Abstract
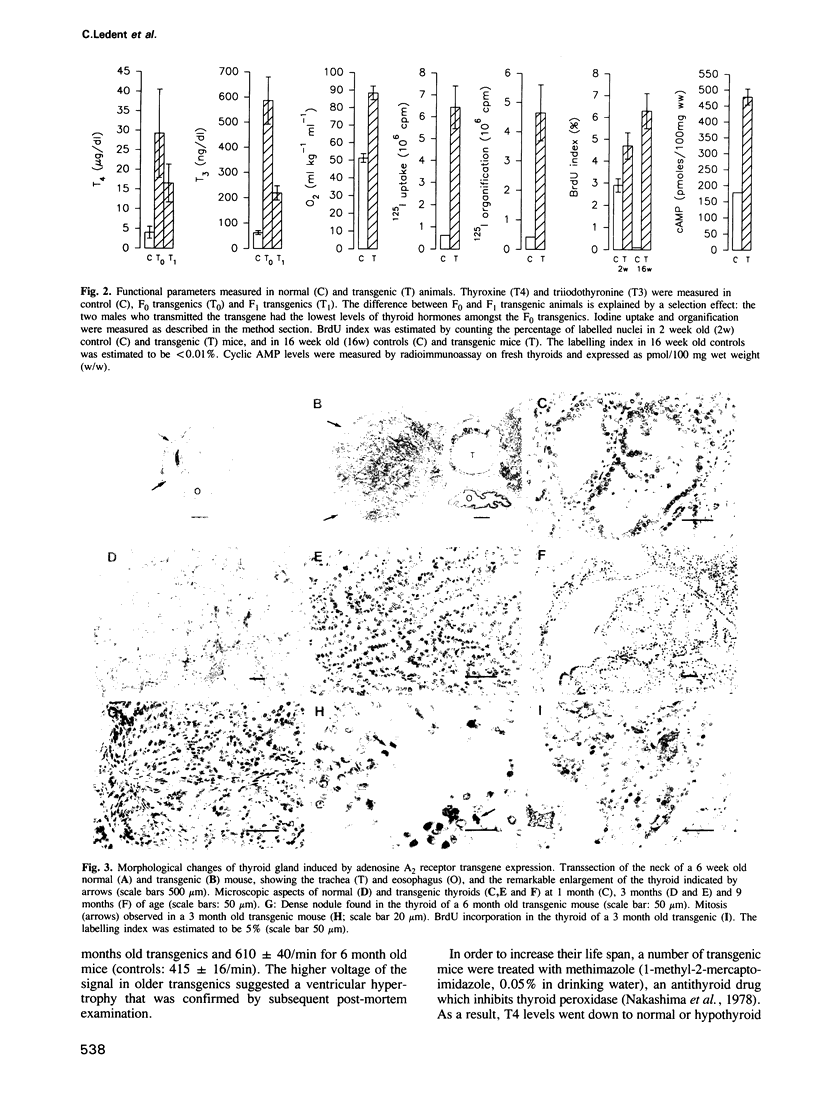
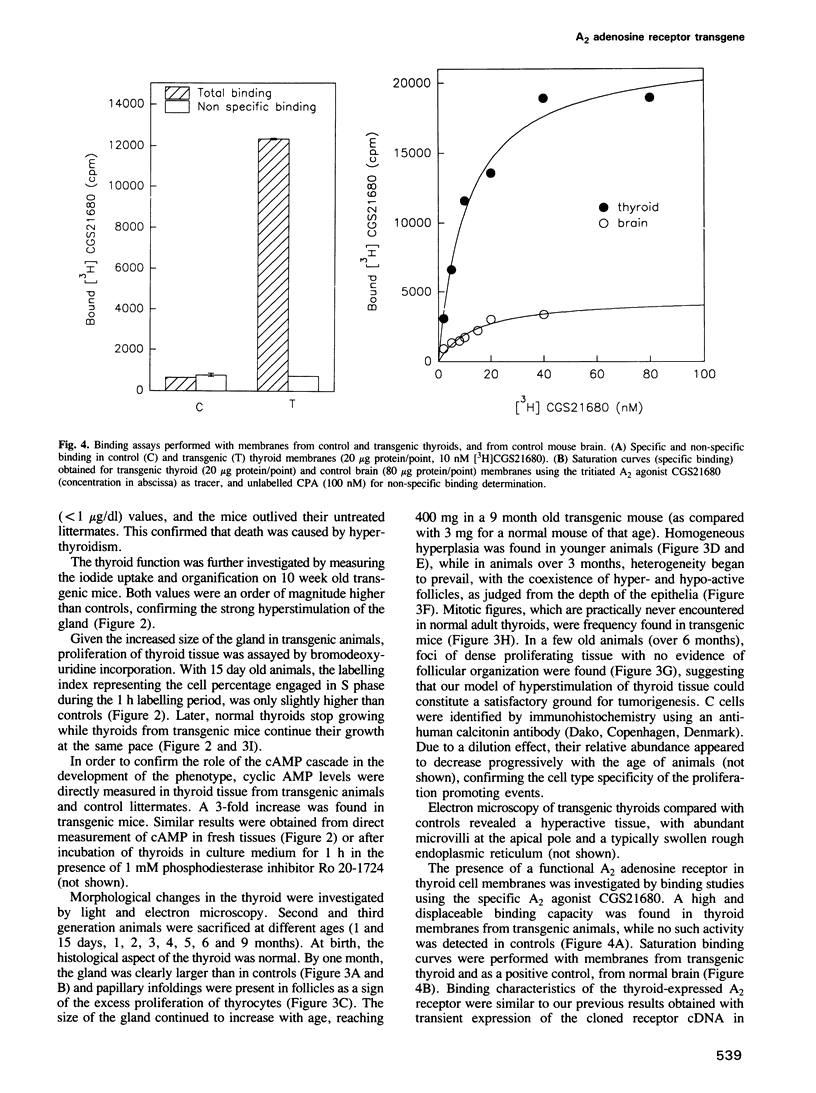
Cyclic AMP (cAMP) is the major intracellular second messenger of thyrotropin (TSH) action on thyroid cells. It stimulates growth as well as the function and differentiation of cultured thyrocytes. The adenosine A2 receptor, which activates adenylyl cyclase via coupling to the stimulating G protein (Gs), has been shown to promote constitutive activation of the cAMP cascade when transfected into various cell types. In order to test whether the A2 receptor was able to function similarly in vivo and to investigate the possible consequences of permanent adenylyl cyclase activation in thyroid cells, lines of transgenic mice were generated expressing the canine A2 adenosine receptor under control of the bovine thyroglobulin gene promoter. Thyroid-specific expression of the A2 adenosine receptor transgene promoted gland hyperplasia and severe hyperthyroidism causing premature death of the animals. The resulting goitre represents a model of hyperfunctioning adenomas: it demonstrates that constitutive activation of the cAMP cascade in such differentiated epithelial cells is sufficient to stimulate autonomous and uncontrolled function and growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Burton F. H., Hasel K. W., Bloom F. E., Sutcliffe J. G. Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature. 1991 Mar 7;350(6313):74–77. doi: 10.1038/350074a0. [DOI] [PubMed] [Google Scholar]
- Dumont J. E., Jauniaux J. C., Roger P. P. The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem Sci. 1989 Feb;14(2):67–71. doi: 10.1016/0968-0004(89)90046-7. [DOI] [PubMed] [Google Scholar]
- Dumont J. E., Roger P., Van Heuverswyn B., Erneux C., Vassart G. Control of growth and differentiation by known intracellular signal molecules in endocrine tissues: the example of the thyroid gland. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:337–342. [PubMed] [Google Scholar]
- Edmonds C. J., Tellez M. Hyperthyroidism and thyroid cancer. Clin Endocrinol (Oxf) 1988 Feb;28(2):253–259. doi: 10.1111/j.1365-2265.1988.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Faglia G., Beck-Peccoz P., Piscitelli G., Medri G. Inappropriate secretion of thyrotropin by the pituitary. Horm Res. 1987;26(1-4):79–99. doi: 10.1159/000180687. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gerard C. M., Lefort A., Libert F., Christophe D., Dumont J. E., Vassart G. Transcriptional regulation of the thyroperoxydase gene by thyrotropin and forskolin. Mol Cell Endocrinol. 1988 Dec;60(2-3):239–242. doi: 10.1016/0303-7207(88)90184-0. [DOI] [PubMed] [Google Scholar]
- Inappropriate secretion of thyroid-stimulating hormone. Ann Intern Med. 1981 Sep;95(3):339–351. doi: 10.7326/0003-4819-95-3-339. [DOI] [PubMed] [Google Scholar]
- Ledent C., Dumont J., Vassart G., Parmentier M. Thyroid adenocarcinomas secondary to tissue-specific expression of simian virus-40 large T-antigen in transgenic mice. Endocrinology. 1991 Sep;129(3):1391–1401. doi: 10.1210/endo-129-3-1391. [DOI] [PubMed] [Google Scholar]
- Ledent C., Parmentier M., Vassart G. Tissue-specific expression and methylation of a thyroglobulin-chloramphenicol acetyltransferase fusion gene in transgenic mice. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6176–6180. doi: 10.1073/pnas.87.16.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Libert F., Schiffmann S. N., Lefort A., Parmentier M., Gérard C., Dumont J. E., Vanderhaeghen J. J., Vassart G. The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J. 1991 Jul;10(7):1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Maenhaut C., Van Sande J., Libert F., Abramowicz M., Parmentier M., Vanderhaegen J. J., Dumont J. E., Vassart G., Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Mazzaferri E. L. Thyroid cancer and Graves' disease. J Clin Endocrinol Metab. 1990 Apr;70(4):826–829. doi: 10.1210/jcem-70-4-826. [DOI] [PubMed] [Google Scholar]
- McKenzie J. M., Zakarija M. Clinical review 3: The clinical use of thyrotropin receptor antibody measurements. J Clin Endocrinol Metab. 1989 Dec;69(6):1093–1096. doi: 10.1210/jcem-69-6-1093. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. M., McDermott D. Functional expression of the human formyl peptide receptor in Xenopus oocytes requires a complementary human factor. J Biol Chem. 1991 Jul 5;266(19):12560–12567. [PubMed] [Google Scholar]
- Nakashima T., Taurog A., Riesco G. Mechanism of action of thioureylene antithyroid drugs: factors affecting intrathyroidal metabolism of propylthiouracil and methimazole in rats. Endocrinology. 1978 Dec;103(6):2187–2197. doi: 10.1210/endo-103-6-2187. [DOI] [PubMed] [Google Scholar]
- Nanoff C., Jacobson K. A., Stiles G. L. The A2 adenosine receptor: guanine nucleotide modulation of agonist binding is enhanced by proteolysis. Mol Pharmacol. 1991 Feb;39(2):130–135. [PMC free article] [PubMed] [Google Scholar]
- Pacini F., Elisei R., Di Coscio G. C., Anelli S., Macchia E., Concetti R., Miccoli P., Arganini M., Pinchera A. Thyroid carcinoma in thyrotoxic patients treated by surgery. J Endocrinol Invest. 1988 Feb;11(2):107–112. doi: 10.1007/BF03350115. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pohl V., Roger P. P., Christophe D., Pattyn G., Vassart G., Dumont J. E. Differentiation expression during proliferative activity induced through different pathways: in situ hybridization study of thyroglobulin gene expression in thyroid epithelial cells. J Cell Biol. 1990 Aug;111(2):663–672. doi: 10.1083/jcb.111.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberg N. H., Vassart G., Lecocq R., Dumont J. E., Refetoff S. Modulation of thyroglobulin messenger RNA accumulation in the rat thyroid. Endocrinology. 1981 Nov;109(5):1650–1656. doi: 10.1210/endo-109-5-1650. [DOI] [PubMed] [Google Scholar]
- Schutte B., Reynders M. M., Bosman F. T., Blijham G. H. Effect of tissue fixation on anti-bromodeoxyuridine immunohistochemistry. J Histochem Cytochem. 1987 Nov;35(11):1343–1345. doi: 10.1177/35.11.3116075. [DOI] [PubMed] [Google Scholar]
- Struthers R. S., Vale W. W., Arias C., Sawchenko P. E., Montminy M. R. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature. 1991 Apr 18;350(6319):622–624. doi: 10.1038/350622a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heuverswyn B., Streydio C., Brocas H., Refetoff S., Dumont J., Vassart G. Thyrotropin controls transcription of the thyroglobulin gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5941–5945. doi: 10.1073/pnas.81.19.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sande J., Dumont J. E. Effects of thyrotropin, prostaglandin E1 and iodide on cyclic 3',5'-AMP concentration in dog thyroid slices. Biochim Biophys Acta. 1973 Jul 28;313(2):320–328. doi: 10.1016/0304-4165(73)90031-7. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]