Abstract
Background:
Imbalance leading to falls is common in people with multiple sclerosis (PwMS).
Objective:
To evaluate the effects of a balance group exercise programme (CoDuSe) on balance and walking in PwMS (Expanded Disability Status Scale, 4.0–7.5).
Methods:
A multi-centre, randomized, controlled single-blinded pilot study with random allocation to early or late start of exercise, with the latter group serving as control group for the physical function measures. In total, 14 supervised 60-minute exercise sessions were delivered over 7 weeks. Pretest–posttest analyses were conducted for self-reported near falls and falls in the group starting late. Primary outcome was Berg Balance Scale (BBS). A total of 51 participants were initially enrolled; three were lost to follow-up.
Results:
Post-intervention, the exercise group showed statistically significant improvement (p = 0.015) in BBS and borderline significant improvement in MS Walking Scale (p = 0.051), both with large effect sizes (3.66; −2.89). No other significant differences were found between groups. In the group starting late, numbers of falls and near falls were statistically significantly reduced after exercise compared to before (p < 0.001; p < 0.004).
Conclusion:
This pilot study suggests that the CoDuSe exercise improved balance and reduced perceived walking limitations, compared to no exercise. The intervention reduced falls and near falls frequency.
Keywords: Accidental falls, exercise, multiple sclerosis, postural balance, core stability
Introduction
Impaired balance1 and trunk control2 and difficulty to perform dual tasks are common in people with multiple sclerosis (PwMS);3 moreover, approximately 56% reports fall (Expanded Disability Status Scale (EDSS) score, 0–6.5).4 Balance function can improve by specific exercise, thus possibly reducing fall frequency.5–7 Freeman et al.8 reported a case series (n = 8) in which an 8-week core stability exercise programme (16 sessions) improved balance and mobility skills. Another study compared core stability exercise (referred to as Pilates) to ‘standard exercise’ and ‘relaxation sessions’ (controls) in a randomized controlled trial (RCT) including 100 PwMS (EDSS, 4.0–6.5).9 The results showed that 12 individualized 30-minute exercise sessions, enhanced with an individualized 15-minute daily home exercise programme, did not significantly improve walking capacity or perceived balance confidence.9 Exercise in different sensory contexts7 including visuo-proprioceptive training10 seems relevant to improve balance in PwMS. Nilsagård et al.6 developed an exercise concept called CoDuSe, built on core stability8 in combination with dual task and sensorimotor challenges. Seven weeks of twice-weekly physiotherapist-led 60-minute sessions of group-based balance exercise did not only improve balance but also reduced number of falls and fallers in a sample (n = 32) with mild to moderate multiple sclerosis (MS).6 Other studies have shown that a 12-week home-based exercise programme for PwMS offers a safe and effective way to improve balance and reduce the risk of falls in people with mild to moderate MS (EDSS scores, 2.5–6.5 and 2–6, respectively).11,12 To achieve a sustainable habit, a combination of home- and community-based programmes has been suggested.13 The effects on balance exercise in PwMS with higher EDSS scores are less studied, since using falls as outcome measure is rare. The aim of this pilot study was to evaluate the effects of the CoDuSe exercise concept for PwMS with EDSS score 4.0–7.5, during 7 weeks of twice-weekly, physiotherapist-led 60-minute sessions in groups of two to five people with the addition of an individually designed home exercise programme.
Patients and methods
Eligible participants (⩾18 years) were identified by the research physiotherapist (PT) at each centre, either in primary health care centres or in hospitals, using personal knowledge and/or access to the Swedish Neuro Register (http://neuroreg.se/). Inclusion criteria were (1) MS diagnosed according to the McDonald criteria,14 (2) walking ability not exceeding 200 m (with or without a walking aid) and (3) ability to transfer between a wheelchair and a plinth with only slight assistance (in order to be able to participate in the intervention).
Exclusion criteria were (1) cognitive symptoms making it difficult to understand the study information, follow instructions or fill in rating scales; (2) having sought medical care related to impaired walking during the past 3 months in order to not be in a current or recent relapse; (3) having participated in balance exercise administered by health care personnel during the past 30 days; and (4) having started or changed medication with 4-aminopyridine during the past 30 days (a medication that brands itself on improving walking and is a potential bias).
A multi-centre, randomized, controlled pilot study was conducted, across seven centres (Örebro, Västerås, Eskilstuna, Linköping, Nyköping, Gävle and Karlskoga) in five different County Council areas in Sweden. Using a waiting list design, participants were randomly allocated to intervention with either early or late start (control group). The participants allocated to late start were urged to maintain their present physical activity levels. The study was registered in the Swedish Clinical Trials database (ID: 153691) and in ClinicalTrials.Gov (NCT 02209467).
The study followed the Declaration of Helsinki and was approved by the Regional Ethics Committee in Uppsala-Örebro (2014/302).
Potential participants received written information about the study together with an invitation to participate and were also contacted by phone to address any questions. Those who agreed to participate were scheduled for baseline testing; written consent was obtained.
An external statistician conducted a computerized random allocation sequence with varied block sizes (2–6). Concealed allocation was achieved using sealed envelopes, which were opened right after baseline measure by the PT in charge at each site. Raters blinding was accomplished with the raters travelling to different centres, unaware of allocation. Testing was conducted a week before the intervention (week 0), the week after its completion (week 8) and 7 weeks after completion (week 16).
Intervention
The CoDuSe balance exercise is a concept including core stability exercise inspired by Freeman et al.,8 dual tasking and sensory strategies.6 The exercises were customized to fit the disability level of the sample throughout an extensive interactive process, including both discussions and practical training to ensure consistency with experienced PTs from all participating centres. The 60-minute group-based balance exercise was given in small groups (two to five people) twice weekly during a 7-week period with at least one PT present. The first 30 minutes were primarily focused on core stability exercise, that is, controlled leg movements. The participants were then encouraged to maintain focus on core stability while performing the remaining exercises, which included dual tasking and sensory strategies such as carrying something while walking or walking on an uneven surface (see Supplementary Appendix 1).Throughout the intervention period, the participants were encouraged and instructed by the PTs to progress to more challenging exercises when suitable. The programme is available from the corresponding author (A.C.).
In addition, they were given an individually tailored home exercise programme with two to five exercises. Progression of the exercises was continuously adjusted by the PTs.
Standardized protocols were used to register compliance and adverse events, that is, falls during group sessions or test occasions. A study-specific diary was used for daily self-registration of home exercises.
Outcome measures
Clinically administered and patient-reported outcomes were used in a standardized order (The Fatigue Scale for Motor and Cognitive Functions (FSMC), Trunk Impairment Scale (TIS), The Timed Sit-To-Stand test (TstS), Postural Sway, Berg Balance Scale (BBS), Falls Efficacy Scale–International (FES-I), the 12-item MS Walking Scale (MSWS), Timed Up and Go (TUG), 10-m walk test (10WT) and 2-minute walk test (2MWT)). Four PTs were in charge of the testing procedure. Each participant was always measured by the same PT. Prior to study start, data collectors were trained in order to minimize systematic differences in rating and measuring the participants’ performance. The used walking aid for the walking test was consistent for the participants between each assessment. Initially, TUG was considered as primary outcome measure. However, prior to the analysis, the primary outcome measure was changed to the BBS as in line with that suggested by Cattaneo et al.15
The BBS, measures static and dynamic balance using 14 items rated from 0 to 4, giving a maximum score of 56. The BBS is a valid and reliable test for PwMS.16,17
TIS measures trunk stability while sitting using three subcategories (static, dynamic and coordination) for a maximum total score of 23. It is valid for PwMS.18
TstS measures time while performing five repeated transfers from sitting to standing.19 The test was slightly modified for safety reasons; instead of crossing arms over the chest, hand support was allowed.
Postural sway in standing without shoes was measured using a sway meter,20 where an area from dash of the pen occurs. Different conditions were used either with eyes open or with eyes closed for 30 seconds.20
TUG measures basic mobility21 and is valid and reliable for PwMS.17 Time is registered from when the person arises, walks 3 m, turns around, walks back and sits down again. A test trial was allowed, and one attempt was registered.
10WT, time to walk quickly but safely from a still standing position was registered. Two attempts were performed and the mean value was used in further calculations.
2MWT22 was measured using a 15-m pathway in a quiet corridor. Walking speed during 2 minutes and metres walked during that time both have a discriminatory property for degree of MS severity.23
FSMC contains 20 items with a maximum total score of 100. It is reliable and discriminates between PwMS and healthy controls.24
FES-I is a valid and reliable self-rating scale measuring concerns about falls in 16 everyday situations (maximum score = 64).25,26
MSWS is a valid and reliable scale where participants rate the extent to which MS has limited their walking ability during the past 2 weeks (maximum score = 100).27
Falls and near falls were prospectively reported daily from baseline to follow-up using a diary. A fall was defined as ‘an unexpected event in which participants come to rest on the ground, floor, or lower level’28 and a near fall as ‘an occasion on which an individual felt that they were about to fall but did not actually fall’.29 Weekly reminders to fill in the diary were given either by the PT after each session or by text message from the study leader. The diaries were collected by the PT in charge of the intervention, collected by the rater PT at each centre during measurement occasions or posted to the study leader in a pre-paid addressed envelope.
Disease burden in the study sample was measured using the patient-administered EDSS,30 and the ratings were interpreted by a neurologist (M.G.) to determine a current EDSS. Cognitive functioning was measured using the Symbol Digits Modalities Test (SDMT).31
To detect a three-point reduction in BBS, with a two-sided 5% significance level and a power of 80%, a sample size of 45 was required. We aimed to enrol at least 50 individuals to account for possible drop-outs.
Statistical methods
The between-group comparisons were performed on the intention-to-treat population with a mixed covariance pattern model for repeated measures data with unstructured covariance matrix with adjustment for sex, age and MS subtype at baseline. This analysis takes care of missing data in an optimal way.32 Effect size (ES) for change between two groups was calculated (mean difference/pooled standard deviation (SD)) and for change within groups (mean differences/SD for the differences).
The overall trend for number of falls and near falls in the late-start group was estimated using the slope from a linear regression within each patient. The overall trend was tested with the Wilcoxon signed-rank test over the participants. For comparison over time, the Wilcoxon signed-rank test was used for continuous variables.
Results
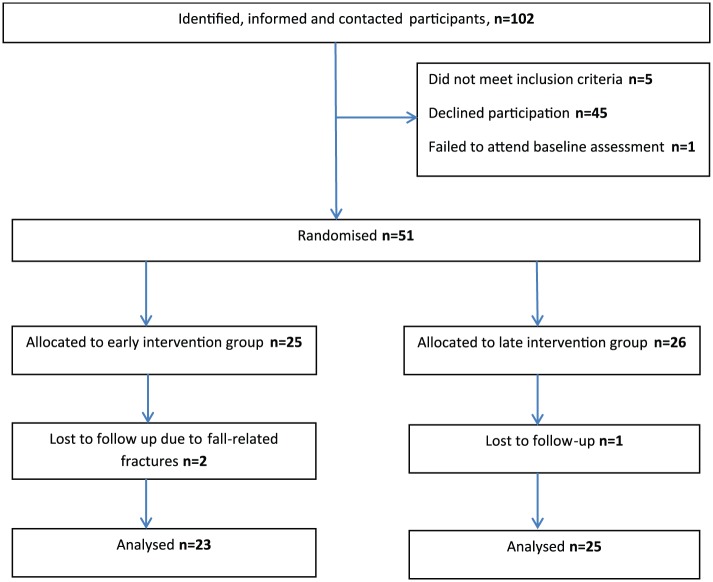
A total of 51 participants were included (Figure 1) (mean age 58 years, median EDSS score 6.0 and female:male ratio 2.2:1). Data were collected between August 2014 and February 2015. Three were lost to follow-up. The groups differed at baseline in terms of age, gender and MS subtype (Table 1). Compliance with the supervised exercise (median 13 of 14 possible sessions, min 7; max 14) was excellent. Compliance to home exercise was in mean 16.28 sessions (min 0; max 75); 48% achieved the goal of performing home exercise twice weekly. After completing the supervised exercise, the mean number of completed home exercise sessions decreased slightly to 13.81 (min 0; max 71) and 38.5% achieved the goal of twice-weekly home exercises.
Figure 1.
Flow chart.
Table 1.
Demographics and baseline characteristics in the intention-to-treat population.
| Variable | Total (n = 51) | Intervention group (n = 25) | Control group (n = 26) |
|---|---|---|---|
| Age (years) | 58 (10.24) 55.5 (31; 78) n = 51 |
61.64 (11.25) 64 (31; 77) n = 25 |
54.73 (8.16) 54 (41; 78) n = 26 |
| Gender | |||
| Male | 16 (31.4%) | 6 (24.0%) | 10 (38.5%) |
| Female | 35 (68.6%) | 19 (76.0%) | 16 (61.5%) |
| Subtype | |||
| Relapsing-remitting | 6 (11.8%) | 0 (0.0%) | 6 (23.1%) |
| Secondary progressive | 32 (62.7%) | 17 (68.0%) | 15 (57.7%) |
| Primary progressive | 13 (25.5%) | 8 (32.0%) | 5 (19.2%) |
| EDSS | 6.11 (0.49) 6.00 (4.00; 7.50) n = 51 |
6.16 (0.45) 6.00 (5.00; 7.50) n = 25 |
6.06 (0.54) 6.00 (4.00; 7.00) n = 26 |
| Falls (retrospective) | |||
| No falls | 24 (47.1%) | 12 (48.0%) | 12 (46.2%) |
| One fall | 13 (25.5%) | 8 (32.0%) | 5 (19.2%) |
| Multiple falls | 14 (27.5%) | 5 (20.0%) | 9 (34.6%) |
| Walking aid indoors | |||
| None | 7 (13.7%) | 2 (8.0%) | 5 (19.2%) |
| Unilateral | 8 (15.7%) | 3 (12.0%) | 5 (19.2%) |
| Crutches/canes bilateral | 2 (3.9%) | 1 (4.0%) | 1 (3.8%) |
| Walker | 17 (33.3%) | 10 (40.0%) | 7 (26.9%) |
| Wheelchair | 5 (9.8%) | 3 (12.0%) | 2 (7.7%) |
| Other | 12 (23.5%) | 6 (24.0%) | 6 (23.1%) |
| Walking aid outdoors | |||
| None | 1 (2.0%) | 0 (0.0%) | 1 (3.8%) |
| Unilateral | 6 (11.8%) | 0 (0.0%) | 6 (23.1%) |
| Crutches/canes bilateral | 1 (2.0%) | 0 (0.0%) | 1 (3.8%) |
| Walker | 15 (29.4%) | 9 (36.0%) | 6 (23.1%) |
| Wheelchair | 9 (17.6%) | 5 (20.0%) | 4 (15.4%) |
| Other | 19 (37.3%) | 11 (44.0%) | 8 (30.8%) |
| Family | |||
| Single | 20 (39.2%) | 8 (32.0%) | 12 (46.2%) |
| Living with partner | 31 (60.8%) | 17 (68.0%) | 14 (53.8%) |
| Accommodation | |||
| Flat | 26 (51.0%) | 11 (44.0%) | 15 (57.7%) |
| House | 25 (49.0%) | 14 (56.0%) | 11 (42.3%) |
| Communal support | |||
| No | 46 (90.2%) | 23 (92.0%) | 23 (88.5%) |
| Yes | 5 (9.8%) | 2 (8.0%) | 3 (11.5%) |
| Support from assistant | |||
| No | 49 (96.1%) | 24 (96.0%) | 25 (96.2%) |
| Yes | 2 (3.9%) | 1 (4.0%) | 1 (3.8%) |
| Years since diagnosis | 20.85 (12) 19 (2; 50) n = 51 |
21.56 (13.84) 19 (2; 50) n = 25 |
20.19 (10.35) 19 (6; 46) n = 26 |
SD: standard deviation; EDSS: Expanded Disability Status Scale.
For categorical variables, n (%) is presented.
For continuous variables, mean (SD)/median (min; max)/n is presented.
A statistically significant improvement (mean, 3.65 points; 95% confidence interval (CI), 0.75 to 6.54) in favour of the intervention group (p = 0.015) was shown for the primary outcome (Table 2). ES value (2.53) suggested a high practical significance. A borderline significant reduction of perceived limitations in walking due to MS was found in favour of the intervention group (7.21 points; 95% CI, −14.46 to 0.03, p = 0.051). There were no significant between-group differences for other outcomes.
Table 2.
Intention-to-treat analysis of within-group and between-group changes.
| Intervention group (n = 25) |
Control group (n = 26) |
Difference intervention – control |
|||||
|---|---|---|---|---|---|---|---|
| Baseline, mean (95% CI) | Follow-up, mean (95% CI) | Change, mean (SEM) 95% CI Effect size p-value |
Baseline, mean (95% CI) | Follow-up, mean (95% CI) | Change, mean (SEM) 95% CI Effect size p-value |
Change, mean (SEM) 95% CI Effect size p-value |
|
| Berg Balance Scale (0–56) | 31.32 (9.41; 53.23) | 35.89 (13.89; 57.89) | 4.57 (1.04) (2.47; 6.66) 4.40 p < .0001 |
34.80 (15.17; 54.44) | 35.72 (15.99; 55.45) | 0.92 (1.00) (−1.08; 2.92) 0.92 p = 0.36 |
3.65 (1.44) (0.75; 6.54) 3.66 p = 0.015 |
| Timed Up and Go, seconds | 50.90 (7.25; 94.55) | 49.95 (6.94; 92.97) | −0.95 (2.30) (−5.57; 3.68) −0.41 p = 0.68 |
48.93 (10.14; 87.71) | 43.57 (5.47; 81.68) | −5.35 (2.18) (−9.75; −0.96) −2.45 p = 0.018 |
4.41 (3.17) (−1.97; 10.79) 2.00 p = 0.17 |
| 2-minute walk test, m | 56.19 (−5.11; 117.50) | 55.96 (−5.45; 117.38) | −0.23 (2.46) (−5.19; 4.73) −0.09 p = 0.93 |
54.99 (0.70; 109.29) | 58.00 (3.60; 112.40) | 3.01 (2.31) (−1.64; 7.65) 1.30 p = 0.20 |
−3.24 (3.37) (−10.03; 3.56) −1.39 p = 0.34 |
| The timed sit-to-stand test, seconds | 25.24 (2.89; 47.59) | 25.57 (3.43; 47.70) | 0.33 (1.56) (−2.81; 3.47) 0.21 p = 0.83 |
23.53 (3.47; 43.58) | 23.61 (3.73; 43.50) | 0.09 (1.44) (−2.82; 2.99) 0.06 p = 0.95 |
0.24 (2.12) (−4.03; 4.52) 0.17 p = 0.91 |
| 10-m walk test, seconds | 90.40 (−12.15; 192.96) | 91.33 (−11.33; 193.98) | 0.92 (2.80) (−4.72; 6.56) 0.33 p = 0.74 |
84.44 (−6.39; 175.27) | 83.86 (−7.06; 174.79) | −0.57 (2.63) (−5.86; 4.72) −0.22 p = 0.83 |
1.49 (3.84) (−6.24; 9.23) 0.56 p = 0.70 |
| Postural sway, eyes open, area (mm2) | 3012.00 (−1566.48; 7590.48) | 3033.57 (−1511.33; 7578.48) | 21.57 (376.67) (−737.84; 780.99) 0.06 p = 0.95 |
2626.74 (−1538.56; 6792.05) | 2521.45 (−1620.42; 6663.32) | −105.30 (369.94) (−851.39; 640.79) −0.28 p = 0.78 |
126.87 (527.94) (−937.68; 1191.43) 0.35 p = 0.81 |
| Postural sway, eyes closed, area (mm2) | 3887.39 (−1751.69; 9526.47) | 3385.55 (−2115.69; 8886.78) | −501.85 (687.95) (−1898.01; 894.32) −0.73 p = 0.47 |
3631.77 (−1214.45; 8477.98) | 2770.12 (−1874.41; 7414.64) | −861.65 (647.71) (−2179.09; 455.79) −1.33 p = 0.19 |
359.81 (944.99) (−1560.05; 2279.66) 0.55 p = 0.71 |
| Trunk Impairment Scale (0–23) | 16.22 (10.58; 21.86) | 17.24 (11.59; 22.89) | 1.02 (0.66) (−0.31; 2.35) 1.54 p = 0.13 |
16.26 (11.20; 21.32) | 16.24 (11.17; 21.31) | −0.02 (0.64) (−1.30; 1.26) −0.03 p = 0.97 |
1.04 (0.92) (−0.81; 2.88) 1.63 p = 0.26 |
| Falls Efficacy Scale–International (16–64) | 29.99 (9.86; 50.11) | 29.67 (9.48; 49.85) | −0.32 (1.72) (−3.79; 3.14) −0.19 p = 0.85 |
27.76 (9.72; 45.80) | 29.10 (10.99; 47.20) | 1.34 (1.65) (−1.99; 4.66) 0.81 p = 0.42 |
−1.66 (2.39) (−6.46; 3.14) −1.00 p = 0.49 |
| MS Walking Scale (0–100) | 95.32 (63.67; 126.96) | 88.67 (56.83; 120.50) | −6.65 (2.60) (−11.88; −1.42) −2.56 p = 0.014 |
83.60 (55.27; 111.94) | 84.16 (55.62; 112.70) | 0.56 (2.49) (−4.45; 5.57) 0.22 p = 0.82 |
−7.21 (3.60) (−14.46; 0.03) −2.89 p = 0.051 |
| Fatigue Scale for Motor and Cognitive functions, total (0–100) | 65.99 (31.31; 100.67) | 63.56 (28.95; 98.16) | −2.43 (2.04) (−6.53; 1.67) −1.19 p = 0.24 |
65.78 (34.69; 96.88) | 64.80 (33.78; 95.82) | −0.98 (1.96) (−4.92; 2.96) −0.50 p = 0.62 |
−1.45 (2.83) (−7.14; 4.24) −0.74 p = 0.61 |
| Fatigue Scale for Motor and Cognitive functions, motor (0–50) | 37.45 (22.17; 52.72) | 35.25 (19.99; 50.50) | −2.20 (1.16) (−4.52; 0.13) −1.90 p = 0.063 |
37.50 (23.80; 51.19) | 36.89 (23.21; 50.56) | −0.61 (1.11) (−2.84; 1.63) −0.55 p = 0.59 |
−1.59 (1.60) (−4.82; 1.64) −1.43 p = 0.33 |
CI: confidence interval; SEM: standard error of mean.
Prospectively reported falls
The late-start group reported a total of 245 falls and 2220 near falls during the study period, giving a fall rate of 1.28/person/month and a near fall rate of 11.64/person/month. An overall positive statistically significant trend with a successive reduction of falls was found for number of falls and near falls before, during and after the balance exercise period. Mean falls dropped from 4.18 before intervention to 1.68 after, and near falls from 23.2 before to 8.64 after (Table 3).
Table 3.
Number of falls and near falls before, during and after intervention period.
| Variable | Before (n = 22) | During (n = 24) | After (n = 22) | Change from before to during |
Change from before to after |
Change from during to after |
Overall trend |
Rate |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-value | p-value | p-value | p-value | Person/month | ||||||||
| Number of falls | 4.18 (5.28) 2.00 (0.00; 22.00) n = 22 |
2.17 (4.22) 0.00 (0.00; 15.00) n = 24 |
1.68 (4.30) 0.00 (0.00; 18.00) n = 22 |
−1.82 (3.02) −2.00 (−8.00; 6.00) n = 22 |
0.0061 | −2.48 (3.31) −2.00 (−12.00; 3.00) n = 21 |
0.0011 | −0.682 (2.835) 0.000 (−11.000; 4.000) n = 22 |
0.31 | −1.24 (1.66) −1.00 (−6.00; 1.50) n = 21 |
0.0006 | 1.28 |
| Number of near falls | 23.2 (34.8) 7.0 (0.0; 130.0) n = 22 |
18.0 (23.5) 9.0 (0.0; 78.0) n = 24 |
8.64 (11.03) 2.00 (0.00; 31.00) n = 22 |
−5.27 (14.94) −1.00 (−52.00; 22.00) n = 22 |
0.052 | −16.5 (29.6) −3.0 (−107.0; 6.0) n = 21 |
0.0038 | −11.0 (16.5) −2.5 (−55.0; 4.0) n = 22 |
0.0024 | −8.24 (14.78) −1.50 (−53.50; 3.00) n = 21 |
0.0018 | 11.64 |
Only participants in the late-start group are included, as they had fall and near fall frequencies registered before the intervention period.
Mean (SD)/median (min;max)/n is presented.
Two adverse events (both falls) occurred during intervention, neither fall was injurious.
Discussion
This pilot study suggests that the CoDuSe balance group exercise programme improves balance, as measured using the BBS and perceived limitations in walking due to MS (EDSS, 4.0–7.5). It also reduces the number of falls as well as near falls. These results are in line with those presented in a previous study and imply that CoDuSe intervention is a promising intervention in disease stages characterized by significant loss of walking ability.6 Besides statistical significance, clinical relevance of changes should be pondered. Minimal detectable change (MDC) for the BBS has been suggested to vary between 3 and 7 points for PwMS.33,34 The studied samples are varying in size (n = 24–120) and the EDSS varies from 0 to 6.5. A recent study investigating sensory integration rehabilitation (EDSS median 3.0) used the MDC of 3 points.35 In the studied sample, 89% were expected to have a progressive MS. We therefore settled for a 3-point change33 that was exceeded (3.65 points). The improvement in MSWS exceeded (mean, 7.21 points) previously suggested MDC at 5.136 and is considered as clinical important, suggesting that gains of participating in CoDuSe exercise transfers to everyday life. This result is in line with that reported after twelve 30-minute exercise sessions during 12 weeks of Pilates group exercise, enhanced with individualized daily home exercise programme.9
The CoDuSe intervention led to a reduction of falls which is important, as falls can lead to injury37 and activity curtailment.38 The CoDuSe intervention has previously been reported to reduce the number of prospectively reported falls after fourteen 60-minute exercise sessions during 7 weeks.6 The ability to reproduce these results in a new sample strengthens that the intervention is effective to reduce falls at least in a shorter time period. Long-term effect still needs to be evaluated.
The number of prospectively reported near falls was also reduced. Being able to manage imbalance without falling can be seen as a successful strategy. The participants may have learnt to maintain stability in situations where they had previously lost balance or may have gained knowledge about their own limits and therefore avoided risks. As this measure is a combination of close calls and good balance corrections, it is difficult to know exactly what this self-report measure adds to actual falls.
Initially, TUG was registered as primary outcome since trips and slips while walking leading to falls have been reported.39 However, prior to analysis, the primary outcome was altered to the BBS since it was believed to reflect the content of the CoDuSe exercise in a more accurate way than TUG would. CoDuSe encourages awareness of trunk stability and mindful movements resulting in moving slower rather than moving faster. BBS contains items challenging both static and dynamic balance and was considered as more appropriate as primary outcome, hence the change.
Balance confidence did not improve despite the reduction of falls and near falls. The FES-I is associated with falls history in PwMS40 but has, to our knowledge, not been correlated with prospectively collected falls during exercise intervention. A possible reason for lack of change may be the short follow-up period, as behavioural changes are known to take time. The remaining outcome measures addressing aspects such as walking ability did not show statistically significant improvement.
The drop-out was low in this study, three of 51 participants. Reasons for drop-out were fractures not associated with intervention (n = 2), and personal reasons. The high compliance on the supervised exercises suggests that the intervention was feasible to conduct and perceived by the participants as worth investing time in. Compliance with the home exercise varied and was lower than reported by Fox et al.9 during the time for the intervention and were reduced further after completing supervised group exercise. Reasons for low compliance were not explored but could reveal important information to support patients to comply better. The social aspects of group exercise are probably of importance. The addition of home exercise provided an opportunity to increase the amount of exercise without investing time in travelling or demanding any clinical resources. Home exercise is likely to be of great value to maintain gained abilities, and the transition to individual exercise responsibility was initiated early.
A multi-centre design was a prerequisite to avoid type-II error but induces a risk of inconsistency in conducting the intervention. To avoid this, the PTs from all sites met several times to discuss and share both theoretical and practical issues. Their clinical expertise assured tailoring of the intervention for each participant for maximum effect while still considering safety aspects. This may be even more crucial considering the high EDSS. Small groups were one safety precaution.
Strengths of this study
This is a clinically originated, multi-centre, randomized, controlled pilot study possible to conduct in most clinical settings. The sample was not restricted by type of MS or cognitive impairments. The use of intention-to-treat analyses eliminates problems with non-compliance and missing outcomes.
The primary outcome measure is valid, reliable, well known and frequently used in research and in clinical praxis. Falls are the ultimate consequence of imbalance and therefore highly relevant as an outcome measure. Prospectively collected data on falls and near falls enhance validity and diminish recall bias. Accurate reporting was emphasized by standardized procedures, face-to-face or text messages.
Limitations of this study
A limitation is the lack of pre-intervention data on falls and near falls. A potential risk in reporting near falls is that these may be such a common part of the person’s everyday life that they go unnoticed.
Heterogeneity is difficult to foresay in samples of PwMS and the study may be underpowered. A power calculation on the basis of present means and SDs on the primary outcome measure BBS suggests that 33 persons in each group would be a more appropriate sample size.
The intention of MS care is to encourage people to be as active as possible. It is a limitation that the actual activity level was not documented. Differences between groups are harder to show when it is likely that the control group maintained some level of activity.
Conclusion
To conclude, this pilot multi-centre, randomized controlled single-blinded study seems promising since showing the CoDuSe group exercise programme improved balance and reduced perceived walking limitations compared to the control group. The intervention also reduced frequency of falls and near falls.
Supplementary Material
Acknowledgments
The authors thank the assisting PTs: Ellinor Cedergren, Department of Physiotherapy at Örebro University Hospital; Steven Allen, Brickegårdens Primary Health Care Centre in Karlskoga; Ian Eide and Martin Göransson, Karlskoga Hospital in Karlskoga; Oskar Davidsson and Lisbeth Franzén, Physiotherapy Clinic at Nyköping Hospital; Marie Fredriksen and Sara Hedström, Physiotherapy Primary Health Care Centre in Linköping; Malin Andreasson and Helena Vesterlin, NeuroRehab, Mälarhospital in Eskilstuna; Ingrid Lundström and Margareta Wibom, Rehab Clinic at Västerås Hospital; and Sara Keisu, Helena Jensen and Maria Svedjebrant, Physical Therapy Special Care, Gävle Hospital.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by grants from the Uppsala-Örebro Regional Research Committé, the research committee of Örebro County Council and the Norrbacka-Eugenia Foundation.
Contributor Information
Anna Carling, University Healthcare Research Centre, Faculty of Medicine and Health, Örebro University, Örebro, Sweden/Department of Physiotherapy, School of Medical Sciences, Örebro University, Örebro University Hospital, Örebro, Sweden.
Anette Forsberg, University Healthcare Research Centre, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
Martin Gunnarsson, Department of Neurology, School of Medical Sciences, Örebro University, Örebro, Sweden.
Ylva Nilsagård, University Healthcare Research Centre, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
References
- 1. Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006; 12: 620–628. [DOI] [PubMed] [Google Scholar]
- 2. Lanzetta D, Cattaneo D, Pellegatta D, et al. Trunk control in unstable sitting posture during functional activities in healthy subjects and patients with multiple sclerosis. Arch Phys Med Rehabil 2004; 85: 279–283. [DOI] [PubMed] [Google Scholar]
- 3. Hamilton F, Rochester L, Paul L, et al. Walking and talking: An investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler 2009; 15: 1215–1227. [DOI] [PubMed] [Google Scholar]
- 4. Nilsagard Y, Gunn H, Freeman J, et al. Falls in people with MS – An individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult Scler 2015; 21: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunn H, Markevics S, Haas B, et al. Systematic review: The effectiveness of interventions to reduce falls and improve balance in adults with multiple sclerosis. Arch Phys Med Rehabil 2015; 96: 1898–1912. [DOI] [PubMed] [Google Scholar]
- 6. Nilsagård YE, von Koch LK, Nilsson M, et al. Balance exercise program reduced falls in people with multiple sclerosis: A single-group, pretest-posttest trial. Arch Phys Med Rehabil 2014; 95: 2428–2434. [DOI] [PubMed] [Google Scholar]
- 7. Cattaneo D, Jonsdottir J, Zocchi M, et al. Effects of balance exercises on people with multiple sclerosis: A pilot study. Clin Rehabil 2007; 21: 771–781. [DOI] [PubMed] [Google Scholar]
- 8. Freeman JA, Gear M, Pauli A, et al. The effect of core stability training on balance and mobility in ambulant individuals with multiple sclerosis: A multi-centre series of single case studies. Mult Scler 2010; 16: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 9. Fox EE, Hough AD, Creanor S, et al. The effects of ‘Pilates’ based core stability training in ambulant people with multiple sclerosis: A multi-centre, randomised, assessor-blinded, controlled trial. Phys Ther 2016; 96: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 10. Prosperini L, Leonardi L, De Carli P, et al. Visuo-proprioceptive training reduces risk of falls in patients with multiple sclerosis. Mult Scler 2010; 16: 491–499. [DOI] [PubMed] [Google Scholar]
- 11. Sosnoff JJ, Finlayson M, McAuley E, et al. Home-based exercise program and fall-risk reduction in older adults with multiple sclerosis: Phase 1 randomized controlled trial. Clin Rehabil 2014; 28: 254–263. [DOI] [PubMed] [Google Scholar]
- 12. Hoang P, Schoene D, Gandevia S, et al. Effects of a home-based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis – A randomized controlled trial. Mult Scler 2015; 22: 94–103. [DOI] [PubMed] [Google Scholar]
- 13. Gunn H, Cattaneo D, Finlayson M, et al. Home or away? Choosing a setting for a falls-prevention program for people with multiple sclerosis. Int J MS Care 2014; 16: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 15. Cattaneo D, Jonsdottir J, Coote S. Targeting dynamic balance in falls-prevention interventions in multiple sclerosis: Recommendations from the International MS Falls Prevention Research Network. Int J MS Care 2014; 16: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cattaneo D, Jonsdottir J, Repetti S. Reliability of four scales on balance disorders in persons with multiple sclerosis. Disabil Rehabil 2007; 29: 1920–1925. [DOI] [PubMed] [Google Scholar]
- 17. Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil 2006; 28: 789–795. [DOI] [PubMed] [Google Scholar]
- 18. Verheyden G, Nuyens G, Nieuwboer A, et al. Reliability and validity of trunk assessment for people with multiple sclerosis. Phys Ther 2006; 86: 66–76. [DOI] [PubMed] [Google Scholar]
- 19. Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med 1985; 78: 77–81. [DOI] [PubMed] [Google Scholar]
- 20. Sturnieks DL, Arnold R, Lord SR. Validity and reliability of the swaymeter device for measuring postural sway. BMC Geriatr 2011; 11: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Podsiadlo D, Richardson S. The timed ‘Up & Go’: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 22. Butland RJ, Pang J, Gross ER, et al. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J 1982; 284: 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gijbels D, Dalgas U, Romberg A, et al. Which walking capacity tests to use in multiple sclerosis? A multicentre study providing the basis for a core set. Mult Scler 2012; 18: 364–371. [DOI] [PubMed] [Google Scholar]
- 24. Penner IK, Raselli C, Stocklin M, et al. The Fatigue Scale for Motor and Cognitive Functions (FSMC): Validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler 2009; 15: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 25. Yardley L, Beyer N, Hauer K, et al. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005; 34: 614–619. [DOI] [PubMed] [Google Scholar]
- 26. Van Vliet R, Hoang P, Lord S, et al. Falls efficacy scale-international: A cross-sectional validation in people with multiple sclerosis. Arch Phys Med Rehabil 2013; 94: 883–889. [DOI] [PubMed] [Google Scholar]
- 27. Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: The 12-item MS Walking Scale (MSWS-12). Neurology 2003; 60: 31–36. [DOI] [PubMed] [Google Scholar]
- 28. Lamb SE, Jorstad-Stein EC, Hauer K, et al. Development of a common outcome data set for fall injury prevention trials: The Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005; 53: 1618–1622. [DOI] [PubMed] [Google Scholar]
- 29. Stack E, Ashburn A. Fall events described by people with Parkinson’s disease: Implications for clinical interviewing and the research agenda. Physiother Res Int 1999; 4: 190–200. [DOI] [PubMed] [Google Scholar]
- 30. Cheng EM, Hays RD, Myers LW, et al. Factors related to agreement between self-reported and conventional Expanded Disability Status Scale (EDSS) scores. Mult Scler 2001; 7: 405–410. [DOI] [PubMed] [Google Scholar]
- 31. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Mult Scler 2007; 13: 52–57. [DOI] [PubMed] [Google Scholar]
- 32. Brown H, Prescott R. Applied mixed models in medicine. 2nd ed. Chichester: John Wiley & Sons, 2006. [Google Scholar]
- 33. Paltamaa J, Sarasoja T, Leskinen E, et al. Measuring deterioration in international classification of functioning domains of people with multiple sclerosis who are ambulatory. Phys Ther 2008; 88: 176–190. [DOI] [PubMed] [Google Scholar]
- 34. Learmonth YC, Paul L, McFadyen AK, et al. Reliability and clinical significance of mobility and balance assessments in multiple sclerosis. Int J Rehabil Res 2012; 35: 69–74. [DOI] [PubMed] [Google Scholar]
- 35. Gandolfi M, Munari D, Geroin C, et al. Sensory integration balance training in patients with multiple sclerosis: A randomized, controlled trial. Mult Scler 2015; 21: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 36. Hobart J, Blight AR, Goodman A, et al. Timed 25-foot walk: Direct evidence that improving 20% or greater is clinically meaningful in MS. Neurology 2013; 80: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 37. Gunn H, Creanor S, Haas B, et al. Frequency, characteristics, and consequences of falls in multiple sclerosis: Findings from a cohort study. Arch Phys Med Rehabil 2014; 95: 538–545. [DOI] [PubMed] [Google Scholar]
- 38. Nilsagard Y, Carling A, Forsberg A. Activities-specific balance confidence in people with multiple sclerosis. Mult Scler Int 2012; 2012: 613925 (8 pp.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsuda PN, Shumway-Cook A, Bamer AM, et al. Falls in multiple sclerosis. PM R 2011; 3: 624–632; quiz 32. [DOI] [PubMed] [Google Scholar]
- 40. Van Vliet R, Hoang P, Lord S, et al. Multiple sclerosis severity and concern about falling: Physical, cognitive and psychological mediating factors. NeuroRehabilitation 2015; 37: 139–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.