Abstract
Background
Fingolimod (FTY) and dimethyl fumarate (DMF) are multiple sclerosis (MS) oral therapies that became available in 2010 and 2013, respectively.
Objective
The objective of this article is to compare discontinuation rates, efficacy, and adverse events (AEs) of FTY and DMF over two years.
Methods
Patients prescribed FTY or DMF at the Rocky Mountain MS Center at University of Colorado prior to October 2013 were identified. Clinician-reported data were retrospectively collected. Primary outcome was discontinuation of drug by the end of year two. Reasons for discontinuation were evaluated.
Results
A total of 271 FTY and 342 DMF patients were evaluated. Patients had a mean age of 42.5 (FTY) and 45.8 (DMF) years and were predominantly female (72.0% FTY; 69.6% DMF) and white (86.3% FTY; 82.2% DMF). At ≤24 months, 93 (34.3%) and 161 (47.1%) discontinued FTY and DMF, respectively, with an unadjusted odds ratio (OR) of 1.70 (1.23–2.37, p = 0.002), or 1.69 (1.16–2.46, p = 0.006) for the doubly robust propensity score weighted estimator. Primary reason for discontinuation was AEs, which were less likely for FTY 46 (17.0%) compared to DMF 82 (24.0%) (OR 1.54, 1.03–2.31, p = 0.035). Discontinuation due to disease activity (FTY (10%) DMF (11.1%); OR 1.13, 0.67–1.90, p = 0.647) and breakthrough disease activity, regardless of discontinuation (FTY (34.7%) DMF (33.6%); OR 0.95, 0.68–1.34, p = 0.783), were similar.
Conclusions
The odds of discontinuation were less for FTY than DMF, and were driven by AEs for both drugs.
Keywords: Comparative efficacy, disease-modifying therapy, Gilenya, Tecfidera, real world
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system characterized by demyelination and axonal damage resulting in one of the leading causes of disability among young adults.1 With numerous disease-modifying therapies (DMTs) now on the market varying in efficacy and side effects, patients and providers face a substantial challenge when considering between various agents to find the most appropriate treatment. Establishing consistent long-term treatment with an efficacious DMT has been associated with decreased risk of relapse, disease progression, and higher patient satisfaction.2,3 Adverse events (AEs) including tolerability issues reduce adherence and promote early discontinuation of DMTs, ultimately effecting affecting disease outcomes, as described extensively with interferon β (IFNβ) and glatiramer acetate (GA).3–6
The dramatic increase in available treatment options has included the introduction of oral DMTs such as fingolimod (FTY) and dimethyl fumarate (DMF). FTY (0.5 mg orally once daily), a sphingosine-1 receptor modulator, was the first oral DMT approved for the treatment of relapsing–remitting MS (RRMS) by the United States Food and Drug Administration (FDA) in 2010, followed by the approval of DMF (240 mg orally twice daily) in 2013.7–9 FTY and DMF have similar efficacy measures compared to placebo in clinical trials. Four separate phase 3 trials demonstrated reductions in the annualized relapse rate (ARR) of 48%–54% for FTY and 44%–53% for DMF, compared to placebo.9–12 FTY and DMF have also shown superior efficacy when compared to injectable DMTs.13–15 FTY reduced ARR by 52% vs intramuscular IFNβ-1a while DMF reduced the number of new and enlarging T2 lesions compared to GA.8,9
While efficacy is similar, the tolerability profiles for FTY and DMF have notable differences. FTY has been associated most commonly with nasopharyngitis, headaches, fatigue, and diarrhea.8,16 In addition, FTY requires eye examinations because of associations with macular edema, and a six-hour monitoring period after receiving the first dose because of risk of rare cardiovascular AEs during treatment initiation.8,17 However, evidence suggests greater adherence to FTY compared to IFNs possibly due to an improved tolerability profile and oral route of administration.18 The tolerability profile of DMF has proved to be more challenging because of flushing and gastrointestinal (GI)-related issues. Common AEs associated with DMF include flushing, nausea, diarrhea, and vomiting, which are worst in the first weeks of treatment.9,11
There are currently limited comparative efficacy data for FTY and DMF in the real-world clinical setting over an extended time-period.19,20 We compared efficacy measures and discontinuation rates, including reasons for discontinuation, during the first two years of treatment with FTY and DMF at a large academic center, with inclusion of pediatric patients under the age of 18 and patients over the age of 55.
Materials and methods
Patient population
Patients who completed enrollment forms and initiated the approval process through their insurance companies for FTY or DMF in the Rocky Mountain Multiple Sclerosis Center at the University of Colorado (RMMSC at CU) were identified as potential study participants. Patients were eligible for inclusion in this study if they were diagnosed with any form of MS and began taking FTY or DMF prior to October 2013.
Study design
All data were collected through retrospective chart reviews of patient medical records. The index date was defined as the date of first drug administration. For each participant included in this study, the index date was identified and all RMMSC at CU encounters were reviewed for up to 24 months after index date. Clinician-reported data were collected from these encounters by BV and included relapse history, magnetic resonance imaging (MRI) outcomes, medications, AEs, MS disease history, and patient characteristics. Quality checks were completed for outliers to confirm accuracy and consistency of data collection.
Outcomes
The primary outcome was discontinuation of FTY or DMF, defined as no longer taking study drug at 24 months after index date and/or starting any other DMT for the treatment of MS during the 24-month follow-up period after the index date. It was noted that some patients withheld taking medication for a period of time, for example, to alleviate AEs or because of travel, without being considered a discontinuation if the patient reinitiated the medication without interruption by any other MS DMT.
Secondary outcomes assessed included (1) reasons for discontinuation, classified as disease activity, AEs, issues with insurance coverage, loss to follow-up or any other reason, (2) clinical relapse activity, (3) MRI activity consisting of contrast-enhancing lesions and new T2 lesions, and (4) a composite efficacy measure combining clinical relapse and MRI activity. Discontinuing due to disease activity, for the purpose of this study, was defined as one or any combination of clinical relapse activity, MRI activity or progression of disability observed in progressive forms of MS leading to discontinuation as noted in the clinical notes. The composite efficacy measure was defined as clinical relapse, contrast-enhancing lesions and/or new T2 lesion. Clinical relapses for the purpose of this study were defined as clinician reported per patient chart notes as new or worsening neurological symptoms lasting greater than 24 hours. All efficacy outcomes were on treatment measures collected at any time point available ≤24 months from index date. Because of the retrospective nature of this study, no consistent measure of disability was available, therefore disability was not included in the composite efficacy measure.
Statistical analysis
Statistical analyses were conducted using SAS Version 9.4 and STATA Version 13.1. Cohen’s D effect size plots were created using R version 3.1.0. All two-tailed p values < 0.05 were considered as significant. For baseline characteristics and secondary outcomes, differences between groups were assessed using T-tests or Wilcoxon ranks sum tests for continuous variables, Poisson or negative binomial methods for count outcomes, and Chi-squared or Fisher’s exact tests for categorical data. Multiple methods were used to assess the primary outcome (discontinuation due to any reason at ≤24 months) and select secondary outcomes (discontinuation due to AEs and composite efficacy measure) in order to account for imbalances between the FTY and DMF groups and evaluate the consistency of the findings. These methods included crude logistic regression, adjusted logistic regression, logistic regression on sample group 1:1 greedy matched by propensity scores without replacement, 1:2 nearest neighbor sample group matched by propensity scores with replacement, and ATT doubly robust weighting estimator using propensity scores. These methods represent different attempts to convert observational data into a pseudo-randomized control trial that are commonly employed in analyzing retrospective chart reviews. The DMT observations were matched to patients on FTY.
Propensity scores were created with a logistic regression model for probability of receiving FTY. Adjusting covariates used to create propensity scores were chosen a priori and included age, gender (female/male), disease duration, diagnosis (RRMS/secondary progressive MS/primary progressive MS), previous natalizumab use in six months prior to index (yes/no), and contrast enhancement on baseline MRI (yes/no/no MRI available). The same covariates were used for the adjusted logistic regression model for the primary outcome and select secondary outcomes. Covariates were selected to represent patient demographics, disease history, and baseline disease activity. Previous use of natalizumab in the six months prior to index date was included as a covariate to account for potential rebound disease associated with ending natalizumab treatment, which could increase odds of discontinuation if the patient or clinician does not feel the new medication is adequately efficacious.
Results
Baseline characteristics
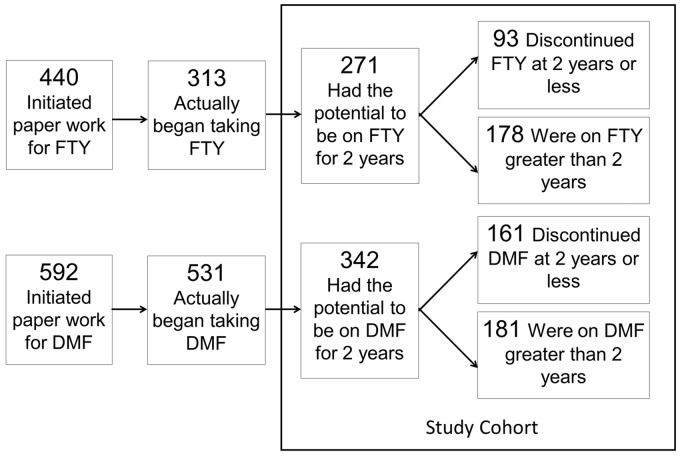
A total of 1032 patients were evaluated for potential inclusion. Figure 1 demonstrates the identification process for each study cohort. For FTY, 440 patients initiated the approval process with their insurance company. Of these, 313 began taking the medication and 271 met the inclusion criteria for this study, which mainly concerned the potential for having been on medication for two years. For DMF, 592 patients initiated the approval process with 531 starting to take the medication and 342 meeting the inclusion criteria.
Figure 1.
Study sample identification. FTY: fingolimod, DMF: dimethyl fumarate.
Table 1 presents the baseline characteristics of each study cohort. FTY and DMF patients did not differ significantly by disease duration, gender, race or ethnicity. Notable differences between the two groups include FTY patients being more likely to have RRMS, be previously on natalizumab, have contrast-enhancing lesions on baseline MRI, and have increased disease severity according to baseline MRI as described by the neuroradiology reports. When assessing time off DMT for breaks in the study drug, one (0.4%) FTY patient and 11 (3.2%) DMF patients exceeded breaks in medication greater than one month.
Table 1.
Baseline characteristics of study cohort.
| Fingolimod (N = 271) |
Dimethyl fumarate (N = 342) |
||||
|---|---|---|---|---|---|
| N or mean | % or SD | N or mean | % or SD | p value | |
| Disease duration (years, SD) | 11.5 | 7.5 | 11.1 | 7.4 | 0.259 |
| Age (years, SD) | 42.5 | 11.4 | 45.8 | 12.2 | <0.001 |
| <18 years old | 5 | 1.8% | 5 | 1.5% | 0.710 |
| >55 years old | 39 | 14.4% | 81 | 23.7% | 0.004 |
| Gender, female | 195 | 72.0% | 238 | 69.6% | 0.523 |
| Race | 0.186 | ||||
| White | 233 | 86.3% | 281 | 82.2% | |
| Black, African American | 4 | 1.5% | 15 | 4.4% | |
| Other | 11 | 4.1% | 13 | 3.8% | |
| Not available | 23 | 8.1% | 33 | 9.6% | |
| Ethnicity | 0.589 | ||||
| Hispanic | 15 | 5.5% | 15 | 4.4% | |
| Non-Hispanic | 226 | 83.4% | 284 | 83.0% | |
| Not available | 30 | 11.1% | 43 | 12.6% | |
| Type of multiple sclerosis | <0.001 | ||||
| Relapsing–remitting | 244 | 90.0% | 265 | 77.5% | |
| Secondary progressive | 23 | 8.5% | 54 | 15.8% | |
| Primary progressive | 4 | 1.5% | 23 | 6.7% | |
| Previous DMTa | <0.001 | ||||
| Interferons | 36 | 13.3% | 49 | 14.3% | |
| Glatiramer acetate | 49 | 18.1% | 106 | 31.0% | |
| Natalizumab | 115 | 42.4% | 65 | 19.0% | |
| Rituximab | 1 | 0.4% | 9 | 2.6% | |
| Fingolimod | N/A | N/A | 24 | 7.0% | |
| Dimethyl fumarate | 1 | 0.4% | N/A | N/A | |
| None | 66 | 24.4% | 84 | 24.6% | |
| Other | 3 | 1.1% | 5 | 1.5% | |
| Mean time between Previous DMT and study drug (SD) | 1.05 (N = 205) | 1.22 | 0.75 (N = 258) | 1.29 | <0.001 |
| Baseline MRI available for review | 0.004 | ||||
| Available | 235 | 86.7% | 320 | 93.6% | |
| Unavailable | 36 | 13.3% | 22 | 6.4% | |
| Contrast enhancement on baseline MRI | 57 (N = 232) | 24.6% | 44 (N = 302) | 14.6% | 0.003 |
| Disease burden on baseline MRI | 0.001 | ||||
| Mild | 100 | 36.9% | 170 | 49.7% | |
| Moderate | 76 | 28.0% | 94 | 27.5% | |
| Severe | 45 | 16.6% | 29 | 8.5% | |
| Missing | 50 | 18.5% | 49 | 14.3% | |
Within six months prior to starting study drug.
DMT: disease-modifying therapy; MRI: magnetic resonance imaging.
Discontinuation outcomes
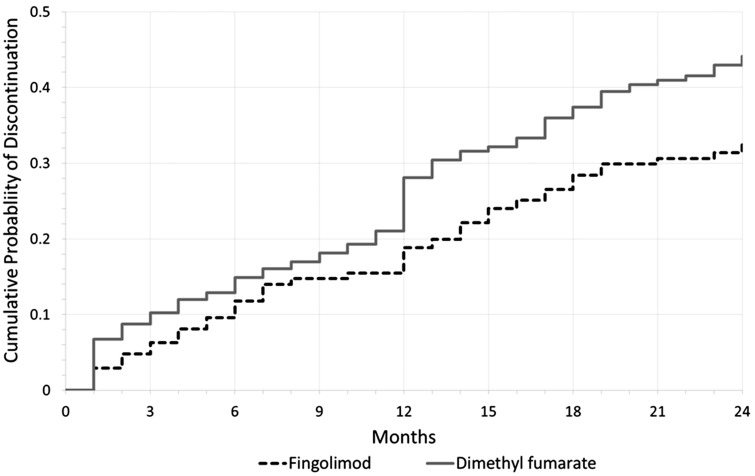
Table 2 shows the unadjusted discontinuation outcomes assessed in this study. Results demonstrate a greater proportion of DMF patients discontinuing the drug at 24 months or less. When assessing the reason for discontinuation, there was no significant difference between FTY and DMF patients as to disease activity, insurance issues, loss to follow-up, or other. The most commonly cited reasons for discontinuations that were classified as “other” include patient preference due to non-adherence and attempting pregnancy for both groups (as determined from patient records). However, a significant difference was observed for AEs/tolerability leading to discontinuation with 24.0% of DMF patients citing this as the primary reason compared to 17.0% of FTY patients (p = 0.034). Figure 2 demonstrates a Kaplan-Meier failure curve for the cumulative probability of discontinuation over time for all patients.
Table 2.
Unadjusted discontinuation outcomes.
| Fingolimod (N = 271) |
Dimethyl fumarate (N = 342) |
||||
|---|---|---|---|---|---|
| N or mean | % or SD | N or mean | % or SD | p value | |
| Discontinued drug ≤24 months | 93 | 34.3% | 161 | 47.1% | 0.001 |
| Disease activitya | 27 | 10.0% | 38 | 11.1% | 0.647 |
| Adverse events | 46 | 17.0% | 82 | 24.0% | 0.034 |
| Insurance | 2 | 0.7% | 4 | 1.2% | 0.699 |
| Lost to follow-up | 15 | 5.5% | 27 | 7.9% | 0.251 |
| Other | 3 | 1.1% | 10 | 2.9% | 0.161 |
| Mean time to discontinuation (months)b | 10.3 | 7.1 | 10.0 | 7.2 | 0.539 |
Includes discontinuation of drug because of clinical relapse, magnetic resonance imaging activity or disease progression.
For those who discontinue.
Figure 2.
Kaplan-Meier failure curve demonstrating cumulative probability of discontinuation over time.
Populations not included in the clinical trials (younger than 18 or older than 55) were examined. Patients older than 55 had proportions of discontinuations similar to the overall study cohort at 30.8% and 44.4% for FTY and DMF, respectively. Fewer patients over the age of 55 discontinued because of disease activity compared to the overall cohort for both drugs (FTY: 5.1% of >55 vs 10.0% of entire cohort, DMF: 5.0% of >55 years vs 11.1% of entire cohort). Although the pediatric population was small and it is difficult to draw conclusions, two of five patients under 18 discontinued the drug both for FTY and DMF. When observing RRMS patients only, DMF consistently had a significantly greater proportion of overall discontinuations; however, discontinuations due to AEs/tolerability no longer differed significantly because of the loss of power (16.8% FTY, 23.4% DMF, p = 0.064) (Supplementary Tables S1–S3).
Clinicians may gain experience and alter methods in mitigating side effects as time passes after a drug comes to market. Therefore we evaluated if counseling concerning tolerability issues would result in fewer discontinuations over time. We assessed discontinuation differences for any reason among those who initiated the drug in the first half of the observation period compared to the second half to investigate if the proportion of discontinuations varied over time. We found no significant differences for either FTY or DMF patients (Supplementary Table S5 and S6).
Efficacy outcomes
Table 3 demonstrates unadjusted efficacy outcomes assessed in this study. There was no significant difference in efficacy between FTY and DMF patients, including clinical relapse (p = 0.116), contrast enhancement (p = 0.293) or new T2 lesions (p = 0.419) on follow-up MRI, individually and as a combined composite measure (p = 0.783). Results are consistent when observing only RRMS patients (Supplementary Table S4).
Table 3.
Unadjusted efficacy outcomes.
| Fingolimod (N = 271) |
Dimethyl fumarate (N = 342) |
||||
|---|---|---|---|---|---|
| N or mean | % or SD | N or mean | % or SD | p value | |
| Patients with a relapse during first two years of study drug | 24 | 8.9% | 44 | 12.9% | 0.116 |
| MRI available while on drug in first two years | 214 | 79.0% | 260 | 76.0% | 0.387 |
| Mean number of available MRIs | 1.64 | 0.69 | 1.63 | 0.68 | 0.895 |
| Patients with contrast enhancement | 28 | 13.1% | 26 | 10.0% | 0.293 |
| Patients with new T2 lesions | 75 | 35.1% | 82 | 31.5% | 0.419 |
| Composite efficacy measurea | 94 | 34.7% | 115 | 33.6% | 0.783 |
Patients who had a clinical relapse, contrast enhancement or a new T2 lesion on follow-up MRI.
MRI: magnetic resonance imaging.
Adjusted outcomes
Table 4 exhibits the unadjusted and adjusted odds of DMF vs FTY for discontinuation for any reason at ≤24 months, discontinuation due to AEs only and the composite efficacy measure. For discontinuation for any reason, all methods of adjustment demonstrate consistent, significant results of an odds ratio (OR) ranging from 1.69 for the doubly robust estimator to 1.86 for the adjusted logistic regression, and with an OR of 1.74 for the 1:2 matching with replacement, indicating increased odds of discontinuing DMF compared to FTY. For discontinuing due to AEs, 1:1 greedy matching was the only adjustment method demonstrating increased odds of discontinuing DMF due to AEs compared to FTY (p = 0.027). For the composite efficacy measure, all methods of adjustment demonstrate no significant difference between DMF and FTY. All results were consistent after removing 2.5% of patients from each end with extreme propensity scores to ensure that outliers were not greatly influencing significance.
Table 4.
Unadjusted and adjusted odds ratios for discontinuation for any reason at ≤24 months, discontinuation due to adverse events only and disease activity (DMF vs FTY).
| Discontinuation |
Efficacy |
||||||
|---|---|---|---|---|---|---|---|
| Due to any reason |
Due to adverse events |
Composite measureb |
|||||
| N | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Simple logistic regression | 613 | 1.70 (1.23, 2.37) | 0.002 | 1.54 (1.03, 2.31) | 0.035 | 0.95 (0.68, 1.34) | 0.783 |
| Adjusted logistic regressiona | 613 | 1.86 (1.29, 2.68) | 0.001 | 1.52 (0.99, 2.35) | 0.055 | 1.06 (0.72, 1.54) | 0.765 |
| Propensity matching with 1:1 greedy matching without replacementa | 542 | 1.74 (1.23, 2.46) | 0.002 | 1.61 (1.05, 2.44) | 0.027 | 1.10 (0.78, 1.56) | 0.591 |
| Propensity matching with 1:2 nearest neighbor matching with replacementa | 813 (481 unique) | 1.74 (1.12, 2.69) | 0.013 | 1.61 (0.95, 2.72) | 0.076 | 1.10 (0.70, 1.72) | 0.673 |
| ATT doubly robust weighting estimatora | 613 | 1.69 (1.16, 2.46) | 0.006 | 1.41 (0.90, 2.21) | 0.134 | 1.17 (0.81, 1.70) | 0.408 |
Controlling for age, disease duration, type of MS, previous natalizumab use, gender, and contrast enhancement on baseline MRI.
Includes clinical relapse, new T2 lesion on follow-up MRI, or contrast enhancement on follow-up MRI regardless of the event leading to discontinuation of drug.
DMF: dimethyl fumarate; FTY: fingolimod; MS: multiple sclerosis; MRI: magnetic resonance imaging.
All matching and weighting methods demonstrated achieving well-balanced groups. Of the various adjustment methods used, 1:2 nearest neighbor matching had the lowest measure of effect size (Cohen’s D) for covariates between the two medications and showed the greatest overlap in propensity scores, followed by ATT doubly robust weighting (Supplementary Figure S1 and S2).
AEs/Tolerability
Table 5 identifies AEs leading to discontinuation of FTY and DMF, with the most common AE being cited as GI-related issues both for FTY (23.9%) and DMF (80.5%) patients. The second most commonly cited AE contributing to discontinuations was headaches and flushing, rashes or hot flashes for FTY (17.4%) and DMF (30.5%), respectively. FTY had a wider range of AEs affecting various systems in comparison to DMF, which were overwhelmingly GI-related issues.
Table 5.
Adverse events leading to discontinuation.
| Adverse event | Fingolimod |
Dimethyl fumarate |
||
|---|---|---|---|---|
| N | Percentage | N | Percentage | |
| GI issues | 11 | 23.9% | 66 | 80.5% |
| Flushing/Rash/Hot flashes | 0 | 0.0% | 25 | 30.5% |
| Lymphopenia | 7 | 15.2% | 6 | 7.3% |
| Infections | 7 | 15.2% | 4 | 4.9% |
| Headaches | 8 | 17.4% | 1 | 1.2% |
| Elevated LFTs | 5 | 10.9% | 1 | 1.2% |
| Arrhythmia | 4 | 8.7% | 0 | 0.0% |
| Hair loss | 2 | 4.3% | 2 | 2.4% |
| Bradycardia | 3 | 6.5% | 0 | 0.0% |
| Hypertension | 3 | 6.5% | 0 | 0.0% |
| Shortness of breath | 3 | 6.5% | 0 | 0.0% |
| Tachycardia | 3 | 6.5% | 0 | 0.0% |
| Muscle spasms/weakness | 1 | 2.2% | 3 | 3.7% |
| Mood issues | 2 | 4.3% | 1 | 1.2% |
| Taste and vision changes | 2 | 4.3% | 1 | 1.2% |
| Reported pain (other than abdominal) | 1 | 2.2% | 2 | 2.4% |
| Weight gain | 1 | 2.2% | 1 | 1.2% |
| Alveolar hemorrhage | 1 | 2.2% | 0 | 0.0% |
| Palpitations | 1 | 2.2% | 0 | 0.0% |
| Pancytopenia | 1 | 2.2% | 0 | 0.0% |
| Reduced LFTs | 1 | 2.2% | 0 | 0.0% |
| Seizures | 1 | 2.2% | 0 | 0.0% |
GI: gastrointestinal; LFTs: liver function tests.
Discussion
A critical aspect to managing MS is establishing consistent, long-term treatment with an appropriate DMT, so as to reduce risk of relapse and disability progression. This has become more challenging for patients and providers as the number of treatment options has increased with limited comparative efficacy data available. In this retrospective cohort study we addressed this gap through investigating the real-world clinical experience for patients on FTY and DMF over two years, specifically discontinuation rates and efficacy outcomes. This was achieved through analysis of a large sample size consisting of patients from a single academic center.
These results demonstrate increased odds of discontinuing DMF vs FTY at 24 months or less. Results are consistently significant using various adjustment methods, including propensity matching and weighting, with OR ranging from 1.69 to 1.86. These consistent results through multiple methods and overlapping of propensity scores between groups are suggestive of achieving balanced groups. In addition, these results confirm trends in previous real-world retrospective studies completed over shorter time periods, also demonstrating increased odds of discontinuing DMF vs FTY, but are contradictory to a claims data study showing no discontinuation differences at one year.19–21 With broader inclusion criteria and less motivation than in controlled studies, it is not surprising that this study observed a greater proportion of discontinuations for FTY and, particularly, DMF than what has been previously observed in clinical trials (FTY: 34.3% vs 32% and 18.8% in phase 3 clinical trials for 0.5 mg daily, DMF: 47% vs 30% and 31% in phase 3 clinical trials).9–12 This finding highlights the importance of investigating the real-world experience following clinical trials.
Disease activity as a reason for discontinuation was similar between FTY and DMF patients at 10.0% and 11.1%, respectively, and did not appear to be a driving force in the difference observed in odds of discontinuation. Interestingly, discontinuing because of disease activity accounted for an even smaller percentage of patients in those above the age of 55, at 5.1% and 5.0% for FTY and DMF, respectively, which is consistent with a decrease in disease activity with age.22,23 When assessing efficacy measures for all patients, there is no significant difference between FTY and DMF in clinical relapses, MRI activity, including contrast enhancement and new T2 lesions, or the composite efficacy measure. The OR varied from 0.95 to 1.17, suggesting that if there is a difference in efficacy, this is likely small. This is not completely unexpected because of the similarity in reduction of ARR seen in phase 3 clinical trials.24 Furthermore, a shorter-term discontinuation study demonstrated no significant difference in regards to breakthrough disease activity leading to discontinuation, and claims data demonstrated no significant difference between FTY and DMF in comparative effectiveness.19,21
In addition, the proportion of patients experiencing disease activity appears to be lower when compared to clinical trials. For example, phase 3 clinical trials demonstrate 28.5%–29.6% of FTY patients and 27%–29% of DMF patients experiencing a relapse compared to 8.9% and 12.9% in our study, respectively.9–12 Differences in disease activity are likely due to the phase 3 clinical trial inclusion criteria consisting of only RRMS patients with recent disease activity, as well as younger patients (FTY: 42.5 vs 40.6 and 36.6 years in clinical trials, DMF: 45.8 vs 37.8 and 38.1 years in clinical trials). Additionally, differences in definitions and data acquisition may also play a role.9–12
The observed difference in the odds of discontinuation in our study was driven by AEs/tolerability, consistent with previous short-term studies, and this was the only significant difference among the reasons leading to discontinuation.19,20 After adjustment/matching (OR 1.41–1.61), these results are no longer statistically significant for three of the four methods examined.
The most common AEs/tolerability issues leading to DMF discontinuation were GI-related issues followed by flushing, rashes, and hot flashes. This is consistent with previous literature as the most common AEs associated with DMF.9 Although the most common AE leading to discontinuation was identical for both drugs, the percentage among patients with AEs experiencing GI-related issues was much higher in DMF patients (80.5%) compared to FTY patients (23.9%) (p value < 0.0001). Previous literature demonstrated GI-related issues to be common in the first weeks of treatment of DMF specifically, therefore we expected a rapid increase in the cumulative probability of discontinuation for DMF in the first few months.9,11 However, Figure 2 contradicts this expectation, with the largest increase in discontinuations occurring at 12 months for DMF patients. This may be due to AEs continuing past the first few months and being unable to control them after trials of multiple prevention methods, such as taking DMF with food to reduce GI-related AEs.
Previous literature has demonstrated an association between poor adherence and AEs.2,20 Furthermore, increased compliance has been shown to improve patient outcomes and reduce health care costs in MS patients.5 Long-term consistent care can reduce the risk of relapse or disability progression with less time untreated and fewer delays in efficacy, which often result from switching DMTs. Therefore, DMTs with improved tolerability profiles have the potential to improve MS disease outcomes and reduce health care costs through achieving consistent long-term care.
Our study consists of a robust analysis on a large sample size similar in number to phase 3 clinical trials. Furthermore, with no age limitations and an age range from 13 to 75 years old, our results are consistent with examination of real-world data, with inclusion of pediatric patients and patients over the age of 55. This is unlike phase 3 clinical trials previously reported for FTY and DMF consisting of only patients ages 18–55. In addition, our retrospective chart review design allows for the inclusion of MRI and clinical data not available through analysis of insurance claims data or reports generated from MSBase, a longitudinal, observational registry for MS researchers.
However, this study does have limitations. As a retrospective chart review cohort study, we are limited to already existing clinician-reported data and are unable to seek clarification if any inconsistencies in the patient chart arise. To avoid recall bias, particularly in the case of inconsistent patient chart notes, data were used from the chart note closest to the date of the event in question. Furthermore, while our adjustment methods do achieve well-balanced groups, there may be hidden biases that available covariates do not address. For example, the prescribing physician is not measured and adjusted for, but may have an effect on odds of discontinuation. However, we believe our adjusted methods are adequate and consistent with previous literature. Additionally, this is a single-site study. At the RMMSC at CU, clinicians counsel patients on methods for reducing tolerability issues, such as nausea, vomiting or flushing. However, the amount and type of counseling on this topic may vary at other clinics. Finally, our study does not investigate adherence or compliance, which may affect efficacy outcomes.
In conclusion, our study demonstrates lower discontinuation rates for FTY compared to DMF, and these were mainly driven by fewer AEs/tolerability issues. We expect that if there are differences in efficacy between FTY and DMF, this will become evident only in either much larger or longer studies.
Supplementary Material
Conflicts of Interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Brandi Vollmer has nothing to declare.
Kavita V Nair: grants from Novartis, Biogen, and Gilead Sciences; and consulting for Astellas and Genentech.
Stefan H Sillau has nothing to declare.
John Corboy: research grants from the National MS Society, Patient Centered Outcomes Research Institute, Novartis, Biogen, and MedDay; medical legal work; editor for Neurology: Clinical Practice; and board member of the National Multiple Sclerosis Society, Colorado-Wyoming chapter.
Timothy Vollmer: research grants/studies from Genzyme, Teva Neuroscience, National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS), Rocky Mountain MS Center, EMD Serono, Biogen Idec, Ono Pharmaceuticals, Acorda Pharmaceuticals, and MedImmune; and advisory boards, lectures, and consultancy with Abbvie Inc, Alcimed, American Academy of Neurology, CB Partners, Capmaels and Rasford, Celestial Intas Pharmaceuticals Ltd, Compass Learning, Genentech/Roche, Genzyme/Sanofi, Goodwin Procter LLP, IMS Consulting Group, Medscape, Novartis Pharmaceuticals, Oxford Pharmagenesis, Patient Centered Outcomes Research Institute, Sommer Consulting, Teva Neuroscience, and Xenoport Inc.
Enrique Alvarez: research grants/studies from Genzyme, Biogen, Rocky Mountain MS Center, Novartis, and Acorda; and consulting for Genzyme, Genentech, Novartis, Acorda, and Biogen.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010; 9: 520–532. [DOI] [PubMed] [Google Scholar]
- 2.Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing–remitting multiple sclerosis: The THEPA-MS survey. Ther Adv Neurol Disord 2016; 9: 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011; 28: 51–61. [DOI] [PubMed] [Google Scholar]
- 4.Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): A multicenter observational study on adherence to disease-modifying therapies in patients with relapsing–remitting multiple sclerosis. Eur J Neurol 2011; 18: 69–77. [DOI] [PubMed] [Google Scholar]
- 5.Patti F. Optimizing the benefit of multiple sclerosis therapy: The importance of treatment adherence. Patient Prefer Adherence 2010; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menzin J, Caon C, Nichols C, et al. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm 2013; 19(1 Suppl A): S24–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002; 277: 21453–21457. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 9.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing–remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 11.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 12.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 13.Filippini G, Del Giovane C, Vacchi L, et al. Immunomodulators and immunosuppressants for multiple sclerosis: A network meta-analysis. Cochrane Database Syst Rev 2013, pp. CD008933. [DOI] [PubMed] [Google Scholar]
- 14.Fogarty E, Schmitz S, Tubridy N, et al. Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: Systematic review and network meta-analysis. Mult Scler Relat Disord 2016; 9: 23–30. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson M, Fox RJ, Havrdova E, et al. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing–remitting multiple sclerosis: A systematic review and mixed treatment comparison. Curr Med Res Opin 2014; 30: 613–627. [DOI] [PubMed] [Google Scholar]
- 16.Comi G, O’Connor P, Montalban X, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler 2010; 16: 197–207. [DOI] [PubMed] [Google Scholar]
- 17.Paolicelli D, Manni A, Direnzo V, et al. Long-term cardiac safety and tolerability of fingolimod in multiple sclerosis: A postmarketing study. J Clin Pharmacol 2015; 55: 1131–1136. [DOI] [PubMed] [Google Scholar]
- 18.Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: A retrospective US claims database analysis. J Med Econ 2014; 17: 696–707. [DOI] [PubMed] [Google Scholar]
- 19.Hersh CM, Love TE, Cohn S, et al. Comparative efficacy and discontinuation of dimethyl fumarate and fingolimod in clinical practice at 12-month follow-up. Mult Scler Relat Disord 2016; 10: 44–52. [DOI] [PubMed] [Google Scholar]
- 20.Wicks P, Rasouliyan L, Katic B, et al. The real-world patient experience of fingolimod and dimethyl fumarate for multiple sclerosis. BMC Res Notes 2016; 9: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boster A, Nicholas J, Wu N, et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: Analysis of a large health insurance claims database. Neurol Ther 2017; 6: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013; 136: 3609–3617. [DOI] [PubMed] [Google Scholar]
- 23.Manouchehrinia A, Westerlind H, Kingwell E, et al. Age related Multiple Sclerosis Severity Score: Disability ranked by age. Mult Scler. Epub ahead of print 1 January 2017. DOI: 10.1177/1352458517690618. [DOI] [PMC free article] [PubMed]
- 24.Fox RJ, Chan A, Zhang A, et al. Comparative effectiveness using a matching-adjusted indirect comparison between delayed-release dimethyl fumarate and fingolimod for the treatment of multiple sclerosis. Curr Med Res Opin 2017; 33: 175–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.