Abstract
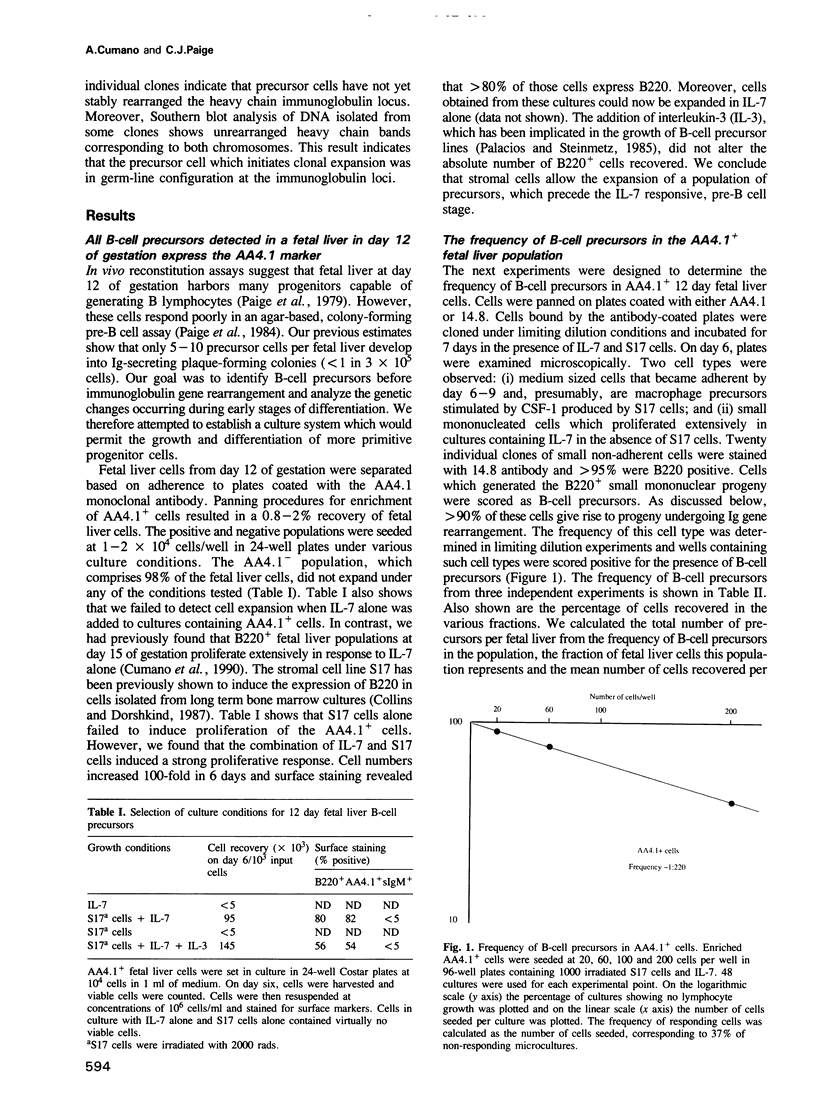
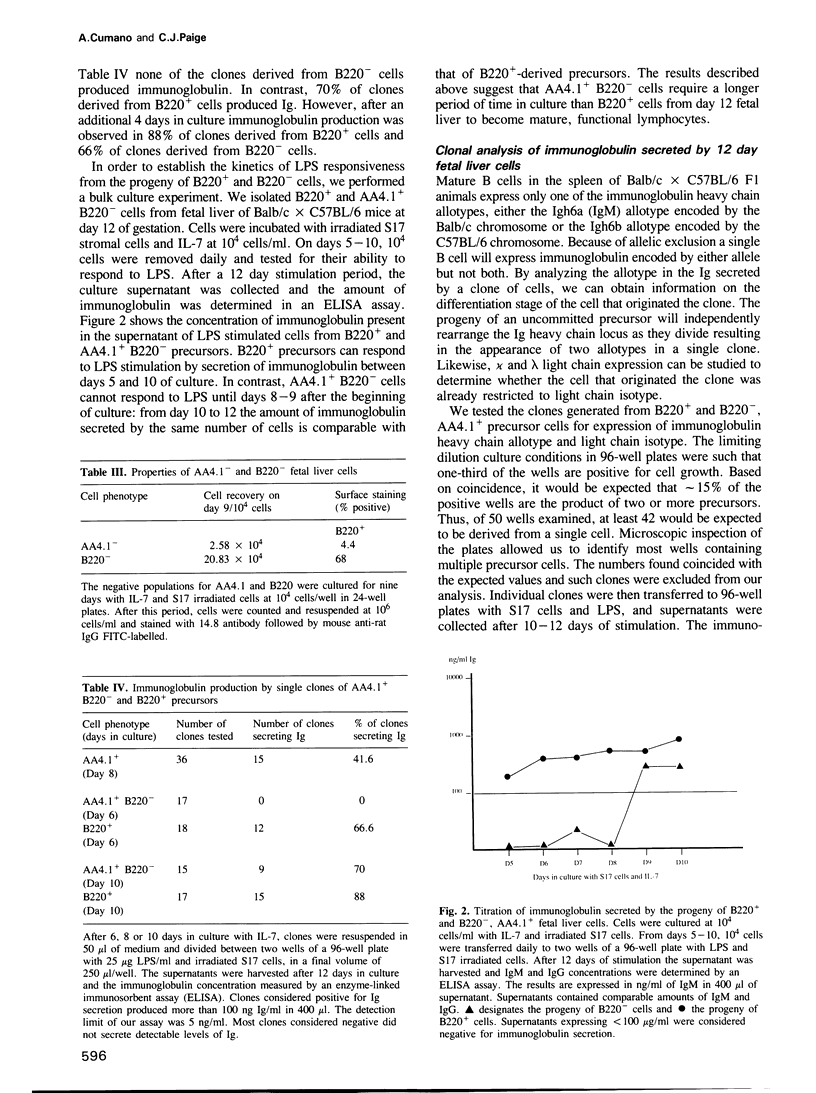
We describe an assay system that allows precursor cells, uncommitted for heavy and light chain immunoglobulin expression, to develop into B lymphocytes that can differentiate to antibody-producing cells. Some precursors have the immunoglobulin loci in germ-line configuration. Approximately 200-1500 precursor cells are present in one fetal liver by day 12 of gestation; they express the surface marker AA4.1. Most precursors do not express the B220 marker. Commitment to heavy chain immunoglobulin expression occurs after an average of two cell division; commitment to light chain expression takes place after two additional rounds of division. DNA analysis from the progeny of single precursor cells shows that: (i) most B220- precursor cells have not completed D-J rearrangement (9/11) and some were in germ line configuration (4/11); and (ii) most B220+ precursor cells exhibit two D-J rearrangements (4/5 samples). These experiments define two types of B-lymphocyte precursor cells in fetal liver: the first, B220+ AA4.1+, acquires the capacity to respond to mitogens only after 5 days in culture, and does not have productive V-D-J rearrangements but might exhibit two stable D-J rearrangements; the second, B220- AA4.1+, acquires the capacity to respond to mitogens only after 9 days in culture and can be in germ-line configuration in the Ig loci, and undergoes rearrangement of heavy and light chain genes in vitro. Both precursor types require interaction with stromal cells before becoming responsive to interleukin 7.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Caton A. J. A single pre-B cell can give rise to antigen-specific B cells that utilize distinct immunoglobulin gene rearrangements. J Exp Med. 1990 Sep 1;172(3):815–825. doi: 10.1084/jem.172.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. S., Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987 Feb 15;138(4):1082–1087. [PubMed] [Google Scholar]
- Cumano A., Dorshkind K., Gillis S., Paige C. J. The influence of S17 stromal cells and interleukin 7 on B cell development. Eur J Immunol. 1990 Oct;20(10):2183–2189. doi: 10.1002/eji.1830201006. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Kunisada T., Ogawa M., Sudo T., Kodama H., Suda T., Nishikawa S., Nishikawa S. Stepwise progression of B lineage differentiation supported by interleukin 7 and other stromal cell molecules. J Exp Med. 1990 May 1;171(5):1683–1695. doi: 10.1084/jem.171.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kincade P. W. Experimental models for understanding B lymphocyte formation. Adv Immunol. 1987;41:181–267. doi: 10.1016/s0065-2776(08)60032-2. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- McKearn J. P., McCubrey J., Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7414–7418. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F. B lymphocyte development in fetal liver. II. Frequencies of precursor B cells during gestation. Eur J Immunol. 1977 Jul;7(7):482–486. doi: 10.1002/eji.1830070715. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg C. E., Whitlock C. A., Weissman I. L. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986 Feb 28;44(4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Gisler R. H., McKearn J. P., Iscove N. N. Differentiation of murine B cell precursors in agar culture. Frequency, surface marker analysis and requirements for growth of clonable pre-B cells. Eur J Immunol. 1984 Nov;14(11):979–987. doi: 10.1002/eji.1830141104. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Moore M. A., Lee G. The fate of fetal and adult B-cell progenitors grafted into immunodeficient CBA/N mice. J Exp Med. 1979 Sep 19;150(3):548–563. doi: 10.1084/jem.150.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J. Surface immunoglobulin-negative B-cell precursors detected by formation of antibody-secreting colonies in agar. Nature. 1983 Apr 21;302(5910):711–713. doi: 10.1038/302711a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Rolink A., Kudo A., Karasuyama H., Kikuchi Y., Melchers F. Long-term proliferating early pre B cell lines and clones with the potential to develop to surface Ig-positive, mitogen reactive B cells in vitro and in vivo. EMBO J. 1991 Feb;10(2):327–336. doi: 10.1002/j.1460-2075.1991.tb07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüppel R., Wilke J., Weiler E. Monoclonal anti-allotype antibody towards BALB/c IgM. Analysis of specificity and site of a V-C crossover in recombinant strain BALB-Igh-Va/Igh-Cb. Eur J Immunol. 1987 May;17(5):739–741. doi: 10.1002/eji.1830170527. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]
- Sudo T., Ito M., Ogawa Y., Iizuka M., Kodama H., Kunisada T., Hayashi S., Ogawa M., Sakai K., Nishikawa S. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989 Jul 1;170(1):333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P. A., Burrows P. D., Namen A., Gillis S., Cooper M. D. Bone marrow stromal cells and interleukin-7 induce coordinate expression of the BP-1/6C3 antigen and pre-B cell growth. Int Immunol. 1990;2(8):697–705. doi: 10.1093/intimm/2.8.697. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N., Radbruch A., Rajewsky K. Ig gene rearrangement and expression in the progeny of B-cell progenitors in the course of clonal expansion in bone marrow cultures. EMBO J. 1987 Sep;6(9):2735–2741. doi: 10.1002/j.1460-2075.1987.tb02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




