoA synthetase (ACSS1 and ACSS2) converts acetate to acetyl-CoA, which can be catabolized in the TCA cycle. In IDH1WT glioma, ACSS2 showed an elevated mRNA expression compared to IDH1MUT glioma (Figure 3), suggesting a higher contribution of acetate to the acetyl-CoA pool in IDH1WT glioma. As indicated previously, higher contribution of acetate is in line with higher expression levels of MCT1 and MCT4 transporters in IDH1WT glioma.
Taken together, we show that relative to IDH1WT glioma, IDH1MUT glioma use lactate and glutamate (but lesser so glutamine) for the TCA cycle, by maintaining high expression of LDHB, PC and GLUD and silencing BCAT. In contrast, in IDH1WT glioma glycolysis is more prominent and acetate is used to fuel the TCA cycle.
Inhibition of the IDH1MUT decreases oxygen consumption and increases lactate acidification
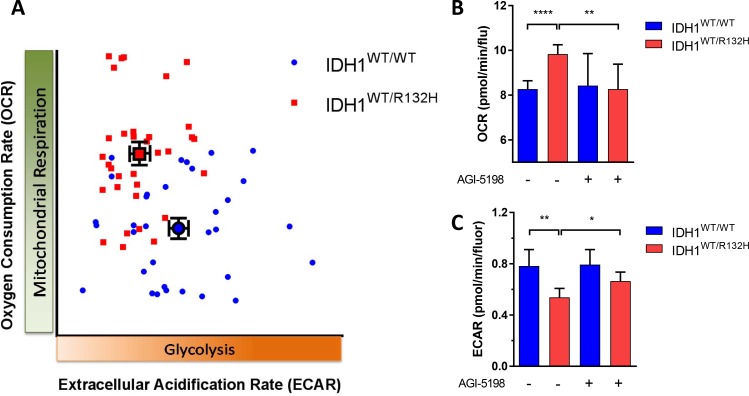
There is a scarcity of relevant models to study metabolic effects of IDH mutations in vitro [31]. As an alternative we used the HCT116-IDH1R132H knock in cell line and its parental counterpart to investigate whether metabolic changes in IDH1R132H gliomas were reflected in this isogenic cell line pair too. We first measured oxygen consumption in HCT116 IDH1WT/R132H and HCT116 IDH1WT/WT cells in the Seahorse [32]. The basal respiration was significantly higher in HCT116 IDH1WT/R132H cells than in HCT116 IDH1WT/WT cells, showing that also in this system IDH1MUT cells have higher oxygen consumption and are more dependent on OXPHOS (Figure 4). To determine whether the IDH1MUT is causally involved in the higher respiration rates, we tested the effects of the specific IDH1R132H inhibitor AGI-5198. In line with expectations after 14 days of culture in the presence of the IDH1MUT inhibitor AGI-5198 the respiration rate of HCT116 IDH1WT/R132H cells was significantly decreased and comparable to levels of HCT116 IDH1WT/WT cells (Figure 4B).
Figure 4. The metabolic phenotype of IDH1MUT and IDH1WT.
(A) Characterization of metabolic phenotype; HCT116 IDH1WT/R132H cells (red) are dependent on OXPHOS, whereas HCT116 IDH1WT/WT cells (blue) cells are glycolytic. (B) The basal oxygen consumption rate (OCR) response of the HCT116 cell lines to 10 mM glucose, 2 mM glutamine and 1.5 mM pyruvate, with or without pretreatment with the IDH1MUT inhibiter AGI-5198 (1 μM, 14 days of incubation). (C) Extracellular acidification rate (ECAR) response of HCT116 cells to glucose (25 mM), with or pretreatment with of AGI-5198 (1 μM, 14 days of incubation). All data were expressed as pmol of O2 per minute and normalized by cell number measured by fluorochrome binding to nucleic acids. A representative experiment out of 4 is shown here, each data point represents mean ± SEM. Plots are visualized with 95% confidence intervals and significance levels are shown by (*P < 0.05), (**P < 0.01) and (****P < 0.0001).
In order to investigate the glycolytic activity, we measured the extracellular acidification rate (ECAR) that determines the glycolytic phenotype of cells and is linearly related to lactate production [33, 34]. In HCT116 IDH1WT/R132H cells, we found a lower ECAR response to glucose compared to HCT116 IDH1WT/WT cells, indicating a lower glycolytic rate in IDH1MUT cells (Figure 4). The glycolytic capacity was also significantly lower in HCT116 IDH1WT/R132H cells compared to HCT116 IDH1WT/WT cells (data not shown). After 14 days incubation in the presence of AGI-5198, we measured a slight increase of ECAR in HCT116 IDH1WT/R132H cells, indicating a shift of metabolism to glycolysis by inhibiting IDH1MUT (Figure 4C). Taken together, we show that relative to IDH1WT cells, IDH1MUT cells are dependent on OXPHOS and inhibition of the IDH1MUT decreases the oxygen consumption leading to a glycolytic phenotype.
DISCUSSION
The present study is the first to investigate the metabolic rewiring that is associated with IDH1MUT in glioma in a comprehensive and integrated fashion. A summary of this metabolic rewiring is shown in Figure 5. We correlated the expression of key glycolytic, TCA cycle and glucose/lactate/glutamate/acetate anaplerosis enzymes with the IDH1MUT status obtained from a large database containing data on both LGG and glioblastoma patients. We show that IDH1WT glioma overexpress enzymes that play key roles in anaerobic glycolysis, i.e. genes that are typically upregulated in Warburg-like cancer cells. In contrast, expression of genes that encode for glycolytic metabolism are downregulated in IDH1MUT glioma. Based on the axiom that gene expression levels are correlated with pathway activity, our data suggest that IDH1MUT glioma predominantly catabolize glutamate, and to a lesser extent glucose, in the TCA cycle for α-KG production. In support of this axiom, we confirmed these observations in functional studies by measuring lactate production and oxygen consumption in HCT116 IDH1WT/WT and HCT116 IDH1WT/R132H cells.
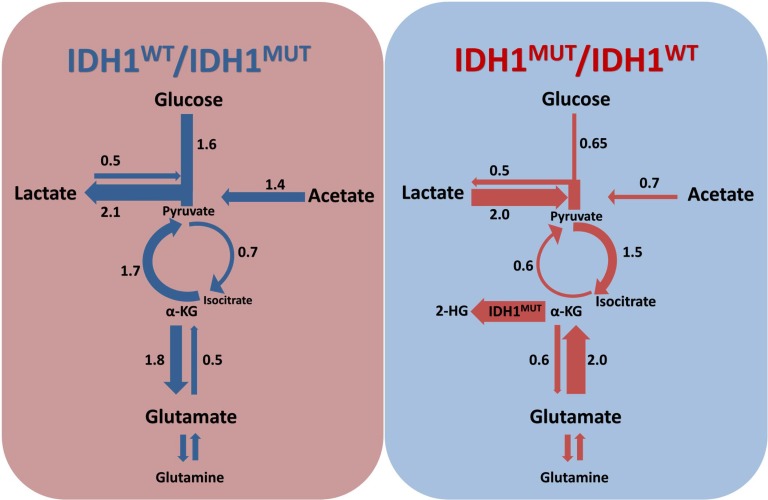
Figure 5. Rewiring of metabolism by IDH1MUT.
IDH1WT/IDH1MUT and IDH1MUT/IDH1WT ratios indicate contribution of a particular pathway as calculated on the basis of gene expression levels in IDH1WT and IDH1MUT, respectively. IDH1WT glioma catabolize glucose in the glycolysis with lactate as end product, whereas IDH1MUT glioma prefers production of pyruvate from lactate and use the TCA cycle to generate isocitrate. Glucose, lactate and glutamate replenish the TCA cycle in IDH1MUT glioma, to facilitate α-KG production for consumption by IDH1MUT to generate 2-HG, whereas acetate anaplerosis is important in IDH1WT glioma.
Glycolysis and lactate production are considered to be the major metabolic ways of processing of glucose in cancer cells as it generates both energy and carbon for macromolecular biosynthesis, required for sustained cell proliferation [35]. In particular, GLUT3, HK2, PKM2 and LDHA play critical roles in initiating and maintaining the high glycolytic rates of rapidly proliferating cancer cells, and are associated with greater dependence on glycolysis than on OXPHOS [36–40]. Our findings are in agreement with the existence of the Warburg effect in IDH1WT glioma, whereas our data indicate that glycolysis and lactate production occur to a lesser extent in IDH1MUT glioma. In line with this notion, silencing of HK2 increases oxygen consumption and decreases lactate production in glioblastoma cells [41]. The elevated levels of LDHB in IDH1MUT gliomas support the findings in previous studies that showed that IDH1MUT glioma have reduced intracellular lactate levels compared to IDH1WT glioma, [42, 43]. Moreover, a recent study showed that the conversion rate of pyruvate into lactate was similar in IDH1MUT glioma and normal brain, whereas IDH1WT glioma showed a higher lactate production than normal brain [44]. Our results also support previous reports that show that IDH1MUT gliomas silence LDHA expression through hypermethylation of its promoter [43] resulting in a low LDHA/LDHB ratio compared with IDH1WT glioma or normal brain tissue [45]. Reduced MCT expression in IDH1MUT glioma is consistent with reduced glycolytic production of lactate as well as silencing of LDHA expression; cells that produce less lactate do not require an increase in its export and are less dependent on MCT expression.
Lactate production by IDH1WT glioma cells has been suggested to acidify the extracellular space [46, 47] resulting in the death of surrounding normal brain cells and increasing the infiltrative potential of cancer cells [48]. Our results suggest that IDH1MUT glioma produce and secrete less lactate than IDH1WT glioma which may correlate with a normalized tissue pH in IDH1MUT glioma and the less aggressive behavior of IDH1MUT glioma as compared to IDH1WT glioma [49].
Compared with IDH1WT glioma, IDH1MUT glioma showed elevated expression levels of TCA cycle genes that function upstream of isocitrate whereas expression of TCA cycle genes downstream of isocitrate were decreased. In line with these observations, a recent study reported high expression of CS and ACO2 in IDH1MUT glioma versus IDH1WT glioma [45]. We have shown previously that IDH enzymatic activity in IDH1MUT glioblastoma is reduced compared to IDH1WT glioblastoma [25]. Consistent with these findings, a previous report demonstrated increased levels of isocitrate (2.7-fold) and D-2-HG (28-fold) in IDH1MUT cancer cells versus IDH1WT cancer cells [8, 50]. In addition to decreased SDH expression in IDH1MUT glioma, a recent study demonstrated elevated succinate levels and protein succinylation in IDH1MUT glioma samples [51]. Taken together, the changes in gene expression levels in IDH1MUT suggest that mitochondrial isocitrate production is maximized, providing substrate for the cytosolic IDH1WT/IDH1MUT dimer complexes to generate α-KG and D-2-HG, respectively.
Due to the lack of stable glioma cell cultures carrying an endogenous IDH1R132H allele, we used in vitro HCT116 colorectal carcinoma cells for functional studies. IDH1MUT occur in 0.5% of colorectal carcinomas [52]. Because IDH1R132H functions as a heterodimer with IDH1WT, 1:1 IDH1R132H:IDH1WT expression in IDH1WT/R132H HCT116 cells is more relevant than IDH1R132H overexpression models that are frequently used. Previous reports have shown that HCT116 IDH1MUT cells have reduced growth rates as compared to IDH1WT cells under conditions of low oxygen tension suggesting that these are more dependent on OXPHOS [53].
Another major contributor to TCA metabolism in cancer cells is glutamine, which provides α-KG via the glutaminolysis pathway. Moreover, 80% of D-2-HG generated by heterozygous IDH1WT/MUT HCT116 colorectal cancer cells is derived from glutamine [53, 54], rendering this an important metabolite for D-2-HG generation. Whereas glutamine has been suggested to be an important carbon source in several types of cancers, experiments in xenograft models revealed that glioblastoma cells take up glutamine but do not directly oxidize it in the TCA cycle [55]. Our analysis suggests an increased contribution of glutamate, rather than glutamine, to the TCA cycle in IDH1MUT glioma, by maintaining high expression levels of GLUD1/2 and silencing or downregulation of BCAT expression. Our results support the findings of previous studies that demonstrated increased conversion of glutamate to α-KG by GLUD [45, 56] and reduced conversion of α-KG to glutamate by BCAT1 in IDH1MUT glioma, which is in line with promoter methylation of BCAT1 in IDH1MUT glioma [29, 57].
In line with our observations, a recent study demonstrated that IDH1MUT overexpression in astrocytes results in increased PC expression and increased fractional flux through PC, suggesting that direct oxaloacetate production from pyruvate is critical for IDH1MUT cancer cells to maintain TCA activity [58]. Furthermore, metabolic flux studies in IDH1MUT glioma models with [1–13C]-glucose showed that 20% of 2-HG was derived from glucose, suggesting that glucose is a less relevant donor of carbon for 2-HG production than glutamate [54]. On the other hand, we observed a high expression of PCK2 that shuttles oxaloacetate into glycolysis suggesting increased contribution from the TCA cycle to glycolysis in IDH1WTglioma. In line with this observation, a study also reported high expression of PCK2 in IDH1WT glioma versus IDH1MUT glioma [45].
Finally, acetate metabolism can replenish the TCA cycle at the level of acetyl-CoA and this is an important anaplerosis pathway in the majority of glioblastoma [55]. Our analyses suggest that this phenomenon is more important in IDH1WT glioma than in IDH1MUT glioma. Mashimo et al. did not stratify their glioblastoma cohort based on IDH1MUT status but demonstrated higher expression of ACSS2 in glioblastoma than in grade II and III glioma and high ACSS2 expression in LGG patients with poor survival as opposed to low ACSS2 expression in LGG patients with prolonged survival. This resonates with the high frequency of IDH1MUT in LGG cohorts and clarifies the observed association of ACSS2 expression and LGG patient survival, potentially reflecting the differences in ACSS2 expression and patient survival between IDH1MUT and IDH1WT glioma.
Our transcriptome-wide approach study was performed on a large number of LGG and glioblastoma samples derived from TCGA. These data have been analyzed previously in an effort to improve the pathological classification of glioma. It was proposed to categorize LGG in 3 molecular classes, based on IDH1, 1p/19q and TP53 status, instead of histology [59]. In addition, IDH1WT LGG appeared to be molecularly and clinically more similar to IDH1WT glioblastoma than to IDH1MUT LGG. Our studies support these conclusions in more detail, based on the profound similarities in metabolic transcript profiles both in IDH1WT gliomas and in IDH1MUT gliomas, irrespective of WHO grade. Of note, our data are confirmed by independent studies showing lower activity of HK, SDH and ACSS2 and higher activity of CS in LGG compared to glioblastoma [41, 55, 60]. Considering the fact that IDH1MUT is more prevalent in LGG than in glioblastoma (80% versus 12%), this suggests that IDH1MUT is an important determinator in histological analyses and that metabolic analyses should be stratified by IDH1MUT status and not histology.
These insights are relevant for rational development of metabolic anti-glioma therapies, because our data predict that inhibitors of glycolysis, such as HK2/3 and PKM2 [61, 62] may be the most promising for IDH1WT glioma, whereas inhibitors of the TCA cycle and the glutamatolysis pathway such as metformin, chloroquine and epigallocatechin-3-gallate [3, 56] may possess a therapeutic index in IDH1MUT glioma.
MATERIALS AND METHODS
Patient selection
Gene expression analysis of LGG and glioblastoma data from TCGA (http://cancergenome.nih.gov) was conducted on processed data of the UCSC Cancer Genome Browser portal [63] and the cBioPortal for Cancer Genomics [64, 65]. The molecular data of 399 IDH1MUT versus 112 IDH1WT LGG and 157 IDH1WT versus 9 IDH1MUT glioblastoma samples were subsetted according to the IDH1 mutational status and analyzed for expression and methylation of metabolic enzymes. Another 16 LGG and 198 glioblastoma tumors were excluded because of unknown IDH1 mutational status or lack of molecular data.
In silico analysis
Relative mRNA expression (RNAseq, IlluminaHiSeq) values were identified for relevant genes encoding for metabolic enzymes involved in glycolysis, the TCA cycle, glutamine-glutamate cycle and acetate cycle (Supplementary Table 1). Annotations were obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG). Mean normalized z-scores for mRNA levels were determined for IDH1MUT and IDH1WT datasets. The expression of each gene in each sample was normalized separately. The returned value indicated the number of standard deviations away from the population mean. In the methylation data, the probes with the strongest negative correlation between the methylation signal and gene expression were included.
Cell culture
HCT116 IDH1WT/R132H knock-in cells, generated by AAV targeting technology GENESIS (28), were kindly provided by Horizon Discovery (Cambridge, UK). IDH1WT/R132H and IDH1WT/WT HCT116 cells were cultured in McCoy's 5A medium (Gibco, Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA) in 5% CO2 at 37°C. Media was supplemented with 10% fetal bovine serum (HyClone, Thermo Fisher Scientific), 100 units/ml penicillin and 100 μg/ml streptomycin (both Gibco).
Reagents
AGI-5198 was purchased from MedChemExpress (Monmouth Junction, NJ, USA), olygomycin, carbonyl-cyanide-(trifluoromethoxy)phenylhydrazone (FCCP), antimycin A, rotenone, 2-deoxyglucose, glucose, L-glutamine and sodium pyruvate were purchased from Sigma-Aldrich. (St. Louis, MO, USA).
Measurement of oxygen consumption rate and extracellular acidification rates
HCT 116 cells, with and without pre-treatment of 1μM AGI-5198, were grown in 5% CO2 at 37°C and OCR and ECAR was measured using an XFe96 analyzer (Seahorse Bioscience, North Billerica, MA, USA). HCT116 cells were plated in XF96 cell culture plates, 2.0*104 IDH1WT/R132H HCT116 cells or 1.75*104 IDH1WT/WT cells (to account for a more rapid proliferation of IDH1WT/WT HCT116 cells relative to IDH1WT/R132H HCT116 cells ([53] and our own observation), were seeded in each well of 96-well assay plates and incubated for 48 h prior to conducting the assay. For determination of OCR, medium was changed to DMEM supplemented with 10 mM glucose, 2 mM glutamine and 1.5 mM pyruvate. For the glycolytic flux, medium was changed to DMEM supplemented with 2 mM L-glutamine and the concentration of glucose added to initiate glycolysis was 25 mM. Four independent replicates were conducted with 10 technical replicates per cell line. Data were expressed as pmol of O2 per minute and normalized by cell number measured by the CyQUANT Cell proliferation kit (Invitrogen™), which is based on a fluorochrome binding to nucleic acids. Fluorescence was measured in a microplate luminometer (ClarioStar BMG Labtech, Cary, NC, USA) with excitation wavelength at 485 ± 10 nm and emission detection wavelength at 530 ± 12.5 nm.
Statistical analysis
Statistical analysis included two-way Mann-Whitney tests to determine significance of expression differences observed between IDH1MUT versus IDH1WT samples and Kaplan–Meier estimates of survival with log-rank tests among strata. Relative expression data were visualized as box plots, depicting the 25th, 50th and 75th percentile, with 95% confidence intervals and significance levels are shown by (*P < 0.05), (**P < 0.01), (***P < 0.001) and (****P < 0.0001).
SUPPLEMENTARY MATERIALS FIGURE AND TABLE
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing interests.
FUNDING
M.K., K.L, C.J.F.v.N. and W.P.L. were supported by the Dutch Cancer Society (KWF-UVA 2014-6839). R.J.M. was supported by an AMC PhD scholarship.
REFERENCES
- 1.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 2.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 3.Molenaar RJ, Botman D, Smits MA, Hira VV, van Lith SA, Stap J, Henneman P, Khurshed M, Lenting K, Mul AN, Dimitrakopoulou D, van Drunen CM, Hoebe RA, et al. Radioprotection of IDH1-mutated cancer cells by the IDH1-mutant inhibitor AGI-5198. Cancer Res. 2015;75:4790–802. doi: 10.1158/0008-5472.CAN-14-3603. [DOI] [PubMed] [Google Scholar]
- 4.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 6.Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta. 2014;1846:326–41. doi: 10.1016/j.bbcan.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–7. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, Tallman MS, Sun Z, Wolniak K, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, Leung IK, Li XS, Woon EC, Yang M, McDonough MA, King ON, Clifton IJ, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–9. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaelin WG., Jr Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335–45. doi: 10.1101/sqb.2011.76.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Lith SA, Molenaar R, van Noorden CJ, Leenders WP. Tumor cells in search for glutamate: an alternative explanation for increased invasiveness of IDH1 mutant gliomas. Neuro Oncol. 2014;16:1669–70. doi: 10.1093/neuonc/nou152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, Ding J, Lei Q, Guan KL, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–5. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, Losman JA, Joensuu P, Bergmann U, Gross S, Travins J, Weiss S, Looper R, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–8. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 18.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 20.Oudard S, Arvelo F, Miccoli L, Apiou F, Dutrillaux AM, Poisson M, Dutrillaux B, Poupon MF. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74:839–45. doi: 10.1038/bjc.1996.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fack F, Espedal H, Keunen O, Golebiewska A, Obad N, Harter PN, Mittelbronn M, Bahr O, Weyerbrock A, Stuhr L, Miletic H, Sakariassen PO, Stieber D, et al. Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas. Acta Neuropathol. 2015;129:115–31. doi: 10.1007/s00401-014-1352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talasila KM, Rosland GV, Hagland HR, Eskilsson E, Flones IH, Fritah S, Azuaje F, Atai N, Harter PN, Mittelbronn M, Andersen M, Joseph JV, Hossain JA, et al. The angiogenic switch leads to a metabolic shift in human glioblastoma. Neuro Oncol. 2016;19:383–93. doi: 10.1093/neuonc/now175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamans B, Navis AC, Wright A, Wesseling P, Heerschap A, Leenders W. Multivoxel (1)H MR spectroscopy is superior to contrast-enhanced MRI for response assessment after anti-angiogenic treatment of orthotopic human glioma xenografts and provides handles for metabolic targeting. Neuro Oncol. 2013;15:1615–24. doi: 10.1093/neuonc/not129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH, Wesseling P, Hulsebos TJ, Troost D, van Tilborg AA, Leenstra S, Vandertop WP, Bardelli A, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014;16:1263–73. doi: 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleeker FE, Atai NA, Lamba S, Jonker A, Rijkeboer D, Bosch KS, Tigchelaar W, Troost D, Vandertop WP, Bardelli A, Van Noorden CJ. The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119:487–94. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miranda-Goncalves V, Honavar M, Pinheiro C, Martinho O, Pires MM, Pinheiro C, Cordeiro M, Bebiano G, Costa P, Palmeirim I, Reis RM, Baltazar F. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol. 2013;15:172–88. doi: 10.1093/neuonc/nos298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–23. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 28.van Lith SA, Navis AC, Verrijp K, Niclou SP, Bjerkvig R, Wesseling P, Tops B, Molenaar R, van Noorden CJ, Leenders WP. Glutamate as chemotactic fuel for diffuse glioma cells: are they glutamate suckers? Biochim Biophys Acta. 2014;1846:66–74. doi: 10.1016/j.bbcan.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Tonjes M, Barbus S, Park YJ, Wang W, Schlotter M, Lindroth AM, Pleier SV, Bai AH, Karra D, Piro RM, Felsberg J, Addington A, Lemke D, et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med. 2013;19:901–8. doi: 10.1038/nm.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moschen I, Broer A, Galic S, Lang F, Broer S. Significance of short chain fatty acid transport by members of the monocarboxylate transporter family (MCT) Neurochem Res. 2012;37:2562–8. doi: 10.1007/s11064-012-0857-3. [DOI] [PubMed] [Google Scholar]
- 31.Lenting K, Verhaak R, Ter Laan M, Wesseling P, Leenders W. Glioma: experimental models and reality. Acta Neuropathol. 2017;133:263–82. doi: 10.1007/s00401-017-1671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–36. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 33.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Nuebel E, Wisidagama DR, Setoguchi K, Hong JS, Van Horn CM, Imam SS, Vergnes L, Malone CS, Koehler CM, Teitell MA. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc. 2012;7:1068–85. doi: 10.1038/nprot.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 36.Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14:267–74. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–8. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, Xu K, Acevedo PN, Hollern DP, Bellinger G, Dayton TL, et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell. 2015;57:95–107. doi: 10.1016/j.molcel.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 40.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–83. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–26. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izquierdo-Garcia JL, Viswanath P, Eriksson P, Chaumeil MM, Pieper RO, Phillips JJ, Ronen SM. Metabolic reprogramming in mutant IDH1 glioma cells. PLoS One. 2015;10:e0118781. doi: 10.1371/journal.pone.0118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chesnelong C, Chaumeil MM, Blough MD, Al-Najjar M, Stechishin OD, Chan JA, Pieper RO, Ronen SM, Weiss S, Luchman HA, Cairncross JG. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro Oncol. 2014;16:686–95. doi: 10.1093/neuonc/not243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaumeil MM, Lupo JM, Ronen SM. Magnetic Resonance (MR) Metabolic imaging in glioma. Brain Pathol. 2015;25:769–80. doi: 10.1111/bpa.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mustafa DA, Swagemakers SM, Buise L, van der Spek PJ, Kros JM. Metabolic alterations due to IDH1 mutation in glioma: opening for therapeutic opportunities? Acta Neuropathol Commun. 2014;2:6. doi: 10.1186/2051-5960-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho J, de Moura MB, Lin Y, Vincent G, Thorne S, Duncan LM, Hui-Min L, Kirkwood JM, Becker D, Van Houten B, Moschos SJ. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol Cancer. 2012;11:76. doi: 10.1186/1476-4598-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCleland ML, Adler AS, Deming L, Cosino E, Lee L, Blackwood EM, Solon M, Tao J, Li L, Shames D, Jackson E, Forrest WF, Firestein R. Lactate dehydrogenase B is required for the growth of KRAS-dependent lung adenocarcinomas. Clin Cancer Res. 2013;19:773–84. doi: 10.1158/1078-0432.CCR-12-2638. [DOI] [PubMed] [Google Scholar]
- 48.Mirebeau-Prunier D, Le Pennec S, Jacques C, Fontaine JF, Gueguen N, Boutet-Bouzamondo N, Donnart A, Malthiery Y, Savagner F. Estrogen-related receptor alpha modulates lactate dehydrogenase activity in thyroid tumors. PLoS One. 2013;8:e58683. doi: 10.1371/journal.pone.0058683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldock AL, Yagle K, Born DE, Ahn S, Trister AD, Neal M, Johnston SK, Bridge CA, Basanta D, Scott J, Malone H, Sonabend AM, Canoll P, et al. Invasion and proliferation kinetics in enhancing gliomas predict IDH1 mutation status. Neuro Oncol. 2014;16:779–86. doi: 10.1093/neuonc/nou027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuyas E, Fernandez-Arroyo S, Corominas-Faja B, Rodriguez-Gallego E, Bosch-Barrera J, Martin-Castillo B, De Llorens R, Joven J, Menendez JA. Oncometabolic mutation IDH1 R132H confers a metformin-hypersensitive phenotype. Oncotarget. 2015;6:12279–96. doi: 10.18632/oncotarget.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li F, He X, Ye D, Lin Y, Yu H, Yao C, Huang L, Zhang J, Wang F, Xu S, Wu X, Liu L, Yang C, et al. NADP(+)-IDH Mutations promote hypersuccinylation that impairs mitochondria respiration and induces apoptosis resistance. Mol Cell. 2015;60:661–75. doi: 10.1016/j.molcel.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 53.Grassian AR, Parker SJ, Davidson SM, Divakaruni AS, Green CR, Zhang X, Slocum KL, Pu M, Lin F, Vickers C, Joud-Caldwell C, Chung F, Yin H, et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 2014;74:3317–31. doi: 10.1158/0008-5472.CAN-14-0772-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Izquierdo-Garcia JL, Viswanath P, Eriksson P, Cai L, Radoul M, Chaumeil MM, Blough M, Luchman HA, Weiss S, Cairncross JG, Phillips JJ, Pieper RO, Ronen SM. IDH1 Mutation induces reprogramming of pyruvate metabolism. Cancer Res. 2015;75:2999–3009. doi: 10.1158/0008-5472.CAN-15-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, Huang Z, Barnett S, Mickey BE, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–14. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, Wang G, Mao Q, Li S, Xiong W, Lin Y, Ge J. Glutamate dehydrogenase (GDH) regulates bioenergetics and redox homeostasis in human glioma. Oncotarget. 2016 Feb 24; doi: 10.18632/oncotarget.7657. [Epub ahead of print] [DOI] [Google Scholar]
- 57.Chaumeil MM, Larson PE, Woods SM, Cai L, Eriksson P, Robinson AE, Lupo JM, Vigneron DB, Nelson SJ, Pieper RO, Phillips JJ, Ronen SM. Hyperpolarized [1-13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma. Cancer Res. 2014;74:4247–57. doi: 10.1158/0008-5472.CAN-14-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izquierdo-Garcia JL, Cai LM, Chaumeil MM, Eriksson P, Robinson AE, Pieper RO, Phillips JJ, Ronen SM. Glioma cells with the IDH1 mutation modulate metabolic fractional flux through pyruvate carboxylase. PLoS One. 2014;9:e108289. doi: 10.1371/journal.pone.0108289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cancer Genome Atlas Research N. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481–98. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feichtinger RG, Weis S, Mayr JA, Zimmermann F, Geilberger R, Sperl W, Kofler B. Alterations of oxidative phosphorylation complexes in astrocytomas. Glia. 2014;62:514–25. doi: 10.1002/glia.22621. [DOI] [PubMed] [Google Scholar]
- 61.Vander Heiden MG, Christofk HR, Schuman E, Subtelny AO, Sharfi H, Harlow EE, Xian J, Cantley LC. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol. 2010;79:1118–24. doi: 10.1016/j.bcp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vartanian A, Agnihotri S, Wilson MR, Burrell KE, Tonge PD, Alamsahebpour A, Jalali S, Taconne MS, Golbourn B, Aldape KD, Zadeh G. Targeting hexokinase 2 enhances response to radio-chemotherapy in glioblastoma. Oncotarget. 2016;7:69518–69535. doi: 10.18632/oncotarget.11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu J, Sanborn JZ, Benz S, Szeto C, Hsu F, Kuhn RM, Karolchik D, Archie J, Lenburg ME, Esserman LJ, Kent WJ, Haussler D, Wang T. The UCSC Cancer Genomics Browser. Nat Methods. 2009;6:239–40. doi: 10.1038/nmeth0409-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.